World Journal of Emergency Medicine ›› 2022, Vol. 13 ›› Issue (4): 266-273.doi: 10.5847/wjem.j.1920-8642.2022.068
• Original Articles • Previous Articles Next Articles
Xiao-kang Dai1, Zhen-xing Ding1, Yuan-yuan Tan1, Hua-rui Bao1, Dong-yao Wang2,3,4( ), Hong Zhang1(
), Hong Zhang1( )
)
Received:2021-11-02
Accepted:2022-02-24
Online:2022-06-23
Published:2022-07-01
Contact:
Dong-yao Wang,Hong Zhang
E-mail:dywsn@ustc.edu.cn;zhanghong20190628@163.com
Xiao-kang Dai, Zhen-xing Ding, Yuan-yuan Tan, Hua-rui Bao, Dong-yao Wang, Hong Zhang. Neutrophils inhibit CD8+ T cells immune response by arginase-1 signaling in patients with sepsis[J]. World Journal of Emergency Medicine, 2022, 13(4): 266-273.
Add to citation manager EndNote|Ris|BibTeX
URL: http://wjem.com.cn/EN/10.5847/wjem.j.1920-8642.2022.068
Table 1.
Characteristics of sepsis patients and healthy controls
| Characteristic | Healthy controls (n=10) | Sepsis patients (n=10) |
|---|---|---|
| Gender (M/F) | 5/5 | 6/4 |
| Age, years | 27 (19-43) | 31 (23-42) |
| White blood cell count, ×109 /L | 5.05 (4.07-7.46) | 9.46 (6.78-21.30) |
| C-reactive protein, mg/L | 2.07 (1.44-3.56) | 98.30 (70.70-133.50) |
| Procalcitonin, ng/mL | 0.11 (0.03-0.25) | 46.60 (21.70-54.50) |
| Neutrophil count, ×109/L | 3.64 (1.25-7.11) | 23.74 (15.60-36.70) |
| Lymphocyte count, ×109/L | 1.84 (0.76-2.60) | 0.53 (0.32-1.51) |
| Monocyte count, ×109/L | 0.48 (0.38-0.71) | 0.66 (0.39-1.21) |
| SOFA score | 0 | 6.35 (3.00-9.00) |
| Infection with Klebsiella pneumoniae | 8 | 8 |
| Infection with Staphylococcus aureus | 2 | 2 |
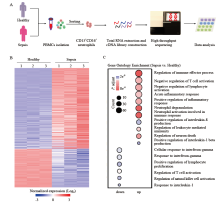
Figure 1.
The transcriptional profiles of neutrophils from patients with sepsis differed from those of healthy controls. A: the protocol of isolation of neutrophils from healthy controls (n=3) and patients with sepsis (n=3); B: the top-200 genes with differential expression (fold change greater than two-fold) in neutrophils from patients with sepsis compared with those in healthy controls were selected for heat map analyses; each column depicts one sample; C: enrichment analyses of differentially expressed genes were performed (using the Gene Ontology database) to evaluate enriched biological processes between neutrophils from patients with sepsis and healthy controls; enrichment of regulation of negative regulation of T cell activation-related biological processes was included.
