INTRODUCTION
Patients with intracranial infections in intensive care units (ICUs) are commonly infected by bacteria, with over 80% of cases caused by gram-positive bacteria.[1] A retrospective study revealed that the incidence of Staphylococcus aureus varies from 4% to 10%, and nearly half of these cases were associated with methicillin-resistant Staphylococcus aureus (MRSA).[2,3] MRSA-associated intracranial infection is one of the most severe diseases and is characterized by a wide range of complications and high mortality.[4,5] Vancomycin is recommended as the first-line therapy for central nervous system (CNS) infections.[6]
The blood-brain barrier (BBB) consists of microvascular endothelial cells and their tight junction proteins (TJPs), as well as astrocytes and pericytes.[7] It protects brain tissue from toxins and pathogens and strictly maintains the stability of the CNS environment.[8] Nevertheless, the BBB also restricts the entry of macromolecules and most small-molecule drugs, which is not conducive to the treatment of intracranial diseases such as intracranial infection, CNS tumors and metabolic disorders.[9,10] As a large hydrophilic molecule with a moderate affinity for plasma proteins, vancomycin is not highly permeable through the BBB. This phenomenon contributes to the low concentration of vancomycin in the brain tissue, which is a major cause of treatment failure in patients with MRSA intracranial infection. Therefore, the development of an effective way to increase BBB permeability and facilitate vancomycin delivery to the BBB is critical to improve treatment outcomes.
Many strategies have been investigated to promote drug delivery to the CNS through the BBB. These strategies include widespread increased permeability of the BBB,[11] intrathecal or intraventricular administration,[12,13] and modification of drug delivery systems,[14] despite their unknown mechanisms. Mannitol is usually used as a dehydrating agent in patients with cerebral edema in clinical practice. Previous animal experiments have confirmed that intravenous administration of mannitol can promote the delivery of various substances, such as antineoplastic drugs and viral vectors, to the CNS, which could substantially delay disease progression and prolong survival.[9,15] These outcomes are likely related to the ability of mannitol to open the BBB.[16,17] Consequently, this study aimed to explore the duration of mannitol-induced BBB opening and further explore the mechanism underlying the decrease in the endothelial glycocalyx layer (EGL) through filamentous actin (F-actin) depolymerization to guide the simultaneous therapy of vancomycin and mannitol and improve the therapeutic effect of MRSA intracranial infection.
METHODS
Animals and groups
All animal procedures were approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital and Guangdong Academy of Medical Sciences, Guangzhou, China (S2023-766-01). Male rats (3‒4 weeks, 180‒210 g) were purchased from Zhuhai Bestest Biotechnology Co., China. The rats were housed at room temperature (25±0.5) °C under a 12 h-12 h light-dark cycle with free access to water and food. The animals were anesthetized via intraperitoneal injection of 3% pentobarbital sodium solution (30 mg/kg) and randomly divided into a sham operation group (group A) and a MRSA infection group (group B). The MRSA-infected rats were then randomly distributed into four groups according to different administrations: the MRSA+vancomycin group (the MRSA+Van group), the MRSA+mannitol group (the MRSA+Man group), the MRSA+simultaneous administration of vancomycin and mannitol group (the MRSA+Van+Man group), and the MRSA+10-hour interval administration of vancomycin and mannitol group (the MRSA+Van+Man [10 h] group). Specifically, vancomycin (9 mg/100 g body weight, Vianex S.A., Greece) and mannitol (200 mg/100 g body weight, Coolaber, China) were administered via tail vein injection every 12 h for three consecutive days (supplementary Figure 1).
Cell culture and groups
Brain microvascular endothelial cells (bEnd3, MeisenCTCC, Meisen CTCC Co., Ltd., China) were cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) solution (Zeta Life, USA) at 37 °C and 5% CO2. These cells were incubated in DMEM (the control group) or DMEM with 5% mannitol for 1, 3, 6, 12 or 24 h in each group (the mannitol group). For further analysis of the association between F-actin and syndecan-1, cytochalasin-D (Cyt D, Abcam, UK) was added to promote F-actin depolymerization, or jasplakinolide (Jask, Abcam, UK) to promote F-actin polymerization. The cells were divided into five groups in accordance with the intervention measures: the control group, the mannitol group, the Cyt D+mannitol group (the Cyt D+mannitol group), the Jask+mannitol group (the Jask+mannitol group), and the DMSO+mannitol group (the DMSO+mannitol group). In the other three groups, the cells were incubated with Cyt D (100 nmol), Jask (15 nmol) or the vehicle DMSO (0.1%, v/v) for 1 h before intervention. The samples were subsequently processed with mannitol for 6 h.
Statistical analysis
All the measurement data are presented as the mean ±standard deviation. Different statistical methods were applied according to different types of data. Student’s t-test was used to compare the data in Figures 1 and 2A and supplementary Figures 2 and 4. One-way analysis of variance (ANOVA) was used to compare the data in Figures 2B, 2C, 2E, 3A-B, 3D-E, 4, and supplementary Figure 3, and multiple comparisons were performed via Tukey’s test. All the data were analyzed via the statistical software SPSS (version 16.0). A P-value <0.05 was considered statistically significant.
Figure 1.
Figure 1.
