INTRODUCTION
Sepsis is a systemic reactive syndrome caused by infection that can further lead to septic shock and multiple organ dysfunction syndrome (MODS).[1] Sepsis is also one of the main causes of death in patients hospitalized in the intensive care unit (ICU).[2,3] A meta-analysis in 2019 estimated that the incidence of ICU-treated sepsis was 58 per 100,000 person-years, of which 41.9% died prior to hospital discharge.[4] It should be noted that acute kidney injury (AKI) is often present in ICU or hospitalized patients.[5] Zhang et al[6] found that 40% of critically ill patients develop sepsis after AKI, suggesting that AKI is likely to increase the risk of sepsis. Meanwhile, sepsis-associated acute kidney injury (S-AKI) greatly increases the mortality of sepsis patients. Due to progression to new chronic kidney disease (CKD), some S-AKI patients need therapy for renal function, although they recover from AKI.[7] Nonetheless, to date, effective prevention and treatment are still needed for S-AKI.
The homeostasis of the endoplasmic reticulum (ER) is impaired after unfolded or misfolded proteins accumulate in the ER after sepsis. This phenomenon is called endoplasmic reticulum stress (ERS), and it may be a factor of S-AKI pathogenesis.[8] Mild ERS can be considered a maintenance mechanism, but prolonged ERS can worsen cell function and even lead to apoptosis.[9] In animal models of sepsis, markers of increased ERS were detected in multiple organs, including the heart and the liver, and these markers were directly correlated with the degree of organ dysfunction, which is likely the primary cause of sepsis inducing multiorgan failure.[10] Moreover, the accumulation of inflammatory factors, reduction of tissue perfusion, ischemia, and hypoxia aggravate ERS in sepsis patients.[11] The transition from the maintenance function of ERS to pathogenesis may cause the organism to enter a vicious cycle. It is suggested that inhibition of ERS is a potential target for the prevention and treatment of sepsis.
Mesencephalic astrocyte-derived neurotrophic factor (MANF) is a kind of neurotrophic factor (NTF) that was found recently and is widely expressed in mammalian tissues.[12] We searched the database “Human Protein Atlas” with MANF as a keyword and found that MANF is detectable in the kidney, blood, heart, lung, liver and brain (supplementary Figure 1). MANF localizes in the ER in cells, and evidence further suggests that MANF is important for the maintenance of ER homeostasis. MANF is particularly high in secretory tissues with extensive protein production.[12] Additional evidence suggests that MANF is involved in the ERS-related diseases.[13] For example, when MANF is lacking, ethanol-induced neuronal apoptosis and ERS are greatly increased.[14] MANF can reduce the content of ERS-related proteins to protect neurons from amyloid β-peptide (Aβ) to improve the symptoms of Alzheimer’s disease (AD).[15] Furthermore, in MANF-knocked-out mice, insulin-deficient diabetes gradually occurs.[16] MANF plays a protective role in hepatic-injury-related diseases.[17,18] MANF also prevents alcohol-induced ERS and cell damage in pancreatic acinar cells.[19]
MANF has been reported to protect cells from stress-induced cell death and protect the heart from ischemic injury after secretion.[20] A previous study showed that ERS up-regulates MANF in podocytes and renal tubules to protect renal function.[13] As an ERS-related protein, MANF has a positive effect on a number of diseases, but no study has explored the role of MANF in S-AKI. The goals of this study were to examine the expression of MANF in S-AKI, evaluate the relationship between MANF and renal function and inflammatory factors.
METHODS
Animals and groups
C57/BL6 mice (male, 6-8 weeks old) were used for the S-AKI model and purchased from Shanghai Laboratory Animal Co., Ltd. They were kept in a specific pathogen-free (SPF) room with a light/dark 12:12 (12-hour light and 12-hour dark) condition and free access to food and water. The room temperature was controlled between 20 and 22 °C.
The animals were randomly divided into four groups with 24 mice in each group. Mice in the control group were injected with sterile normal saline into the abdominal cavity at a dose of 10 mg/kg. The sepsis model was induced by injecting lipopolysaccharide (LPS) (Sigma-Aldrich Inc., USA) at 10 mg/kg intraperitoneally. The S-AKI can be developed in 24 h.[19] After S-AKI induction, 200 μg/kg MANF (ICD02HU, Immuno Clone Biosciences Co., USA) was injected intraperitoneally daily in the S-AKI+MANF and control+MANF groups. Simultaneously, an equal dose of normal saline was administered daily intraperitoneally in the control and S-AKI groups. The serum and kidney tissue specimens were collected at 24 h, 36 h, and 48 h after the S-AKI model was established. All animal experimental procedures were reviewed and approved by the Ethical Committee for Animals of Shanghai Gongli Hospital.
