World Journal of Emergency Medicine ›› 2022, Vol. 13 ›› Issue (3): 182-188.doi: 10.5847/wjem.j.1920-8642.2022.056
• Original Articles • Previous Articles Next Articles
Mei-jia Shen1,2, Li-chao Sun2, Xiao-yu Liu1,2, Meng-chen Xiong2,3, Shan Li2,3, A-ling Tang2,3, Guo-qiang Zhang1,2( )
)
Received:2022-01-15
Accepted:2022-04-20
Online:2022-05-13
Published:2022-05-01
Contact:
Guo-qiang Zhang
E-mail:zhangchong2003@vip.sina.com
Mei-jia Shen, Li-chao Sun, Xiao-yu Liu, Meng-chen Xiong, Shan Li, A-ling Tang, Guo-qiang Zhang. Trichostatin A improves the inflammatory response and liver injury in septic mice through the FoxO3a/autophagy signaling pathway[J]. World Journal of Emergency Medicine, 2022, 13(3): 182-188.
Add to citation manager EndNote|Ris|BibTeX
URL: http://wjem.com.cn/EN/10.5847/wjem.j.1920-8642.2022.056

Figure 1.
TSA improved liver injury and inflammation and promoted autophagy and the expression of FoxO3a in the septic mice. A: the liver injury evaluated by H&E staining (200×); B: electron microscope results of the liver tissue in each group after different treatments (12,000×); C: the expression of LC3, P62, and FoxO3a in the liver tissue measured by Western blotting; D: immunofluorescence results of LC3 in the liver tissue after different treatments (400×). TSA: trichostatin A; CLP: cecal ligation and puncture; H&E: hematoxylin-eosin; DAPI: 4',6-diamidino-2-phenylindole.

Table 1.
Comparison of ALT, AST, IL-6, TNF-α in the serum and LC3 II, P62, and FoxO3a in the liver tissue at 12 h after the intervention in Institute of Cancer Research (ICR) mice
| Parameters | Control group | TSA group | CLP group | CLP+TSA group |
|---|---|---|---|---|
| ALT a, U/L | 54.92±9.68 | 34.36±12.90 | 198.18±27.07** | 128.42±20.55# |
| AST a, U/L | 206.05±17.11 | 100.49±6.39** | 634.98±74.10** | 478.60±32.56# |
| IL-6 a, pg/mL | 371.62±27.69 | 306.04±13.05** | 2,665.27±324.90** | 2,080.26±373.66# |
| TNF-α a, pg/mL | 177.79±24.72 | 135.00±17.40* | 399.01±60.98** | 221.90±46.89## |
| LC3 II b | 1 | 1.46±0.02** | 1.36±0.01** | 1.66±0.04## |
| P62 b | 1 | 0.60±0.02** | 0.87±0.02** | 0.59±0.02## |
| FoxO3a b | 1 | 2.03±0.13** | 1.49±0.02** | 2.89±0.03## |

Figure 2.
FoxO3a was involved in the induction of autophagy and the improvement of liver injury and inflammation in the sepsis cell model by TSA. The expression of IL-6 (A) and TNF-α (B) measured by ELISA; the expression of LC3, P62, and FoxO3a analyzed by Western blotting (C); immunofluorescence results of LC3 (D) and FoxO3a (E) detected by confocal microscopy (400×); the changes of LC3 and P62 expression after FoxO3a gene knocked down in AML12 cells in each group analyzed by Western blotting (F). FoxO3a: forkhead box O3a; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; TSA: trichostatin A; LPS: lipopolysaccharide; ELISA: enzyme-linked immunosorbent assay; DAPI: 4',6-diamidino-2-phenylindole. Compared with control group, *P<0.05, **P<0.01.
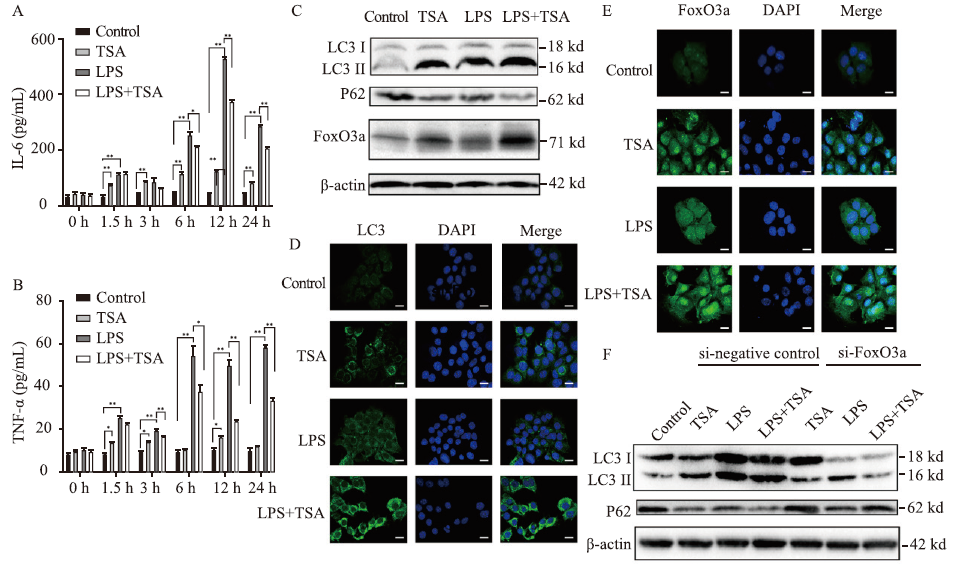
Table 2.