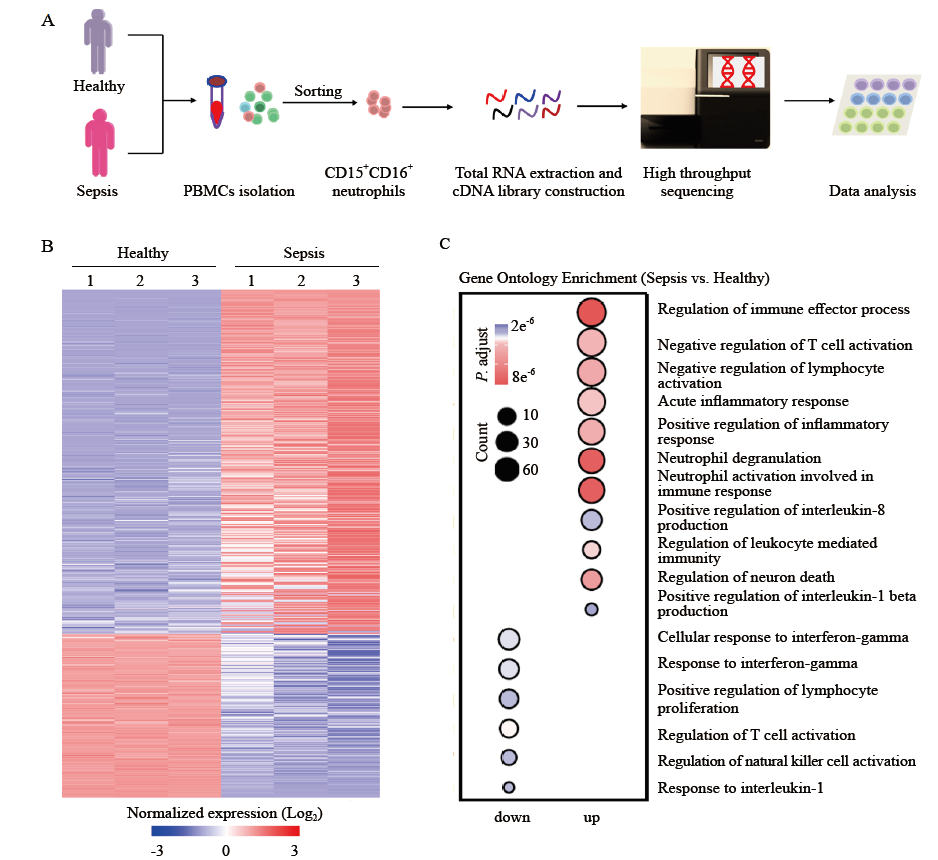
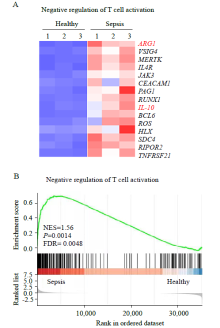
Figure 2.
Differentially expressed genes (DEGs) analysis and gene set enrichment analysis (GSEA) of neutrophils. A: heat map of negative regulation of T cell activation-related genes with differential expression in neutrophils from patients with sepsis compared with those from healthy controls; B: GSEA revealed an increase in negative regulation of T cell activation processes in neutrophils from patients with sepsis compared with that in healthy controls. NES: normalized enrichment score; FDR: false discovery rate.
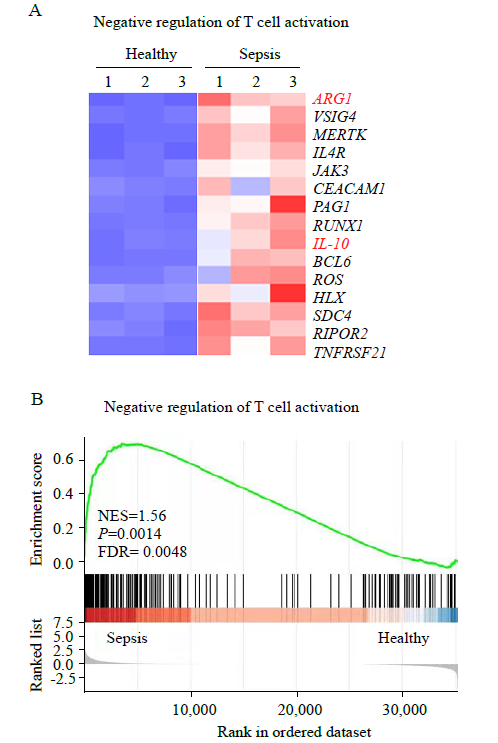

Figure 3.
Neutrophils expression of arginase-1 in patients with sepsis. A-D: ARG1, IL-10, PAG1, and RUNX1 expression in neutrophils from patients with sepsis and healthy controls, as assessed by qRT-PCR. Data were representative of six experiments; E: representative histograms (left) and percentage statistics (right) calculated for the expression of arginase-1 in neutrophils of patients with sepsis (red; n = 6) and healthy controls (blue; n = 6). Data were analyzed by two-tailed unpaired Student’s t-test; data were presented as mean ± standard deviation.