Measurement of MANF expression in the kidney
The expression of MANF in the kidney was measured at 0, 24, 36 and 48 h after induction of S-AKI using Western blotting. The kidney tissue was subjected to protein lysis with a tissue grinder (Tissuelyser-24, Jingxin Industrial Development Co., China). The protein lysate was quantified with an enhanced BCA protein assay kit (P0010, Beyotime, China). Protein was subjected to electrophoresis and transmembrane in turn. The treated polyvinylidene difluoride (PVDF) membrane was subjected to blocking and washing at 4 ℃. The treated membrane was exposed to an antibody against MANF (dilution 1:1000, A305-572A-T, Thermo Fisher Scientific Inc., USA) and then reacted with the secondary antibody. The treated membrane was electrogenerated by chemiluminescence (ChemiQ4600, Clinx Science Instruments, China) to examine protein expression. Finally, the image was processed by ImageJ software (NIH, USA).
Measurement of MANF expression in the serum
We pressed the mice’s neck gently to ensure sufficient congestion of the retroorbital vein after fixing the mice and inserted the needle for serum collection along the inner canthus of the mouse at an angle of 45°. Then, serum samples collected were placed at 4 ℃ for 30 min and centrifuged (3,500 r/min, 10 min) with a centrifuge (MIKRO220R, Hettich, Germany). We tested the MANF in the serum using the MANF ELISA kit (MM-21129R1, MEIMIAN, China).
Measurement of pro-inflammatory cytokines, blood urea nitrogen (BUN), and serum creatinine (SCr)
We also tested inflammatory factors (tumor necrosis factor-α [TNF-α] and interleukin-6 [IL-6]) using enzyme-linked immunosorbent assay (ELISA) (MEIMIAN, China). The BUN and SCr were examined using an automatic biochemical analyzer (HITACHI, Japan). The test methods were operated in accordance with the manufacturer’s instructions.
Pathological examination of the kidney tissue
The mice were sacrificed at 48 h after the S-AKI model was established, and the kidney tissue was prepared for pathological examination using hematoxylin-eosin (H&E) staining. After the kidney tissue was fixed in 10% formalin solution (Chulei, China) for 24 h, the kidney was dehydrated with alcohol and embedded in paraffin. Sections were cut and stained in a fully automatic dyeing machine (ST5010XL, Leica, Germany). Images were examined after staining with a microscope (D-CleverEye, Dipath, China).
Statistical analysis
The measured data are expressed as the mean ± standard deviation followed by analysis of significant differences using SPSS software (v23.0, SPSS Inc., USA). The comparison between two groups was performed by unpaired Student’s t-test, and statistics among multiple groups were carried out using Tukey’s post hoc test following one-way analysis of variance (ANOVA). A P-value <0.05 was considered statistically significant.
RESULTS
MANF expression in the kidney tissue
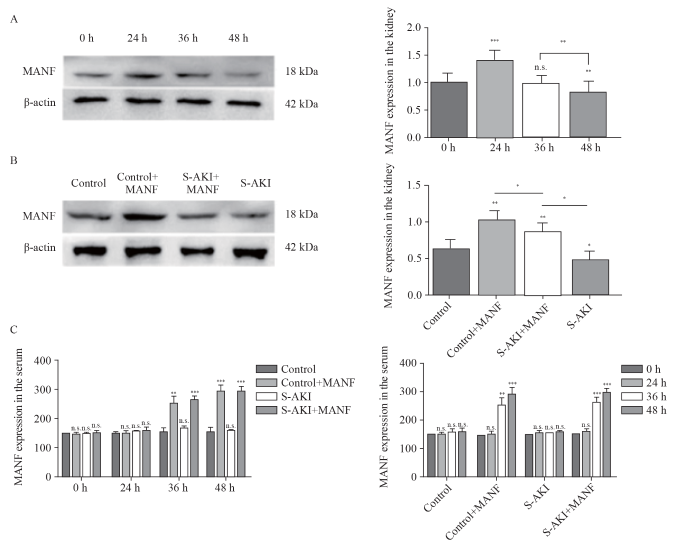
In the S-AKI group, MANF in the kidney tissue was significantly up-regulated at 24 h, but gradually decreased with the prolongation of onset time. After 36 h, MANF returned to a normal level, and it was lower than its baseline level after 48 h (Figure 1A).
Figure 1.
Figure 1.
Expression of MANF in the kidney and serum of mice. A: Western blotting analysis of MANF in protein extracts from the kidney tissue of the S-AKI mice at different time; B: Western blotting analysis of MANF levels in protein extracts from the kidney tissue among different groups at 48 h; C: MANF in the serum at different time detected by the MANF ELISA kit. MANF: mesencephalic astrocyte-derived neurotrophic factor; S-AKI: sepsis-associated acute kidney injury; n.s.: no significance. *P<0.05, **P<0.01, ***P<0.001.
Compared with the control group at 48 h, the MANF in the control+MANF group was up-regulated, while the MANF in the S-AKI group was down-regulated. Compared with that in the S-AKI group, the MANF in the S-AKI+MANF group was up-regulated (Figure 1B).