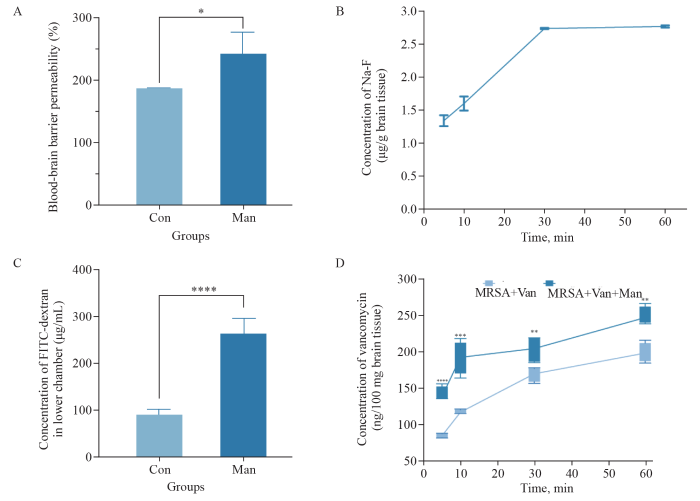
Mannitol promoted BBB opening for 30 min in rats and elevated the concentration of vancomycin in brain tissues. A: the Man group increased BBB permeability compared with the Con group (P<0.05); B: the concentration of NaF rapidly increased during initial 5-min period, and increased over 30 min following mannitol administration; the NaF concentration at 30 min showed no difference compared with that at 60 min; C: the concentration of FITC-dextran in the lower chamber of the Transwell was significantly higher in the Man group than that in the Con group (P<0.05); D: the concentration of vancomycin in the Man+Van group tended to increase at 5, 10, 30 and 60 min compared with that in the Van group (P<0.01). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. BBB: blood-brain barrier; NaF: sodium fluorescein; Man: mannitol; Van: vancomycin; FITC: fluorescein isothiocyanate.
Figure 2.
Figure 2.
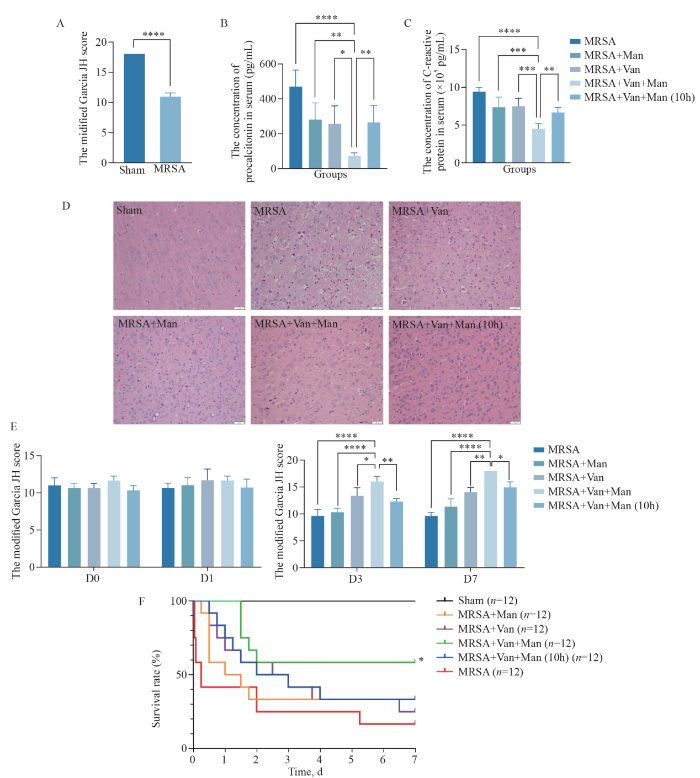
Simultaneous therapy of mannitol and vancomycin attenuated inflammation and improved therapeutic effect in rats with MRSA-induced intracranial infection. A: the modified Garcia JH scores were lower in the MRSA group than in the sham group (P<0.0001); B: the levels of PCT decreased in the MRSA+Van+Man group compared to those in the MRSA group, the MRSA+Man group, the MRSA+Van group and the MRSA+Van+Man (10 h) group significantly (P<0.05); C: the levels of CRP decreased in the MRSA+Van+Man group compared to those in the MRSA group, the MRSA+Man group, the MRSA+Van group and the MRSA+Van+Man (10 h) group significantly (P<0.05); D: the HE staining displayed the inflammatory cell infiltration in the brain tissues of each group; E: the MRSA+Van+Man group had better neurological function compared with the MRSA group, the MRSA+Man group, the MRSA+Van group and the MRSA+Van+Man (10 h) group on days 3 and 7; F: the MRSA+Van+Man group had a better 7-day survival rate than the MRSA group, the MRSA+Man group, the MRSA+Van group, and the MRSA+Van+Man (10 h) group. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. MRSA: Methicillin-resistant Staphylococcus aureus; sham, sham operation; Van: vancomycin; Man: mannitol; PCT: procalcitonin; CRP: C-reactive protein.
Figure 3.
Figure 3.