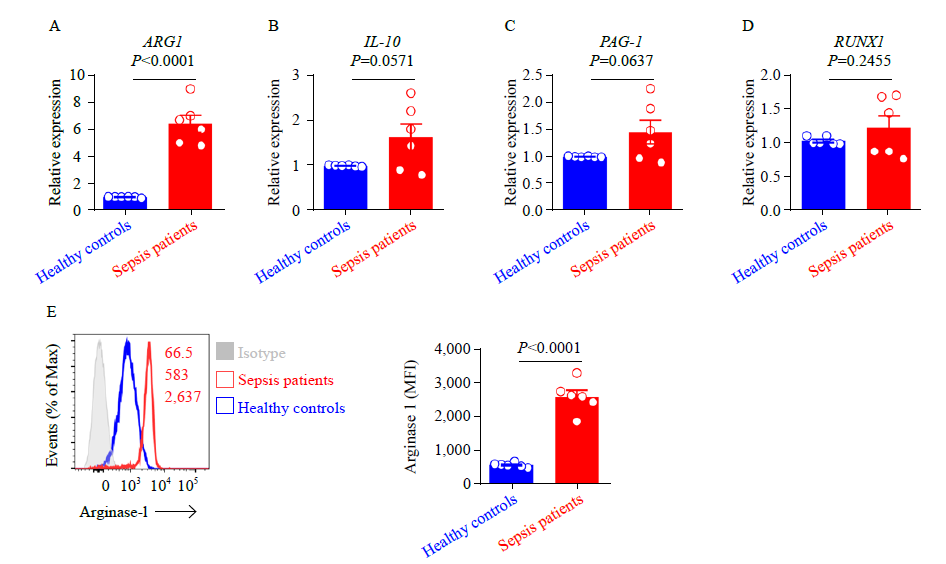
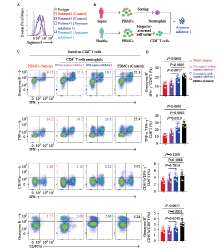
Figure 4.
Inhibition of arginase-1 signaling elevates the percentage of polyfunctional effector CD8+ T cells. A: representative histograms showing the analysis of arginase-1 expression in neutrophils of two patients with sepsis after being treated with arginase inhibitor 1; B: the protocol of the isolation of CD8+ T cells from patients with sepsis and healthy controls; and CD14-CD15+CD16+ neutrophils from patients with sepsis. Purified CD8+ T cells from healthy controls were cocultured with neutrophils (ratio = 3:1), treated with or without arginase inhibitor 1 (10 μmol/L); C and D: PBMCs of septic patients (red), or CD8+ T cells purified from healthy controls and cocultured with neutrophils, and treated with (blue) or without (purple) arginase inhibitor 1 (10 μmol/L) overnight, or PBMCs of healthy controls (black), were stimulated with PMA for 4 h. CD8+ T cells were gated for analysis. Representative flow cytometry plots (C) and pool data (D) of the proportion of IFN-γ and granzyme B coexpression, IFN-γ and TNF-α coexpression, IFN-γ and CD107a coexpression, as well as CD107a and granzyme B coexpression by CD8+ T cells. n 6. For (D), data were analyzed by one-way ANOVA with Tukey’s multiple comparisons test; Data are presented as mean ± standard deviation.

| 1 |
Chen J, Wei HM. Immune intervention in sepsis. Front Pharmacol. 2021; 12: 718089.
doi: 10.3389/fphar.2021.718089 |
| 2 |
Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018; 319(1): 62-75.
doi: 10.1001/jama.2017.17687 pmid: 29297082 |
| 3 |
van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017; 17(7): 407-20.
doi: 10.1038/nri.2017.36 pmid: 28436424 |
| 4 | Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016; 353: i1585. |
| 5 |
Spinella PC, Tucci M, Fergusson DA, Lacroix J, Hébert PC, Leteurtre S, et al. Effect of fresh vs standard-issue red blood cell transfusions on multiple organ dysfunction syndrome in critically ill pediatric patients: a randomized clinical trial. JAMA. 2019; 322(22): 2179-90.
doi: 10.1001/jama.2019.17478 pmid: 31821429 |
| 6 |
Rhee SG. Overview on peroxiredoxin. Mol Cells. 2016; 39(1): 1-5.
doi: 10.14348/molcells.2016.2368 pmid: 26831451 |
| 7 |
Huang M, Cai SL, Su JQ. The pathogenesis of sepsis and potential therapeutic targets. Int J Mol Sci. 2019; 20(21): 5376.