MANF expression in the serum
Compared with the control group, the MANF in the serum of the S-AKI group did not change significantly. The MANF in the serum of the control+MANF and S-AKI+MANF groups was up-regulated after 24 h (Figure 1C).
Changes of pro-inflammatory cytokines, BUN, and SCr
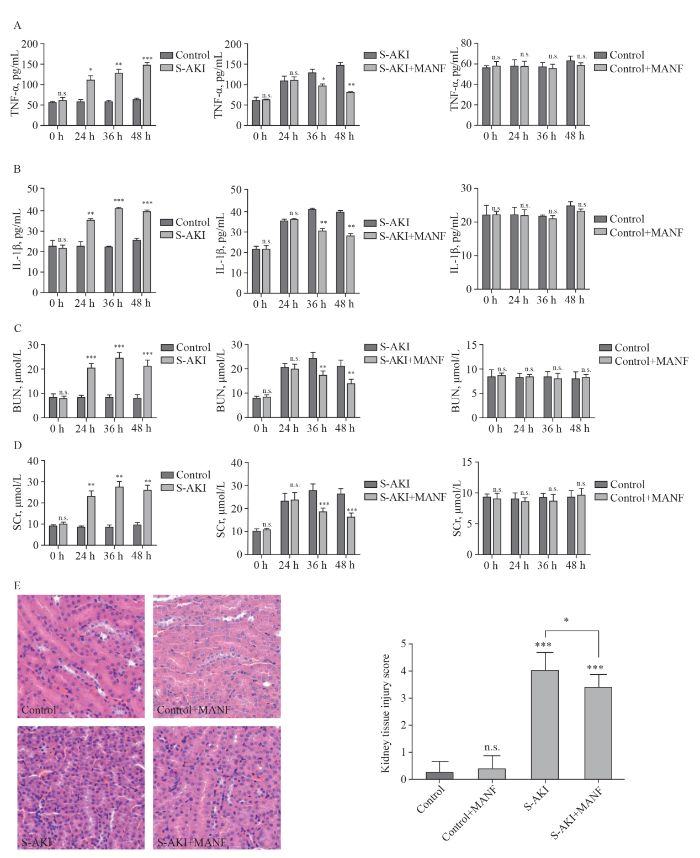
Compared with the control group, there were no significant changes in inflammatory factors (TNF-α and IL-1β) or renal function (BUN and SCr) in the control+MANF group (Figures 2 A-D). In the S-AKI group, SCr and BUN increased gradually, and TNF-α and IL-1β were overexpressed. Compared with the S-AKI group, renal function in the S-AKI+MANF group was gradually improved, and the secretion of the inflammatory factors (TNF-α and IL-1β) was gradually reduced.
Figure 2.
Figure 2.
Changes of pro-inflammatory cytokines,BUN, SCr, and histological findings among different groups. A: changes of TNF-α; B: changes of IL-1β; C: changes of BUN; D: changes of SCr; E: histological findings of the kidney tissue detected by hematoxylin-eosin staining (H&E, ×40) staining. TNF-α: necrosis factor-α; IL-1β: interleukin-1β; MANF: mesencephalic astrocyte-derived neurotrophic factor; S-AKI: sepsis-associated acute kidney injury; BUN: blood urea nitrogen; SCr: serum creatinine. *P<0.05, **P<0.01, ***P<0.001
Pathological changes of the kidney tissue
The kidney tissue in the control and control+MANF groups was normal. There were no pathological changes in the infiltration of renal tubular epithelial cells or inflammatory cells in the renal cortex and renal interstitium. In the S-AKI and S-AKI+MANF groups, the kidney tissue showed the infiltration of inflammatory cells in the renal cortex and stroma, swelling of renal tubular epithelial cells, vacuolar degeneration, and partial brush-like margin shedding. The pathological changes were more extensive in the S-AKI group than in the S-AKI+MANF group (Figure 2E).
DISCUSSION
When the patient suffers from S-AKI, insufficient tissue irrigation, poor renal microcirculation function, and weakening of oxygen and energy intake by the kidney tissue can aggravate ERS and further aggravate renal injury.[21] MANF is an ERS-related protein, and MANF upregulation can reduce and protect pancreatic β cells.[22] Thus, in this study, we detected the MANF in S-AKI mice at different periods. Accordingly, at 24 h after LPS was injected intraperitoneally to induce sepsis, S-AKI occurred in mice. At this time, the MANF in the kidneys increased rapidly. This was likely due to LPS-induced sepsis in mice. The mice had insufficient circulatory perfusion, tissue hypoxia, and massive accumulation of inflammatory cells, which activated ERS.[23] MANF reactivity was initially up-regulated, as reported by Gao et al,[24] and the expression of MANF in the kidney of mice decreased at 36 h and further decreased at 48 h. The renal function of S-AKI mice gradually deteriorated, and the accumulation of inflammatory factors increased. This change in MANF was similar to the results of Yang et al.[25] This may be caused by the fact that when pathogenic factors appear, the unfolded protein response (UPR) appears in the endoplasmic reticulum, triggering ERS, resulting in the activation of MANF and the up-regulation of MANF.[20] However, with the progression of the disease, the lack of irrigation of kidney tissue has become increasingly obvious,[26,27] and other problems have further aggravated ERS.[28,29] MANF may be insufficient to maintain ER homeostasis.[30] The ESR further intensifies after the reduction of MANF, forming a vicious cycle.