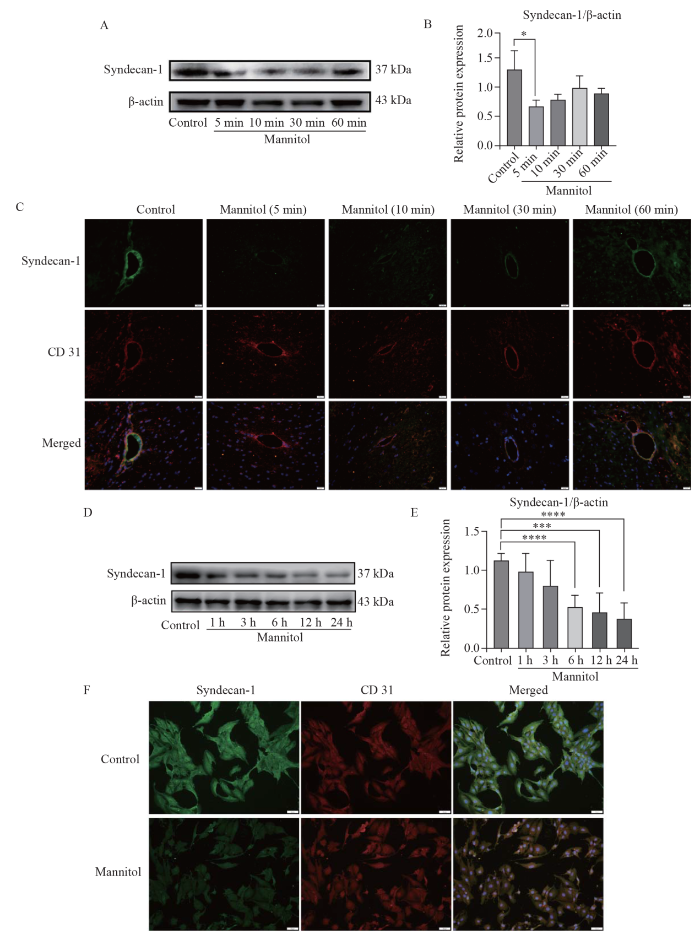
Mannitol reduced the EGL in vivo and in vitro. A, B: following mannitol administration, the expression levels of syndecan-1 in the brain tissue of rats sharply decreased at 5 min (P<0.05) and gradually returned to normal at 10, 30 and 60 min (P>0.05). C: the immunofluorescence signals of syndecan-1 (green) were faded at 5 min after mannitol administration, but were gradually restored at 10, 30 and 60 min; D, E: the expression levels of syndecan-1 of the cells decreased over time at 1, 3, 6, 12 and 24 h after mannitol intervention; F: the immunofluorescence signals of syndecan-1 (green) were faded in the Man group compared with the control group. *P<0.05, ***P<0.001, ****P<0.0001. EGL: endothelial glycocalyx layer.
Figure 4.
Figure 4.
Mannitol weakened EGL via F-actin depolymerization. A, B: the expression of F-actin decreased over time after mannitol intervention; C: the immunofluorescence signals of syndecan-1 (red) were faded in the Man group compared with the Con group; D, F, G: when pre-treated with Cyt D, the expression of F-actin and syndecan-1 reduced in the Cyt D+Man group compared with the Man group (P<0.05); E, H, I: when pre-treated with Jask, the expression of F-actin and syndecan-1 reduced in the Cyt D+Man group compared with the Man group (P<0.05); J: the immunofluorescence staining images showed that when F-actin was depolymerized with Cyt D, the syndecan-1 further declined, while F-actin was polymerized with Jask, the syndecan-1 remained stable. *P<0.05, ***P<0.001, ****P<0.0001. EGL: endothelial glycocalyx layer; Man: mannitol; Con: control; Cyt D: cytochalasin-D.
Additional methods and materials are presented in the supplementary data.
RESULTS
Mannitol promoted BBB opening for 30 min in rats and elevated the concentration of vancomycin in brain tissues
The levels of NaF in brain tissues serve as a reliable indicator of BBB permeability. Compared with the control group, the mannitol group exhibited a significant increase in BBB permeability merely 5 min after mannitol injection (P<0.05, Figure 1A). A detailed time-course analysis of the NaF concentration in the brain tissue was then carried out. During the initial 5-minute period, the NaF concentration increased most rapidly, with a more moderate increase over the following 30 min. Strikingly, at 60 min, there was no difference in NaF concentration compared with that at the 30 min (P>0.05, Figure 1B). These findings demonstrated that mannitol was capable of transiently opening the BBB, with peak permeability occurring at 5 min and lasting for about 30 min.
In vitro, it was observed that with mannitol intervention, a substantially greater amount of fluorescein isothiocyanate-dextran (FITC-dextran) managed to permeate through the single-cell layer in the mannitol group compared to the control group (P<0.05, Figure 1C). These findings provide strong supplementary evidence that mannitol was able to enhance cell barrier permeability.
Given the established influence of mannitol on BBB permeability, we further explored its potential role in facilitating the transport of vancomycin across the BBB. Compared with the Van group (MRSA+Van group), the Van+Man group (MRSA+Van+Man group) consistently demonstrated a tendency for elevated vancomycin concentrations in brain tissue at multiple time points, namely, 5, 10, 30, and 60 min (P<0.05, Figure 1D). These findings indicated that mannitol could effectively promote the entry of vancomycin into the brain, thereby increasing drug availability at the site of infection.
Therapeutic effects of simultaneous administration of mannitol and vancomycin
Twenty-four hours after the intracranial injection of MRSA in a rat model (day 0), the modified Garcia JH scores were lower in the MRSA group than in the sham group (P<0.0001, Figure 2A), indicating that the model was successfully established. Given the transient duration (30 min) of mannitol-induced BBB opening described above, we focused on the differences between simultaneous therapy and 10-hour interval administration of vancomycin and mannitol.