doi: 10.3390/ijms20215376 |
| 8 |
Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016; 193(3): 259-72.
doi: 10.1164/rccm.201504-0781OC |
| 9 |
Zhang H, Zeng L, Xie M, Liu J, Zhou BR, Wu RL, et al. TMEM173 drives lethal coagulation in sepsis. Cell Host Microbe. 2020; 27(4): 556-70.e6.
doi: S1931-3128(20)30112-8 pmid: 32142632 |
| 10 |
Karakike E, Giamarellos-Bourboulis EJ. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front Immunol. 2019; 10: 55.
doi: 10.3389/fimmu.2019.00055 pmid: 30766533 |
| 11 |
Liu L, Sun BW. Neutrophil pyroptosis: new perspectives on Sepsis. Cell Mol Life Sci. 2019; 76(11): 2031-42.
doi: 10.1007/s00018-019-03060-1 pmid: 30877336 |
| 12 |
Group RC, Horby P, Mafham M, Linsell L, Bell JL, Staplin N, et al. Effect of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020; 383(21): 2030-40.
doi: 10.1056/NEJMoa2022926 |
| 13 |
Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019; 133(20): 2178-85.
doi: 10.1182/blood-2018-11-844530 pmid: 30898862 |
| 14 |
Dąbrowska D, Jabłońska E, Garley M, Ratajczak-Wrona W, Iwaniuk A. New aspects of the biology of neutrophil extracellular traps. Scand J Immunol. 2016; 84(6): 317-22.
doi: 10.1111/sji.12494 pmid: 27667737 |
| 15 |
Yamamoto K, Yamada H, Wakana N, Kikai M, Terada K, Wada N, et al. Augmented neutrophil extracellular traps formation promotes atherosclerosis development in socially defeated apoE-/- mice. Biochem Biophys Res Commun. 2018; 500(2): 490-6.
doi: 10.1016/j.bbrc.2018.04.115 |
| 16 |
Ocana A, Nieto-Jiménez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. 2017; 16(1): 137.
doi: 10.1186/s12943-017-0707-7 |
| 17 |
Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014; 14(5): 302-14.
doi: 10.1038/nri3660 |
| 18 |
Sippel TR, Shimizu T, Strnad F, Traystman RJ, Herson PS, Waziri A. Arginase I release from activated neutrophils induces peripheral immunosuppression in a murine model of stroke. J Cereb Blood Flow Metab. 2015; 35(10): 1657-63.
doi: 10.1038/jcbfm.2015.103 |
| 19 |
Nascimento DC, Viacava PR, Ferreira RG, Damaceno MA, Piñeros AR, Melo PH, et al. Sepsis expands a CD39+plasmablast population that promotes immunosuppression via adenosine-mediated inhibition of macrophage antimicrobial activity. Immunity. 2021; 54(9): 2024-41.e8.
doi: 10.1016/j.immuni.2021.08.005 pmid: 34473957 |
| 20 | Darcy CJ, Woodberry T, Davis JS, Piera KA, McNeil YR, Chen YW, et al. Increased plasma arginase activity in human sepsis: association with increased circulating neutrophils. Clin Chem Lab Med. 2014; 52(4): 573-81. |
| 21 |
Reizine F, Lesouhaitier M, Gregoire M, Pinceaux K, Gacouin A, Maamar A, et al. SARS-CoV-2-induced ARDS associates with MDSC expansion, lymphocyte dysfunction, and arginine shortage. J Clin Immunol. 2021; 41(3): 515-25.
doi: 10.1007/s10875-020-00920-5 |
| 22 |
Pang R, Zhou H, Huang YF, Su YB, Chen XH. Inhibition of host arginase activity against staphylococcal bloodstream infection by different metabolites. Front Immunol. 2020; 11: 1639.
doi: 10.3389/fimmu.2020.01639 pmid: 32849560 |
| 23 |
Cong JJ, Wang XW, Zheng XH, Wang D, Fu BQ, Sun R, et al. Dysfunction of natural killer cells by FBP1-induced inhibition of glycolysis during lung cancer progression. Cell Metab. 2018; 28(2): 243-55.e5.
doi: 10.1016/j.cmet.2018.06.021 |
| 24 |
Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019; 50(4): 924-40.