To determine whether MANF can produce drug toxicity and side effects in normal mice, we first injected MANF. It was found that the indexes of renal function and inflammation in mice treated with MANF had no significant changes compared with normal mice. These results indicated that during homeostasis, the increase of MANF would not cause damage to cells. In this study, the MANF in the kidney tissue and serum of normal mice treated with MANF was significantly higher than that of normal mice. This indicates that MANF can be injected intraperitoneally, circulated into the serum, and then be absorbed into the kidney tissue. In contrast, after injection of MANF into S-AKI mice, the renal function and inflammatory indexes of mice did not improve immediately, which may be that time is required to absorb the drug after injection.[31] The renal function of the MANF-treated mice gradually improved, and the inflammatory factors decreased, indicating that after exogenous supplementation with MANF protein, the MANF in kidney tissue increased, thus alleviating the stress state of the endoplasmic reticulum and reducing cell damage. This is consistent with the phenomenon of MANF supplementation in other ERS-related diseases.[32,33]
Regardless of the factors that lead to AKI, even mild AKI patients are at risk of CKD.[34,35] After AKI, the small arterioles and capillaries of the kidney are affected. The reduction in renal blood flow[36] and inflammatory cell infiltration[37] may be the factors affecting the prognosis of patients. There was no obvious damage to the kidney tissue in the control and control+MANF groups. The pathological changes in the kidney in S-AKI and AKI are similar.[38] Kidney injury was significantly less severe in the S-AKI +MANF group than in the S-AKI group. This indicates that MANF not only protects kidney function and reduces inflammation but also appears to protect kidney tissue and slow or reverse kidney damage. However, the precise molecular mechanisms by which MANF affects ERS during kidney injury deserve additional investigation.
CONCLUSION
MANF may play a role in the occurrence and development of S-AKI. For normal mice, the increase in MANF did not lead to injury or kidney tissue changes. For S-AKI mice, MANF treatment may significantly alleviate renal injury, reduce the inflammatory response, and alleviate or reverse kidney tissue damage. This conclusion not only supports the theory of the protective effect of MANF on ERS-related diseases but also shows that MANF may be a new treatment for S-AKI.
Funding: The study was supported by the Health Commission Clinical Characteristic Discipline Construction Program of Pudong New Area, Shanghai (PWYts2021-17) and Youth Science and Technology Project Health and Family Planning Commission of Pudong New Area, Shanghai (PWRq2020-35).
Ethical approval: The stusy was approved by the Ethical Committee for Animals of Shanghai Gongli Hospital.
Conflicts of interest: The authors declare no conflict of interest.
Contributors: SFC and XWH contributed equally to this study. SFC analyzed the data and wrote the article. XWH performed the experiments and organized the data; XYY guided the design of the project. DFG guided and reviewed the article. PLS, GC, and GRL participated in the collection of data for this study.
All the supplementary files in this paper are available at http://wjem.com.cn.
Reference
Is rosuvastatin protective against sepsis-associated encephalopathy? A secondary analysis of the SAILS trial
Application of Kaiser Sepsis Calculator in culture-positive infants with early onset sepsis
DOI:10.1007/s12519-021-00446-9 [Cited within: 1]
Initial venous lactate levels as a predictor of mortality in severe sepsis: a single-center retrospective cohort study
DOI:10.5847/wjem.j.1920-8642.2022.078 PMID:36119767 [Cited within: 1]
Acute kidney injury and fluid resuscitation in septic patients: are we protecting the kidney?
DOI:10.1159/000501748
[Cited within: 1]

Acute kidney injury (AKI) is a common complication in critically ill patients, especially among septic patients. Sepsis and hypovolemia are the 2 most frequent etiologies of AKI in intensive care units and frequently coexist in critically ill patients. Effective fluid resuscitation is crucial for the stabilization of sepsis-induced tissue hypoperfusion or septic shock. However, the lack of a goal-directed therapy targeting kidney oxygenation prevents from optimization of the fluid therapy with regard to improvement of renal oxygen delivery and extraction. Similarly, fluid administration as all therapeutic actions carries adverse effects such as the activation of cytokines, disruption of the capillary glycocalyx, and adverse effects on kidney metabolism and oxygenation. Moreover, a positive fluid balance is associated with an increased risk of AKI and is a negative predictor for recovery of renal function. The role of fluid resuscitation on kidney injury stems from the high renal vulnerability to hypoxemic injury. Indeed, fluids have a poor oxygen solubility and hemodilution decreases blood viscosity both promoting intrarenal shunting and heterogeneity with a decreased capillary density and enhanced intrarenal cortex and medullary hypoxia. The development of physiological biomarkers that are able to detect the early development of AKI specifically aimed at the identification of renal microcirculatory dysfunctions should form a valuable contribution to monitoring therapeutic modalities.