After treatment, the rats in the MRSA+Van+Man group presented significantly decreased procalcitonin (PCT) and C-reactive protein in the peripheral blood compared to rats in the MRSA, MRSA+Van, MRSA+Man, and MRSA+Van+Man (10 h) groups (P<0.05, Figures 2B, C), suggesting the superior anti-inflammatory effects. Hematoxylin-eosin (HE) staining of brain tissue from the MRSA group revealed edema, erythrocyte extravasation, and neutrophil infiltration. However, all the treated groups demonstrated reduced neutrophil infiltration compared to the MRSA group. Notably, the MRSA+Van+Man group presented the least neutrophil infiltration (Figure 2D). This finding further confirmed the potent anti-inflammatory effect of the simultaneous administration of mannitol and vancomycin.
We next investigated the neurological behavior and survival time of the rats to determine the therapeutic effects. Immediately after model establishment, the Garcia JH scores did not differ between the groups on day 0 and day 1 (P>0.05, Figure 2E). On days 3 and 7, the MRSA+Van+Man group had higher scores than the MRSA, the MRSA+Van, the MRSA+Man, and the MRSA+Van+Man (10 h) groups (P<0.05, Figure 2E), indicating enhanced neurological recovery. Furthermore, the MRSA+Van+Man group had a significantly higher 7-day survival rate than the other groups (P<0.05, Figure 2F), while no significant differences were observed between the MRSA, MRSA+Van, MRSA+Man, and MRSA+Van+Man (10 h) groups (P>0.05, Figure 2F). The results strongly suggested that the simultaneous administration of mannitol and vancomycin was efficacious in attenuating both peripheral and cerebral inflammation, and these effects were likely attributable to mannitol’s ability to open the BBB rather than its dehydrating ability, as evidenced by the different outcomes between the MRSA+Van+Man group and the MRSA+Van+Man (10 h) group.
Mannitol reduced the EGL in vivo and in vitro
The EGL, a complex consisting of proteins and polysaccharides (such as hyaluronic acid [HA] and heparan sulfate [HS]), is located inside vessels and is important in regulating vascular permeability.[18] By employing Western blotting and double immunofluorescence staining techniques, we assessed the expression of syndecan-1, a marker of the EGL,[19] in rat brain tissue. Strikingly, 5 min after mannitol administration, the expression of syndecan-1 in the brain tissue significantly decreased (P<0.05; Figures 3A, B). Over the next 10, 30, and 60 min, the expression gradually returned to normal levels (P>0.05; Figures 3A, B).
Immunofluorescence analysis further confirmed these findings. Compared with the control group, the immunofluorescence signal of syndecan-1 was weakened 5 min after mannitol administration but then gradually recovered over the following 10, 30 and 60 min (Figure 3C). Moreover, the levels of HA and HS in the serum increased in the mannitol group compared with that in the control group at 5, 10, 30 and 60 min (P<0.05, supplementary Figures 2A, B), providing evidence that mannitol could downregulate EGL in vivo.
To further validate our in vivo observations, in vitro experiments were carried out using bEnd.3 cells. Following mannitol treatment, syndecan-1 expression tended to decrease at 1, 3, 6, 12 and 24 h (P<0.05, Figures 3D‒F). Moreover, the levels of HA and HS in the supernatant of the mannitol group were notably higher than those in the control group (P<0.05, supplementary Figures 2C, D). Importantly, the expression of TJPs such as occludin, ZO-1 and claudin-1 did not differ between the control group and the mannitol group either in vivo or in vitro (P>0.05, supplementary Figures 3 and 4). These results indicated that mannitol’s effect on BBB permeability was mediated through alterations in the EGL.
Mannitol weakened EGL via F-actin depolymerization
F-actin is an important intracellular component for signal transduction, cell structure support and material transport.[20-
To clarify the relationships between F-actin and EGL, we used Jask to promote polymerization and Cyt D to promote depolymerization. The Western blotting results revealed that when F-actin was depolymerized by Cyt D, the expression of syndecan-1 was lower in the Cyt D+mannitol group than in the mannitol group (P<0.05, Figures 4D, F, G). Conversely, as F-actin was polymerized by Jask, the expression of syndecan-1 was not significantly different between the Jask+mannitol group and the control group (P>0.05, Figures 4E, H, I). Immunofluorescence staining further revealed that as F-actin was depolymerized, the syndecan-1 signal decreased in the Cyt D+mannitol group compared with that in the mannitol group (Figure 4J), whereas F-actin was polymerized, the syndecan-1 signal was not different between the Jask+mannitol group and the mannitol group (Figure 4J). Therefore, we thought that mannitol decreased the EGL through F-actin depolymerization in this study.
DISCUSSION
In this study, mannitol transiently increased BBB permeability for 30 min. When vancomycin was applied for 30 min following mannitol administration, there was an overt increase in the concentration of vancomycin in brain tissues, suggesting that the altered BBB permeability facilitates the entry of drugs into the brain. By comparing simultaneous therapy with a 10-hour interval administration of vancomycin and mannitol, we confirmed the neuroprotective effects of simultaneous therapy in the context of MRSA intracranial infection. These effects included attenuated peripheral inflammation and neuroinflammation, as well as improved neurobehavioral function and increased survival rates.