doi: 10.1016/j.immuni.2019.03.024 |
| 25 |
Lee KH, Kronbichler A, Park DDY, Park Y, Moon H, Kim H, et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: a comprehensive review. Autoimmun Rev. 2017; 16(11): 1160-73.
doi: 10.1016/j.autrev.2017.09.012 |
| 26 |
Bergmann CB, Salyer CE, Beckmann N, Caldwell CC. Intraperitoneal neutrophil IL-10 production is promoted by interferon γ in a murine model of sepsis model in the acute phase of sepsis. Biochem Biophys Res Commun. 2020; 530(1): 278-84.
doi: 10.1016/j.bbrc.2020.07.089 |
| 27 |
Steinhauser ML, Hogaboam CM, Kunkel SL, Lukacs NW, Strieter RM, Standiford TJ. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999; 162(1): 392-9.
pmid: 9886412 |
| 28 |
Fu BQ, Wang DY, Shen XK, Guo C, Liu YY, Ye Y, et al. Immunomodulation induced during interferon-α therapy impairs the anti-HBV immune response through CD24+ CD38hi B cells. Front Immunol. 2020; 11: 591269.
doi: 10.3389/fimmu.2020.591269 |
| 29 |
Wang DY, Zheng XH, Fu BQ, Nian ZG, Qian YB, Sun R, et al. Hepatectomy promotes recurrence of liver cancer by enhancing IL-11-STAT3 signaling. EBioMedicine. 2019; 46: 119-32.
doi: 10.1016/j.ebiom.2019.07.058 |
| 30 |
Park I, Kim M, Choe K, Song E, Seo H, Hwang Y, et al. Neutrophils disturb pulmonary microcirculation in Sepsis-induced acute lung injury. Eur Respir J. 2019; 53(3): 1800786.
doi: 10.1183/13993003.00786-2018 |
| 31 |
Mortaz E, Alipoor SD, Adcock IM, Mumby S, Koenderman L. Update on neutrophil function in severe inflammation. Front Immunol. 2018; 9: 2171.
doi: 10.3389/fimmu.2018.02171 |
| 32 |
Seree-Aphinan C, Vichitkunakorn P, Navakanitworakul R, Khwannimit B. Distinguishing sepsis from infection by neutrophil dysfunction: a promising role of CXCR2 surface level. Front Immunol. 2020; 11: 608696.
doi: 10.3389/fimmu.2020.608696 |
| 33 |
Shen XK, Fu BQ, Liu YY, Guo C, Ye Y, Sun R, et al. NKp30+NK cells are associated with HBV control during pegylated-interferon-alpha-2b therapy of chronic hepatitis B. Sci Rep. 2016; 6: 38778.
doi: 10.1038/srep38778 |
| 34 |
Kurachi M. CD8+ T cell exhaustion. Semin Immunopathol. 2019; 41(3): 327-37.
doi: 10.1007/s00281-019-00744-5 pmid: 30989321 |
| 35 |
Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity. 2018; 48(2): 202-13.
doi: 10.1016/j.immuni.2018.01.007 |
| 36 |
Shen XF, Cao K, Jiang JP, Guan WX, Du JF. Neutrophil dysregulation during sepsis: an overview and update. J Cell Mol Med. 2017; 21(9): 1687-97.