A novel prediction model of acute kidney injury based on combined blood variables in STEMI
DOI:10.1016/j.jacasi.2021.07.013
PMID:36341223
[Cited within: 1]

Development of acute kidney injury (AKI) is associated with poor prognosis in patients with ST-segment elevation myocardial infarction (STEMI).This study sought to investigate whether a combination of pre-procedural blood tests could predict the incidence of AKI in patients with STEMI.A total of 908 consecutive Japanese patients with STEMI who underwent primary percutaneous coronary intervention within 48 hours of symptom onset were recruited and divided into derivation (n = 617) and validation (n = 291) cohorts. A risk score model was created based on a combination of parameters assessed on routine blood tests on admission.In the derivation cohort, multivariate analysis showed that the following 4 variables were significantly associated with AKI: blood sugar ≥200 mg/dL (odds ratio [OR]: 2.07), high-sensitivity troponin I >1.6 ng/mL (upper limit of normal ×50) (OR: 2.43), albumin ≤3.5 mg/dL (OR: 2.85), and estimated glomerular filtration rate <45 mL/min/1.73 m (OR: 2.64). Zero to 4 points were given according to the number of those factors. Incremental risk scores were significantly associated with a higher incidence of AKI in both cohorts ( < 0.001). Receiver-operating characteristic curve analysis of risk models showed adequate discrimination between patients with and without AKI (derivation cohort, area under the curve: 0.754; 95% confidence interval: 0.733-0.846; validation cohort, area under the curve: 0.754; 95% confidence interval: 0.644-0.839).Our novel laboratory-based model might be useful for early prediction of the post-procedural risk of AKI in patients with STEMI.© 2021 The Authors.
Effect of miR-132-3p on sepsis-induced acute kidney injury in mice via regulating HAVCR1/KIM-1
PMID:34377256
[Cited within: 1]

To investigate the effect of miR-132-3p and HAVCR1/kidney injury molecule (KIM)-1 on sepsis-induced acute kidney injury (AKI) in mice.One hundred C57BL/6 mice were divided into five groups with 20 mice in each group: the normal group (normal mice), the model group (mice with sepsis), the miR-132-3p mimic group (miR-132-3p overexpression), the oe-HAVCR1/KIM-1 group (HAVCR1/KIM-1 overexpression), and the miR-132-3p mimic + oe-HAVCR1/KIM-1 group. Dual-luciferase reporter assay was performed to verify the targeting relationship between miR-132-3p and HAVCR1/KIM-1. The expressions of miR-132-3p and HAVCR1/KIM-1 in mice' kidneys, the levels of renal function markers, the expressions of apoptosis-associated proteins, the renal cell apoptosis rate, and the inflammatory factors in serum were all examined.We found that miR-132-3p can target HAVCR1/KIM-1 and regulate its expression. Compared with the normal mice, the septic mice exhibited lower miR-132-3p level and higher HAVCR1/KIM-1 level (both P<0.05). Moreover, the septic mice had higher levels of cleaved caspase-3, Bax, blood urea nitrogen, creatinine, tumor necrosis factor-α, interleukin-1β, and interleukin-6, higher renal cell apoptosis rate, and lower Bcl-2 level than the normal mice (all P<0.05). MiR-132-3p overexpression could improve the renal function of the mice with sepsis and inhibit renal cell apoptosis and inflammatory progression, whereas HAVCR1/KIM1 overexpression exhibited an opposite effect and could block the renal protective effects of miR-132-3p overexpression on the septic mice.MiR-132-3p overexpression can inhibit renal cell apoptosis and inflammatory progression via suppressing HAVCR1/KIM-1 expression, thereby exert renal protective effects on mice with sepsis.AJTR Copyright © 2021.