Mannitol is beneficial for many neurological disorders, such as intracranial tumors and brain metabolic disorders,[9,23,24] but its effects on MRSA-associated intracranial infection deserve further exploration. In addition to its dehydrating effect, mannitol transiently opens the BBB, revealing the neuroprotective effects of the simultaneous administration of vancomycin and mannitol in CNS infection. In this study, increased concentrations of vancomycin in brain tissues were detected in the rats with simultaneous therapy with vancomycin and mannitol compared with those with only vancomycin treatment. Moreover, the MRSA rats subjected to simultaneous administration of mannitol and vancomycin presented attenuated inflammation and improved neurological function and 7-day survival rates compared with those subjected to 10-hour interval administration. Consequently, these results suggested that medication time in mannitol BBB opening period is essential for treatment of MRSA intracranial infection. Interestingly, unlike the intra-arterial injection of hyperosmotic mannitol, as previously reported,[23] the intravenous administration of mannitol in this study strengthened its clinical effect. Additionally, overuse of mannitol may have a long-term impact on BBB leakage, neurological dysfunction and increased pro-inflammatory cytokines,[25,26] indicating that further research on the dose-effect relationship of mannitol is needed. The reported dose of mannitol ranges from 1.0 to 6.9 mg per gram of body weight, among which a dose of 2.0 mg per gram of body weight is the most commonly used.[9,27,28] The concentration of mannitol varies from 20% to 25%, with 20% being the most popular option and being closer to clinical practice.
The traditional view is that hypertension caused by mannitol leads to contraction of endothelial cells, disrupting TJPs between cells, thus leading to increased BBB permeability.[29] However, a study has shown that changes in plasma osmolality do not substantially alter the expression of TPJs, such as claudin-1, occludin, and ZO-1, but instead lead to increased expression of TJP genes.[30] Therefore, it is not sufficient to explain the mechanism of mannitol opening with changes in TPJs alone. EGL plays an essential role in vascular permeability. The main physiological function of the EGL is to form the blood-tissue barrier and regulate the selective permeability of vessels and substance exchange.[19] In this study, we reported that mannitol increases BBB permeability via regulation of the EGL and further demonstrated that mannitol transiently opens the BBB for 30 min via a reduction in the EGL. When microvascular endothelial cells were treated with mannitol, the expression of syndecan-1 decreased significantly. The results of the in vivo experiment were similar to those of mannitol administration; the rats presented evidence of destruction of the EGL of the BBB, as a decrease in the level of syndecan-1 was accompanied by increased BBB permeability at 5 min, but the expression of syndecan-1 and BBB permeability gradually recovered over 30 min.
F-actin plays an essential role in cell barrier function.[22] We found that when F-actin was polymerized, the level of syndecan-1 remained stable after mannitol intervention. In contrast, when F-actin was depolymerized, the expression of syndecan-1 was further downregulated. These findings suggested that the EGL was regulated by F-actin.
CONCLUSION
The results of the present study show that mannitol may increase BBB permeability for 30 min via a reduction in EGL through F-actin depolymerization. Simultaneous administration of vancomycin and mannitol helps increase the concentration of vancomycin in the brain tissue and improves the therapeutic effect in rats with MRSA intracranial infection.
Funding: This study was supported by the National Natural Science Foundation for Young Scientists of China (grant no. 82002074), the Natural Science Foundation of Guangdong Province (2023A1515010267, 2023A1515012665, 2024A1515010073), the China International Medical Foundation Cerebrovascular Disease Youth Innovation Fund (Z-2016-20-2201), and the Medical Leading Talents Fund of Guangdong Province (KJ012019430)..
Ethical approval: This study was reviewed and approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital (S2023-766-01).
Conflicts of interest: The authors declare that they have no competing interests.
Contributors: YW: conceptualization, resources, writing-original draft. ZWS: methodology, investigation. HSZ: methodology, investigation. MTL: formal analysis. ZL: data curation. SYZ: software. SMC: writing-review & editing. JQT: writing-review & editing. HGD: formal analysis. HKZ: conceptualization, supervision. All the authors agree to be accountable for all aspects of the work, ensuring its integrity and accuracy, and all the authors have read and approved the final manuscript.
All the supplementary files in this paper are available at http://wjem.com.cn.
Reference
Risk factors for intracranial infection after craniotomy: a case-control study
Oral bacteria-associated liver abscess in conjunction with pulmonary arteriovenous fistula
How we deal with Staphylococcus aureus (MSSA, MRSA) central nervous system infections
DOI:10.31083/j.fbs1401001
PMID:35320912
[Cited within: 1]

Among central nervous system (CNS) infections (e.g., meningitis, brain abscess, ventriculitis, transverse myelitis), those caused by (SA) are particularly challenging both in management and treatment, with poor clinical outcomes and long hospital stay. It has been estimated that SA is responsible for around 1%-7% of meningitis (up to 19% in healthcare-associated meningitis). Recent neurosurgical procedures and immunocompromisation are major risk factors for SA CNS infections. Hand hygiene, surveillance nasal swabs and perioperative prophylaxis are crucial points for effective SA infections prevention. In case of SA-CNS infections, pending microbiological results, anti-methicillin-resistant SA (MRSA) antibiotic, with good CNS penetration, should be included, with prompt de-escalation as soon as MRSA is ruled out. Consultation with an expert in antimicrobial therapy is recommended as well as prompt source control when feasible. In this narrative review, we reviewed current literature to provide practical suggestions on diagnosis, prevention, management, and treatment of SA CNS infections.© 2022 The Author(s). Published by IMR Press.