Comparison of ALT, AST, LC3 II, P62, and FoxO3a in AML12 cells
| Parameters | Control group | TSA group | LPS group | LPS+TSA group |
|---|---|---|---|---|
| ALT a, U/L | 5.05±0.55 | 14.20±2.20 | 29.42±0.62** | 23.47±0.64## |
| AST a, U/L | 9.55±0.65 | 18.90±3.10 | 63.52±4.50** | 38.51±1.50## |
| LC3 II b | 1 | 1.77±0.02** | 1.39±0.01** | 1.87±0.01## |
| P62 b | 1 | 0.50±0.01** | 0.73±0.01** | 0.38±0.01## |
| Foxo3a b | 1 | 2.23±0.02** | 1.79±0.01** | 3.10±0.07## |
Table 3.
Comparison of ALT, AST, IL-6, TNF-α, LC3 II, and P62 after FoxO3a gene knocked down in AML12 cells among groups
| Parameters | Control group | si-negative control | si-FoxO3a | ||||
|---|---|---|---|---|---|---|---|
| TSA group | LPS group | LPS+TSA group | TSA group | LPS group | LPS+TSA group | ||
| ALT a, U/L | 5.37±0.63 | 14.13±1.80 | 29.27±0.52 | 23.33±0.50 | 14.63±1.03 | 38.57±2.30** | 29.20±1.10** |
| AST a, U/L | 9.07±0.87 | 20.27±3.18 | 57.00±1.59 | 39.57±1.94 | 19.63±3.03 | 71.23±5.85* | 46.70±2.90* |
| IL-6 a, pg/mL | 40.05±1.96 | 111.77±2.53 | 500.50±7.96 | 355.07±9.71 | 132.07±2.76** | 598.57±14.01** | 413.73±26.24* |
| TNF-α a, pg/mL | 10.33±1.25 | 11.93±1.69 | 54.15±2.79 | 36.77±2.39 | 13.47±1.92* | 66.47±3.03* | 50.43±3.35** |
| LC3 II b | 1 | 1.51±0.01 | 2.51±0.02 | 2.57±0.01 | 1.22±0.01** | 1.69±0.01** | 1.28±0.04** |
| P62 b | 1 | 0.48±0.01 | 0.54±0.01 | 0.33±0.01 | 0.94±0.01** | 0.66±0.01** | 0.93±0.02** |
| 1 |
Machado FR, Mazza BF. Improving mortality in sepsis: analysis of clinical trials. Shock. 2010; 34(Suppl 1):54-8.
doi: 10.1097/SHK.0b013e3181e7e8b4 |
| 2 |
Mann EA, Baun MM, Meininger JC, Wade CE. Comparison of mortality associated with sepsis in the burn, trauma, and general intensive care unit patient: a systematic review of the literature. Shock. 2012; 37(1):4-16.
doi: 10.1097/SHK.0b013e318237d6bf |
| 3 |
Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002; 27(6):421-9.
doi: 10.1247/csf.27.421 pmid: 12576635 |
| 4 |
Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011; 469(7330):323-35.
doi: 10.1038/nature09782 |
| 5 |
Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008; 132(1):27-42.
doi: 10.1016/j.cell.2007.12.018 pmid: 18191218 |
| 6 |
Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010; 12(9):823-30.
doi: 10.1038/ncb0910-823 pmid: 20811354 |
| 7 |
Hsiao HW, Tsai KL, Wang LF, Chen YH, Chiang PC, Chuang SM, et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock. 2012; 37(3):289-96.
doi: 10.1097/SHK.0b013e318240b52a |
| 8 |
Shao L, Xiong X, Zhang Y, Miao H, Ren Y, Tang X, et al. IL-22 ameliorates LPS-induced acute liver injury by autophagy activation through ATF4-ATG7 signaling. Cell Death Dis. 2020; 11(11):970.
doi: 10.1038/s41419-020-03176-4 |
| 9 |
Pei L, He L. Hepatoprotective effect of anemoside B4 against sepsis-induced acute liver injury through modulating the mTOR/p70S6K-mediated autophagy. Chem Biol Interact. 2021; 345:109534.