From surviving sepsis to surviving sepsis-associated acute kidney injury: focusing on risk stratification of acute kidney injury/acute kidney disease after sepsis
DOI:10.1016/j.xkme.2021.06.002 URL [Cited within: 1]
Hydrogen alleviated organ injury and dysfunction in sepsis: the role of cross-talk between autophagy and endoplasmic reticulum stress: experimental research
DOI:10.1016/j.intimp.2019.106049 URL [Cited within: 1]
Mediators of endoplasmic reticulum stress-induced apoptosis
DOI:10.1038/sj.embor.7400779
PMID:16953201
[Cited within: 1]

The efficient functioning of the endoplasmic reticulum (ER) is essential for most cellular activities and survival. Conditions that interfere with ER function lead to the accumulation and aggregation of unfolded proteins. ER transmembrane receptors detect the onset of ER stress and initiate the unfolded protein response (UPR) to restore normal ER function. If the stress is prolonged, or the adaptive response fails, apoptotic cell death ensues. Many studies have focused on how this failure initiates apoptosis, as ER stress-induced apoptosis is implicated in the pathophysiology of several neurodegenerative and cardiovascular diseases. In this review, we examine the role of the molecules that are activated during the UPR in order to identify the molecular switch from the adaptive phase to apoptosis. We discuss how the activation of these molecules leads to the commitment of death and the mechanisms that are responsible for the final demise of the cell.
The pathogenesis of sepsis and potential therapeutic targets
DOI:10.3390/ijms20215376
URL
[Cited within: 1]

Sepsis is defined as “a life-threatening organ dysfunction caused by a host’s dysfunctional response to infection”. Although the treatment of sepsis has developed rapidly in the past few years, sepsis incidence and mortality in clinical treatment is still climbing. Moreover, because of the diverse manifestations of sepsis, clinicians continue to face severe challenges in the diagnosis, treatment, and management of patients with sepsis. Here, we review the recent development in our understanding regarding the cellular pathogenesis and the target of clinical diagnosis of sepsis, with the goal of enhancing the current understanding of sepsis. The present state of research on targeted therapeutic drugs is also elaborated upon to provide information for the treatment of sepsis.
Intragastric and atomized administration of canagliflozin inhibit inflammatory cytokine storm in lipopolysaccharide-treated sepsis in mice: a potential COVID-19 treatment
DOI:10.1016/j.intimp.2021.107773 URL [Cited within: 1]
Unconventional neurotrophic factors CDNF and MANF: structure, physiological functions and therapeutic potential
DOI:S0969-9961(16)30171-1
PMID:27425895
[Cited within: 2]

Cerebral dopamine neurotrophic factor (CDNF) and mesencephalic astrocyte-derived neurotrophic factor (MANF) promote the survival of midbrain dopaminergic neurons which degenerate in Parkinson's disease (PD). However, CDNF and MANF are structurally and functionally clearly distinct from the classical, target-derived neurotrophic factors (NTFs) that are solely secreted proteins. In cells, CDNF and MANF localize in the endoplasmic reticulum (ER) and evidence suggests that MANF, and possibly CDNF, is important for the maintenance of ER homeostasis. MANF expression is particularly high in secretory tissues with extensive protein production and thus a high ER protein folding load. Deletion of MANF in mice results in a diabetic phenotype and the activation of unfolded protein response (UPR) in the pancreatic islets. However, information about the intracellular and extracellular mechanisms of MANF and CDNF action is still limited. Here we will discuss the structural motifs and physiological functions of CDNF and MANF as well as their therapeutic potential for the treatment of neurodegenerative diseases and diabetes. Currently available knockout models of MANF and CDNF in mice, zebrafish and fruit fly will increase information about the biology of these interesting proteins.Copyright © 2016 Elsevier Inc. All rights reserved.
Mesencephalic astrocyte-derived neurotrophic factor as a urine biomarker for endoplasmic reticulum stress-related kidney diseases
PMID:26940092
[Cited within: 2]

Endoplasmic reticulum (ER) stress and disrupted proteostasis contribute to the pathogenesis of a variety of glomerular and tubular diseases. Thus, it is imperative to develop noninvasive biomarkers for detecting ER stress in podocytes or tubular cells in the incipient stage of disease, when a kidney biopsy is not yet clinically indicated. Mesencephalic astrocyte-derived neurotrophic factor (MANF) localizes to the ER lumen and is secreted in response to ER stress in several cell types. Here, using mouse models of human nephrotic syndrome caused by mutant laminin β2 protein-induced podocyte ER stress and AKI triggered by tunicamycin- or ischemia-reperfusion-induced tubular ER stress, we examined MANF as a potential urine biomarker for detecting ER stress in podocytes or renal tubular cells. ER stress upregulated MANF expression in podocytes and tubular cells. Notably, urinary MANF excretion concurrent with podocyte or tubular cell ER stress preceded clinical or histologic manifestations of the corresponding disease. Thus, MANF can potentially serve as a urine diagnostic or prognostic biomarker in ER stress-related kidney diseases to help stratify disease risk, predict disease progression, monitor treatment response, and identify subgroups of patients who can be treated with ER stress modulators in a highly targeted manner.Copyright © 2016 by the American Society of Nephrology.