Methicillin-resistant Staphylococcus aureus intracranial abscess: an analytical series and review on molecular, surgical and medical aspects
Methicillin-resistant Staphylococcus aureus central nervous system infections: analysis and outcome
DOI:10.3109/02688697.2015.1006168
PMID:25688639
[Cited within: 1]

Methicillin-Resistant Staphylococcus aureus (MRSA)-associated infections are potentially devastating and fatal. It has two distinct pathogenic mechanisms: postoperative and spontaneous. In the study presented here, we review the epidemiology, clinical features, response to treatment, and outcome of MRSA central nervous system infections at our tertiary referral institute.In this analysis, we reviewed the medical records of all patients who were diagnosed with S. aureus meningitis between January 2010 and December 2012. Clinical information included predisposing factors, past medical history, comorbidities, mode of acquisition of infection, as well as therapeutic management, length of treatment, and clinical outcomes were analyzed.A total of 34 cases of MRSA meningitis were diagnosed during the study period. There were 28 (82.4%) cases of postoperative meningitis and 6 (17.6%) cases of spontaneous meningitis. A majority (24/28) of the patients had one or the other predisposing conditions for the infection. Compared with patients with postoperative meningitis, patients with spontaneous meningitis had a significantly older (31.93 yrs vs 55.8 yrs; p = 0.021) and higher frequency of community-acquired infection (100% vs. 39%; p = 0.007). In patients with postoperative meningitis, the median postoperative day when the infection manifested clinically was day- 19 (range, 3-90 days). A total of 25/34 (74%) patients received definitive antibiotic (vancomycin and/or linezolid based) therapy. Nine patients were continued on empirical antimicrobial therapy (combination of ceftriaxone, amikacin, and metronidazole), as the organism was sensitive to those drugs. There were no in-hospital mortalities in our series, though 3/34 patients (8.8%) were discharged with Glasgow coma score (GCS)< 8 and 8/34 patients (23.5%) were discharged with GCS 9-12 from the hospital.In acute bacterial meningitis, there is a progressive shift from methicillin-sensitive strains to methicillin-resistant strains in recent years. Although most patients have a favorable response to vancomycin and linezolid, the beneficial effect of combined antimicrobial therapy or alternative antibiotics needs to be evaluated.
Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children
Structure and function of the blood-brain barrier (BBB)
Development, maintenance and disruption of the blood-brain barrier
DOI:10.1038/nm.3407
PMID:24309662
[Cited within: 1]

The interface between the blood circulation and the neural tissue features unique characteristics that are encompassed by the term 'blood-brain barrier' (BBB). The main functions of this barrier, namely maintenance of brain homeostasis, regulation of influx and efflux transport, and protection from harm, are determined by its specialized multicellular structure. Every constituent cell type makes an indispensable contribution to the BBB's integrity. But if one member of the BBB fails, and as a result the barrier breaks down, there can be dramatic consequences and neuroinflammation and neurodegeneration can occur. In this Review, we highlight recently gained mechanistic insights into the development and maintenance of the BBB. We then discuss how BBB disruption can cause or contribute to neurological disease. Finally, we examine how this knowledge can be used to explore new possibilities for BBB repair.
Mannitol-facilitated CNS entry of rAAV2 vector significantly delayed the neurological disease progression in MPS IIIB mice
DOI:10.1038/gt.2009.85
PMID:19587708
[Cited within: 4]

The presence of the blood-brain barrier (BBB) presents the most critical challenge in therapeutic development for mucopolysaccharidosis (MPS) IIIB, a lysosomal storage disease with severe neurological manifestation, because of alpha-N-acetylglucosaminidase (NaGlu) deficiency. Earlier, we showed a global central nervous system (CNS) transduction in mice by mannitol-facilitated entry of intravenous (IV)-delivered recombinant adeno-associated viral serotype 2 (rAAV2) vector. In this study, we optimized the approach and showed that the maximal transduction in the CNS occurred when the rAAV2 vector was IV injected at 8 min after mannitol administration, and was approximately 10-fold more efficient than IV delivery of the vector at 5 or 10 min after mannitol infusion. Using this optimal (8 min) regimen, a single IV infusion of rAAV2-CMV-hNaGlu vector is therapeutically beneficial for treating the CNS disease of MPS IIIB in adult mice, with significantly extended survival, improved behavioral performance, and reduction of brain lysosomal storage pathology. The therapeutic benefit correlated with maximal delivery to the CNS, but not peripheral tissues. This milestone data shows the first effective gene delivery across the BBB to treat CNS disease. The critical timing of vector delivery and mannitol infusion highlights the important contribution of this pretreatment to successful intervention, and the long history of safe use of mannitol in patients bodes well for its application in CNS gene therapy.