doi: 10.1016/j.cbi.2021.109534 |
| 10 |
Yu Q, Zou L, Yuan X, Fang F, Xu F. Dexmedetomidine protects against septic liver injury by enhancing autophagy through activation of the AMPK/SIRT1 signaling pathway. Front Pharmacol. 2021; 12:658677.
doi: 10.3389/fphar.2021.658677 |
| 11 |
Zhang JB, Ng S, Wang JG, Zhou J, Tan SH, Yang ND, et al. Histone deacetylase inhibitors induce autophagy through FoxO1-dependent pathways. Autophagy. 2015; 11(4):629-42.
doi: 10.1080/15548627.2015.1023981 |
| 12 |
Kim SJ, Park JS, Lee DW, Lee SM. Trichostatin A protects liver against septic injury through inhibiting toll-like receptor signaling. Biomol Ther (Seoul). 2016; 24(4):387-94.
doi: 10.4062/biomolther.2015.176 |
| 13 |
Cui SN, Chen ZY, Yang XB, Chen L, Yang YY, Pan SW, et al. Trichostatin A modulates the macrophage phenotype by enhancing autophagy to reduce inflammation during polymicrobial sepsis. Int Immunopharmacol. 2019; 77:105973.
doi: 10.1016/j.intimp.2019.105973 |
| 14 |
Guo Y, Li Z, Shi C, Li J, Yao M, Chen X. Trichostatin A attenuates oxidative stress-mediated myocardial injury through the FoxO3a signaling pathway. Int J Mol Med. 2017; 40(4):999-1008.
doi: 10.3892/ijmm.2017.3101 |
| 15 |
Liu YM, Lv J, Zeng QL, Shen S, Xing JY, Zhang YY, et al. AMPK activation ameliorates D-GalN/LPS-induced acute liver failure by upregulating FoxO3a to induce autophagy. Exp Cell Res. 2017; 358(2):335-42.
doi: 10.1016/j.yexcr.2017.07.008 |
| 16 |
Funk DJ, Parrillo JE, Kumar A. Sepsis and septic shock: a history. Crit Care Clin. 2009; 25(1):83-101, viii.
doi: 10.1016/j.ccc.2008.12.003 |
| 17 |
Deitch EA. Rodent models of intra-abdominal infection. Shock. 2005; 24(Suppl 1):19-23.
doi: 10.1097/01.shk.0000191386.18818.0a |
| 18 |
Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006; 25(51):6680-4.
doi: 10.1038/sj.onc.1209954 pmid: 17072321 |
| 19 |
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010; 140(6):805-20.
doi: 10.1016/j.cell.2010.01.022 pmid: 20303872 |
| 20 |
Takano KI, Yamamoto S, Tomita K, Takashina M, Yokoo H, Matsuda N, et al. Successful treatment of acute lung injury with pitavastatin in septic mice: potential role of glucocorticoid receptor expression in alveolar macrophages. J Pharmacol Exp Ther. 2011; 336(2):381-90.
doi: 10.1124/jpet.110.171462 |
| 21 | Oh JE, Lee HK. Pattern recognition receptors and autophagy. Front Immunol. 2014; 5:300. |
| 22 |
Brealey D, Singer M. Mitochondrial dysfunction in Sepsis. Curr Infect Dis Rep. 2003; 5(5):365-71.
doi: 10.1007/s11908-003-0015-9 pmid: 13678565 |
| 23 |
Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012; 13(3):255-63.
doi: 10.1038/ni.2215 |
| 24 |
Guo Y, Li Z, Shi C, Li J, Yao M, Chen X. Trichostatin A attenuates oxidative stress-mediated myocardial injury through the FoxO3a signaling pathway. Int J Mol Med. 2017; 40(4):999-1008.