doi: 10.1111/jcmm.13112 |
| [1] | Xuan Fu, Xue Lin, Samuel Seery, Li-na Zhao, Hua-dong Zhu, Jun Xu, Xue-zhong Yu. Speckle-tracking echocardiography for detecting myocardial dysfunction in sepsis and septic shock patients: A single emergency department study [J]. World Journal of Emergency Medicine, 2022, 13(3): 175-181. |
| [2] | Mei-jia Shen, Li-chao Sun, Xiao-yu Liu, Meng-chen Xiong, Shan Li, A-ling Tang, Guo-qiang Zhang. Trichostatin A improves the inflammatory response and liver injury in septic mice through the FoxO3a/autophagy signaling pathway [J]. World Journal of Emergency Medicine, 2022, 13(3): 182-188. |
| [3] | Hai Hu, Jing-yuan Jiang, Ni Yao. Comparison of different versions of the quick sequential organ failure assessment for predicting in-hospital mortality of sepsis patients: A retrospective observational study [J]. World Journal of Emergency Medicine, 2022, 13(2): 114-119. |
| [4] | Li-wei Duan, Jin-long Qu, Jian Wan, Yong-hua Xu, Yi Shan, Li-xue Wu, Jin-hao Zheng, Wei-wei Jiang, Qi-tong Chen, Yan Zhu, Jian Zhou, Wen-bo Yu, Lei Pei, Xi Song, Wen-fang Li, Zhao-fen Lin. Effects of viral infection and microbial diversity on patients with sepsis: A retrospective study based on metagenomic next-generation sequencing [J]. World Journal of Emergency Medicine, 2021, 12(1): 29-35. |
| [5] | Hai-jiang Zhou, Tian-fei Lan, Shu-bin Guo. Outcome prediction value of National Early Warning Score in septic patients with community-acquired pneumonia in emergency department: A single-center retrospective cohort study [J]. World Journal of Emergency Medicine, 2020, 11(4): 206-215. |
| [6] | Yu-ming Wang, Yan-jun Zheng, Ying Chen, Yun-chuan Huang, Wei-wei Chen, Ran Ji, Li-li Xu, Zhi-tao Yang, Hui-qiu Sheng, Hong-ping Qu, En-qiang Mao, Er-zhen Chen. Effects of fluid balance on prognosis of acute respiratory distress syndrome patients secondary to sepsis [J]. World Journal of Emergency Medicine, 2020, 11(4): 216-222. |
| [7] | Miao Yuan, Ding-yi Yan, Fang-shi Xu, Yi-di Zhao, Yang Zhou, Long-fei Pan. Effects of sepsis on hippocampal volume and memory function [J]. World Journal of Emergency Medicine, 2020, 11(4): 223-230. |
| [8] | Wen-peng Yin, Jia-bao Li, Xiao-fang Zheng, Le An, Huan Shao, Chun-sheng Li. Effect of neutrophil CD64 for diagnosing sepsis in emergency department [J]. World Journal of Emergency Medicine, 2020, 11(2): 79-86. |
| [9] | Shao-hua Liu, Huo-yan Liang, Hong-yi Li, Xian-fei Ding, Tong-wen Sun, Jing Wang. Effect of low high-density lipoprotein levels on mortality of septic patients: A systematic review and meta-analysis of cohort studies [J]. World Journal of Emergency Medicine, 2020, 11(2): 109-116. |
| [10] | Yi-wen Fan, Shao-wei Jiang, Jia-meng Chen, Hui-qi Wang, Dan Liu, Shu-ming Pan, Cheng-jin Gao. A pulmonary source of infection in patients with sepsis-associated acute kidney injury leads to a worse outcome and poor recovery of kidney function [J]. World Journal of Emergency Medicine, 2020, 11(1): 18-26. |
| [11] | Kimberly A. Chambers, Adam Y. Park, Rosa C. Banuelos, Bryan F. Darger, Bindu H. Akkanti, Annamaria Macaluso, Manoj Thangam, Pratik B. Doshi. Outcomes of severe sepsis and septic shock patients after stratification by initial lactate value [J]. World Journal of Emergency Medicine, 2018, 9(2): 113-117. |
| [12] | Ji Yong Jung, Ji Ung Na, Sang Kuk Han, Pil Cho Choi, Jang Hee LEE, Dong Hyuk Shin. Differential diagnoses of magnetic resonance imaging for suspected acute appendicitis in pregnant patients [J]. World Journal of Emergency Medicine, 2018, 9(1): 26-32. |
| [13] | Muhammad Akbar Baig, Hira Shahzad, Erfan Hussain, Asad Mian. Validating a point of care lactate meter in adult patients with sepsis presenting to the emergency department of a tertiary care hospital of a low- to middle-income country [J]. World Journal of Emergency Medicine, 2017, 8(3): 184-189. |
| [14] | Chao Cao, Tao Ma, Yan-fen Chai, Song-tao Shou. The role of regulatory T cells in immune dysfunction during sepsis [J]. World Journal of Emergency Medicine, 2015, 6(1): 5-9. |
| [15] | Kun Chen, Qiu-xiang Zhou, Hong-wei Shan, Wen-fang Li, Zhao-fen Lin. Prognostic value of CD4+CD25+ Tregs as a valuable biomarker for patients with sepsis in ICU [J]. World Journal of Emergency Medicine, 2015, 6(1): 40-43. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