Chemotherapy in pediatric brain tumor and the challenge of the blood-brain barrier
Focused ultrasound/microbubbles-assisted BBB opening enhances LNP-mediated mRNA delivery to brain
The methodology and pharmacokinetics study of intraventricular administration of vancomycin in patients with intracranial infections after craniotomy
Efficacy and safety of intrathecal meropenem and vancomycin in the treatment of postoperative intracranial infection in patients with severe traumatic brain injury
DOI:10.3892/etm.2019.7503
PMID:31086592
[Cited within: 1]

This study investigated the improvement and safety of intrathecal meropenem and vancomycin in the treatment of postoperative intracranial infection in patients with severe traumatic brain injury (STBI). A retrospective analysis was performed on 86 patients with intracranial infections after cranial trauma operation in Tai'an Traditional Chinese Medicine Hospital and Affiliated Hospital of Taishan Medical University from May 2004 to June 2017. The patients were divided into the control group (43 patients) and the experimental group (43 patients) according to the treatment. Patients in the control group were intravenously infused with vancomycin hydrochloride (1.0 g, Q12H) and meropenem (2.0 g, Q8H). After lumbar cistern drainage was performed for the release of cerebrospinal fluid (CSF), patients in the experimental group were slowly given vancomycin 20 mg. After the tube was flushed with 2 ml of 0.9% sodium chloride injection, the patients were slowly given meropenem 20 mg, bid. The clinical efficacy, cure time and treatment cost of patients in the two groups were observed. The adverse reactions and sequelae after 6 months of treatment were recorded. The response rate (RR) of patients in the experimental group was significantly higher than that in the control group (P<0.05). The cure time of patients in the experimental group was significantly lower than that in the control group (P<0.05). The treatment cost of patients in the experimental group was significantly lower than that in the control group (P<0.05). The incidence of adverse reactions of patients, incidence of sequelae of patients in the experimental group was significantly lower than that in the control group (P<0.05). Intrathecal meropenem and vancomycin is more effective than intravenous administration in the treatment of intracranial infection after craniotomy. It can significantly shorten the treatment time and reduce the treatment cost, with better safety.
Key for crossing the BBB with nanoparticles: the rational design
Intracarotid infusion of hypertonic mannitol changes permeability of blood-brain barrier to methotrexate in rats
PMID:11360668
[Cited within: 1]

To study whether infusion of hypertonic mannitol through internal carotid artery could enhance methotrexate (MTX) concentration in rat cortex and to study the time-course of this process.Hypertonic mannitol was infused into the rat left internal carotid artery, ten minutes later, MTX was injected from the left femoral vein (i.v.) or left common carotid artery (ia), and the concentration of MTX was assayed 1 h later. Rats were given MTX at different time interval after infusion of mannitol, and the concentration of MTX was assayed 1 h after drug administration. At the same time, the rat cortex's density was analyzed.After mannitol infusion, MTX's concentration in rat cortex increased 2.54 times (i.v.) and 3.41 times (ia) as compared to control, respectively. After 10 min, such effect reached its peak, and almost disappeared after 6 h. There was no significant change in rat cortex's density.Mannitol can make blood-brain barrier (BBB) reversibly permeable, and increase MTX concentration in brain without any obvious injury to the brain.
Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in malignant brain tumors: the Sherbrooke experience
DOI:10.1002/cncr.21112
PMID:15880378
[Cited within: 1]

The treatment of malignant brain tumors is hampered by the presence of the blood-brain barrier, which limits chemotherapy penetration to the central nervous system (CNS). In recent years, different strategies have been designed to circumvent this physiologic barrier. The osmotic blood-brain barrier disruption (BBBD) procedure is one such strategy, and has been studied extensively in preclinical and clinical studies. The authors detail their experience so far with the procedure in the context of an open Phase II study in the treatment of malignant brain tumors.Patients with histologically proven malignant gliomas, primitive neuroectodermal tumors, primary CNS lymphomas, and metastatic disease to the brain were eligible. Patients enrolled were treated every 4 weeks (1 cycle) for < or = 12 cycles. A methotrexate-based regimen was offered to patients with lymphomas, whereas a carboplatin-based regimen was offered to patients with all other histologies. Before intraarterial chemotherapy infusion, patients were submitted to an osmotic BBBD procedure.Seventy-two patients were included in the current report. The overall median survival times (MST) from treatment initiation for glioblastoma multiforme (GBM), anaplastic oligodendrogliomas, primary CNS lymphomas, and metastases were, respectively, 9.1, 13.9, not reached, and 9.9 months, whereas time to disease progression was 4.1, 9.2, 12.3, and 3.3 months. The MST from diagnosis was 32.2 months for GBM.These encouraging results prompted the authors to further refine their knowledge of the potential contribution of this procedure in the treatment of brain tumors. These authors designed a randomized Phase III study for patients with GBM that is now open.Copyright 2005 American Cancer Society.