doi: 10.3892/ijmm.2017.3101 |
| 25 | Ali T, Rahman SU, Hao Q, Li W, Liu Z, Ali Shah F, et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FoxO3a regulation. J Pineal Res. 2020; 69(2):e12667. |
| 26 | Wu Y, Leng Y, Meng Q, Xue R, Zhao B, Zhan L, et al. Suppression of excessive histone deacetylases activity in diabetic hearts attenuates myocardial ischemia/reperfusion injury via mitochondria apoptosis pathway. J Diabetes Res. 2017; 2017: 8208065. |
| [1] | Xuan Fu, Xue Lin, Samuel Seery, Li-na Zhao, Hua-dong Zhu, Jun Xu, Xue-zhong Yu. Speckle-tracking echocardiography for detecting myocardial dysfunction in sepsis and septic shock patients: A single emergency department study [J]. World Journal of Emergency Medicine, 2022, 13(3): 175-181. |
| [2] | Hai Hu, Jing-yuan Jiang, Ni Yao. Comparison of different versions of the quick sequential organ failure assessment for predicting in-hospital mortality of sepsis patients: A retrospective observational study [J]. World Journal of Emergency Medicine, 2022, 13(2): 114-119. |
| [3] | Li-wei Duan, Jin-long Qu, Jian Wan, Yong-hua Xu, Yi Shan, Li-xue Wu, Jin-hao Zheng, Wei-wei Jiang, Qi-tong Chen, Yan Zhu, Jian Zhou, Wen-bo Yu, Lei Pei, Xi Song, Wen-fang Li, Zhao-fen Lin. Effects of viral infection and microbial diversity on patients with sepsis: A retrospective study based on metagenomic next-generation sequencing [J]. World Journal of Emergency Medicine, 2021, 12(1): 29-35. |
| [4] | Hai-jiang Zhou, Tian-fei Lan, Shu-bin Guo. Outcome prediction value of National Early Warning Score in septic patients with community-acquired pneumonia in emergency department: A single-center retrospective cohort study [J]. World Journal of Emergency Medicine, 2020, 11(4): 206-215. |
| [5] | Yu-ming Wang, Yan-jun Zheng, Ying Chen, Yun-chuan Huang, Wei-wei Chen, Ran Ji, Li-li Xu, Zhi-tao Yang, Hui-qiu Sheng, Hong-ping Qu, En-qiang Mao, Er-zhen Chen. Effects of fluid balance on prognosis of acute respiratory distress syndrome patients secondary to sepsis [J]. World Journal of Emergency Medicine, 2020, 11(4): 216-222. |
| [6] | Miao Yuan, Ding-yi Yan, Fang-shi Xu, Yi-di Zhao, Yang Zhou, Long-fei Pan. Effects of sepsis on hippocampal volume and memory function [J]. World Journal of Emergency Medicine, 2020, 11(4): 223-230. |
| [7] | Wen-peng Yin, Jia-bao Li, Xiao-fang Zheng, Le An, Huan Shao, Chun-sheng Li. Effect of neutrophil CD64 for diagnosing sepsis in emergency department [J]. World Journal of Emergency Medicine, 2020, 11(2): 79-86. |
| [8] | Shao-hua Liu, Huo-yan Liang, Hong-yi Li, Xian-fei Ding, Tong-wen Sun, Jing Wang. Effect of low high-density lipoprotein levels on mortality of septic patients: A systematic review and meta-analysis of cohort studies [J]. World Journal of Emergency Medicine, 2020, 11(2): 109-116. |
| [9] | Yi-wen Fan, Shao-wei Jiang, Jia-meng Chen, Hui-qi Wang, Dan Liu, Shu-ming Pan, Cheng-jin Gao. A pulmonary source of infection in patients with sepsis-associated acute kidney injury leads to a worse outcome and poor recovery of kidney function [J]. World Journal of Emergency Medicine, 2020, 11(1): 18-26. |
| [10] | Kimberly A. Chambers, Adam Y. Park, Rosa C. Banuelos, Bryan F. Darger, Bindu H. Akkanti, Annamaria Macaluso, Manoj Thangam, Pratik B. Doshi. Outcomes of severe sepsis and septic shock patients after stratification by initial lactate value [J]. World Journal of Emergency Medicine, 2018, 9(2): 113-117. |
| [11] | Muhammad Akbar Baig, Hira Shahzad, Erfan Hussain, Asad Mian. Validating a point of care lactate meter in adult patients with sepsis presenting to the emergency department of a tertiary care hospital of a low- to middle-income country [J]. World Journal of Emergency Medicine, 2017, 8(3): 184-189. |
| [12] | Ruo Wu, Luo-gen Peng, Hui-min Zhao. Diverse coagulopathies in a rabbit model with different abdominal injuries [J]. World Journal of Emergency Medicine, 2017, 8(2): 141-147. |
| [13] | Jia-jun Xu, Jian-tao Zhen, Li Tang, Qing-ming Lin. Intravenous injection of Xuebijing attenuates acute kidney injury in rats with paraquat intoxication [J]. World Journal of Emergency Medicine, 2017, 8(1): 61-64. |
| [14] | Chao Cao, Tao Ma, Yan-fen Chai, Song-tao Shou. The role of regulatory T cells in immune dysfunction during sepsis [J]. World Journal of Emergency Medicine, 2015, 6(1): 5-9. |
| [15] | Wei-ping Sun, Guang-xiong Yuan, Yan-juan Hu, Li-zhen Liao, Lin Fu. Effect of low-dose glucocorticoid on corticosteroid insufficient patients with acute exacerbation of chronic obstructive pulmonary disease [J]. World Journal of Emergency Medicine, 2015, 6(1): 34-39. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