Modeling hyperosmotic blood-brain barrier opening within human tissue-engineered in vitro brain microvessels
The glomerular endothelium restricts albumin filtration
Nanomechanics of the endothelial glycocalyx: from structure to function
DOI:S0002-9440(20)30069-9
PMID:32035884
[Cited within: 2]

The negatively charged, brush-like glycocalyx covers the surface layer of endothelial cells. This layer of membrane-bound, carbohydrate-rich molecules covers the luminal surface of the endothelium along the entire vascular tree, mostly comprising glycoproteins and proteoglycans. Together with the underlying actin-rich endothelial cortex, 50 to 150 nm beneath the plasma membrane, the endothelial glycocalyx (eGC) is recognized as a vasoprotective nanobarrier and responsive hub. Importantly, both the eGC and cortex are highly dynamic and can adapt their nanomechanical properties (ie, stiffness and height) to changes in the environment. The constant change between a soft and a stiff endothelial surface is imperative for proper functioning of the endothelium. This review defines the nanomechanical properties of the eGC and stresses the underlying mechanisms and factors leading to a disturbed structure-function relationship. Specifically, under inflammatory conditions, the eGC is damaged, resulting in enhanced vascular permeability, tissue edema, augmented leukocyte adhesion, platelet aggregation, and dysregulated vasodilation. An integrated knowledge of the relationship between the nanomechanical properties, structure, and function of the eGC might be key in understanding vascular function and dysfunction. In this context, the clinical aspects for preservation and restoration of proper eGC nanomechanics are discussed, considering the eGC as a potentially promising diagnostic marker and therapeutic target in the near future.Copyright © 2020 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Podocytopathy: the role of actin cytoskeleton
Oxidation alters myosin-actin interaction and force generation in skeletal muscle filaments
Respiratory syncytial virus disrupts the airway epithelial barrier by decreasing cortactin and destabilizing F-actin
Hyperosmolar blood-brain barrier opening using intra-arterial injection of hyperosmotic mannitol in mice under real-time MRI guidance
Intra-arterial delivery of AAV vectors to the mouse brain after mannitol mediated blood brain barrier disruption
Assessment of mannitol-induced chronic blood-brain barrier dysfunction in vivo using magnetic resonance
Drug delivery strategies to overcome the blood-brain barrier (BBB)
Identification of variable lymphocyte receptors that can target therapeutics to pathologically exposed brain extracellular matrix
Biodegradable polymeric nanoparticles administered in the cerebrospinal fluid: brain biodistribution, preferential internalization in microglia and implications for cell-selective drug release
DOI:S0142-9612(19)30223-6
PMID:31026609
[Cited within: 1]

Cell-selective drug release in the central nervous system (CNS) holds great promise for the treatment of many CNS disorders but it is still challenging. We previously demonstrated that polymeric nanoparticles (NPs) injected intra-parenchyma in the CNS can be internalized specifically in microglia/macrophages surrounding the injection site. Here, we explored NPs administration in the cerebrospinal fluid (CSF) to achieve a wider spreading and increased cell targeting throughout the CNS; we generated new NPs variants and studied the effect of modifying size and surface charge on NPs biodistribution and cellular uptake. Intra-cerebroventricular administration resulted in prevalent localization of the NPs in proximity to stem-cell niches, such as around the lateral ventricles, the subventricular zone and the rostral migratory stream. NPs internalization occurred preferentially in brain myeloid cells/microglia. We demonstrated that brain biodistribution and extent of internalization in microglia are influenced by NPs dimensions and can be improved by applying a transient disruption of the blood-brain barrier with mannitol, leading to NPs internalization in up to 25% of brain myeloid/microglia cells. A fraction of the targeted cells was positive for markers of proliferation or stained positive for stemness/progenitor-cell markers such as Nestin, c-kit, or NG2. Interestingly, through these newly formulated NPs we obtained controlled and selective release of drugs otherwise difficult to formulate (such as busulfan and etoposide) to the target cells, preventing unwanted side effects and the toxicity obtained by direct brain delivery of the not encapsulated drugs. Overall, these data provide proof of concept of the applicability of these novel NP-based drug formulations for achieving internalization not only in mature microglia but also possibly in more immature myeloid cells in the brain and pave the way for brain-restricted microglia-targeted drug delivery regimens.Copyright © 2019 Elsevier Ltd. All rights reserved.
The effects of the Na+/Ca2+exchange blocker on osmotic blood-brain barrier disruption
PMID:11334793
[Cited within: 1]

Osmotic disruption of the blood-brain barrier (BBB) by mannitol is currently being used to enhance drug delivery in human brains. Despite clinical and experimental interest, to date the time course in the early phase of disruption has not been accurately identified. The mechanism in barrier closure also remains elusive. We first studied the rapid change in cerebrovascular permeability after BBB disruption in rats, and then demonstrated that the Na(+)/Ca(++) exchange blocker (KB-R7943) prolongs osmotic disruption. Osmotic BBB disruption was attained by using intra-arterial infusion of hypertonic mannitol in Sprague-Dawley (SD) rats. To measure the changes in cerebrovascular permeability, perfusate containing [14C]-sucrose was infused intra-arterially at different time points following osmotic stress. Cerebrovascular permeability was then measured with the in situ brain perfusion technique. This is the first in vivo study demonstrating that osmotic disruption is prolonged by the Na(+)/Ca(++) exchange blocker, which did not affect the peak level of BBB disruption. The exact time course of cerebrovascular reversibility was studied and the earliest BBB disruption was seen to occur 5 min after osmotic stress. Histopathological examination after osmotic disruption with the Na(+)/Ca(++) exchange blocker showed no neuronal damage in rat brains. Our findings represent important experimental information regarding pharmacological manipulation of BBB disruption. The possibility of prolonging the transient opening of the BBB has major clinical implications.
Effect of high glucose on ocular surface epithelial cell barrier and tight junction proteins