MANF is neuroprotective against ethanol-induced neurodegeneration through ameliorating ER stress
DOI:10.1016/j.nbd.2020.105216 URL [Cited within: 1]
Mesencephalic astrocyte-derived neurotrophic factor (MANF) protects against Aβ toxicity via attenuating Aβ-induced endoplasmic reticulum stress
DOI:10.1186/s12974-019-1429-0 [Cited within: 1]
Mesencephalic astrocyte-derived neurotrophic factor (MANF) is highly expressed in mouse tissues with metabolic function
DOI:10.3389/fendo.2019.00765 URL [Cited within: 1]
Emerging roles for mesencephalic astrocyte-derived neurotrophic factor (MANF) in pancreatic beta cells and diabetes
DOI:10.3389/fphys.2018.01457
PMID:30386256
[Cited within: 1]

Mesencephalic astrocyte-derived neurotrophic factor (MANF) was originally identified as a secreted trophic factor for dopamine neurons in vitro. It protects and restores damaged cells in rodent models of Parkinson's disease, brain and heart ischemia, spinocerebellar ataxia and retina in vivo. However, its exact mechanism of action is not known. MANF is widely expressed in most human and mouse organs with high levels in secretory tissues. Intracellularly, MANF localizes to the endoplasmic reticulum (ER) and ER stress increases it's expression in cells and tissues. Furthermore, increased MANF levels has been detected in the sera of young children with newly diagnosed Type 1 (T1D) diabetes and Type 2 (T2D) diabetic patients. ER stress is caused by the accumulation of misfolded and aggregated proteins in the ER. It activates a cellular defense mechanism, the unfolded protein response (UPR), a signaling cascade trying to restore ER homeostasis. However, if prolonged, unresolved ER stress leads to apoptosis. Unresolved ER stress contributes to the progressive death of pancreatic insulin-producing beta cells in both T1D and T2D. Diabetes mellitus is characterized by hyperglycemia, caused by the inability of the beta cells to maintain sufficient levels of circulating insulin. The current medications, insulin and antidiabetic drugs, alleviate diabetic symptoms but cannot reconstitute physiological insulin secretion which increases the risk of devastating vascular complications of the disease. Thus, one of the main strategies in improving current diabetes therapy is to define and validate novel approaches to protect beta cells from stress as well as activate their regeneration. Embryonic deletion of the Manf gene in mice led to gradual postnatal development of insulin-deficient diabetes caused by reduced beta cell proliferation and increased beta cell death due to increased and sustained ER stress. In vitro, recombinant MANF partly protected mouse and human beta cells from ER stress-induced beta cell death and potentiated mouse and human beta cell proliferation. Importantly, in vivo overexpression of MANF in the pancreas of T1D mice led to increased beta cell proliferation and decreased beta cell death, suggesting that MANF could be a new therapeutic candidate for beta cell protection and regeneration in diabetes.
Hepatocyte-derived MANF is protective for rifampicin-induced cholestatic hepatic injury via inhibiting ATF4-CHOP signal activation
DOI:10.1016/j.freeradbiomed.2020.10.028 URL [Cited within: 1]
MANF protects pancreatic acinar cells against alcohol-induced endoplasmic reticulum stress and cellular injury
DOI:10.1002/jhbp.928
PMID:33644980
[Cited within: 2]

Heavy alcohol drinking is associated with pancreatitis. Pancreatitis is initiated by the damage to the pancreatic acinar cells. The endoplasmic reticulum (ER) stress has been shown to play an important role in alcohol-induced pancreatic damage. Mesencephalic astrocyte-derived neurotrophic factor (MANF) is an ER stress-inducible protein. The aim of the study was to determine whether MANF can ameliorate alcohol-induced ER stress and cellular damages to pancreatic acinar cells.Alcohol-induced damage to mouse pancreatic 266-6 acinar cells was determined by MTT and flow cytometry. MANF expression was down-regulated by MANF siRNA using a Neon Transfection System. The over-expression of MANF was performed by the infection with the adenoviral vector carrying mouse MANF gene. The expression of ER stress markers was determined by immunoblotting and immunofluorescence.Alcohol caused ER stress, oxidative stress and induced apoptosis of 266-6 acinar cells. Recombinant human MANF alleviated alcohol-induced ER stress and cell death by inhibiting IRE1-caspase 12-caspase 3 apoptotic pathway. Overexpression of mouse MANF also protected cells against alcohol-induced apoptosis. In contrast, inhibiting MANF by siRNA exacerbated alcohol-induced cellular damage.MANF was protective against alcohol-induced ER stress and cellular injury in pancreatic acinar cells. The findings suggest a potential therapeutic value of MANF for alcoholic pancreatitis.This article is protected by copyright. All rights reserved.
Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion
DOI:10.1074/jbc.M112.356345
PMID:22637475
[Cited within: 2]

The endoplasmic reticulum (ER) stress protein mesencephalic astrocyte-derived neurotrophic factor (MANF) has been reported to protect cells from stress-induced cell death before and after its secretion; however, the conditions under which it is secreted are not known. Accordingly, we examined the mechanism of MANF release from cultured ventricular myocytes and HeLa cells, both of which secrete proteins via the constitutive pathway. Although the secretion of proteins via the constitutive pathway is not known to increase upon changes in intracellular calcium, MANF secretion was increased within 30 min of treating cells with compounds that deplete sarcoplasmic reticulum (SR)/ER calcium. In contrast, secretion of atrial natriuretic factor from ventricular myocytes was not increased by SR/ER calcium depletion, suggesting that not all secreted proteins exhibit the same characteristics as MANF. We postulated that SR/ER calcium depletion triggered MANF secretion by decreasing its retention. Consistent with this were co-immunoprecipitation and live cell, zero distance, photo affinity cross-linking, demonstrating that, in part, MANF was retained in the SR/ER via its calcium-dependent interaction with the SR/ER-resident protein, GRP78 (glucose-regulated protein 78 kDa). This unusual mechanism of regulating secretion from the constitutive secretory pathway provides a potentially missing link in the mechanism by which extracellular MANF protects cells from stresses that deplete SR/ER calcium. Consistent with this was our finding that administration of recombinant MANF to mice decreased tissue damage in an in vivo model of myocardial infarction, a condition during which ER calcium is known to be dysregulated, and MANF expression is induced.
Sepsis-induced acute kidney injury: a disease of the microcirculation
DOI:10.1111/micc.2019.26.issue-2 URL [Cited within: 1]
Liraglutide protects pancreatic β cells from endoplasmic reticulum stress by upregulating MANF to promote autophagy turnover
DOI:10.1016/j.lfs.2020.117648 URL [Cited within: 1]
Sepsis causes heart injury through endoplasmic reticulum stress-mediated apoptosis signaling pathway
Expression and distribution of mesencephalic astrocyte-derived neurotrophic factor in the retina and optic nerve
DOI:10.3389/fpsyg.2019.00686 URL [Cited within: 1]
Hepatocyte-derived MANF alleviates hepatic ischaemia-reperfusion injury via regulating endoplasmic reticulum stress-induced apoptosis in mice
DOI:10.1111/liv.14697
PMID:33064897
[Cited within: 1]

Endoplasmic reticulum (ER) perturbations are novel subcellular effectors involved in the ischaemia-reperfusion injury. As an ER stress-inducible protein, mesencephalic astrocyte-derived neurotrophic factor (MANF) has been proven to be increased during ischaemic brain injury. However, the role of MANF in liver ischaemia reperfusion (I/R) injury has not yet been studied.To investigate the role of MANF in the process of liver ischaemia-reperfusion, Hepatocyte-specific MANF knockout (MANF ) mice and their wild-type (WT) littermates were used in our research. Mice partial (70%) warm hepatic I/R model was established by vascular occlusion. We detected the serum levels of MANF in both liver transplant patients and WT mice before and after liver I/R injury. Recombinant human MANF (rhMANF) was injected into the tail vein before 1 hour occlusion. AST, ALT and Suzuki score were used to evaluate the extent of I/R injury. OGD/R test was performed on primary hepatocytes to simulate IRI in vitro. RNA sequence and RT-PCR were used to detect the cellular signal pathway activation while MANF knockout.We found that MANF expression and secretion are dramatically up-regulated during hepatic I/R. Hepatocyte-specific MANF knockout aggravates the I/R injury through the over-activated ER stress. The systemic administration of rhMANF before ischaemia has the potential to ameliorate I/R-triggered UPR and liver injury. Further study showed that MANF deficiency activated ATF4/CHOP and JNK/c-JUN/CHOP pathways, and rhMANF inhibited the activation of the two proapoptotic pathways caused by MANF deletion.Collectively, our study unravels a previously unknown relationship among MANF, UPR and hepatic I/R injury.© 2020 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
An Official ATS/ERS/ESICM/SCCM/SRLF Statement: prevention and management of acute renal failure in the ICU patients: an international consensus conference in intensive care medicine
DOI:10.1164/rccm.200711-1664ST URL [Cited within: 1]
Inhibition of Brd4 alleviates renal ischemia/reperfusion injury-induced apoptosis and endoplasmic reticulum stress by blocking FoxO4-mediated oxidative stress
DOI:10.1016/j.redox.2019.101195 URL [Cited within: 1]
Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress
DOI:10.1089/dna.2019.5097
PMID:31809190
[Cited within: 1]

Myocardial ischemic disease affects the prognosis in perioperative patients. Diabetes can aggravate myocardial injury. The purpose of this research is to investigate the effect of ferroptosis in the process of diabetes mellitus (DM) myocardial ischemia/reperfusion (I/R) injury (IRI). Endoplasmic reticulum stress (ERS) is investigated whether aggravates cardiomyocytes injury. Rat DM+I/R (DIR), cell high glucose (HG), hypoxia reoxygenation (H/R), and high-glucose H/R (HH/R) models were established. Ferroptosis inhibitor Ferrostatin-1, ferroptosis agonist Erastin, ERS inhibitor Salubrinal, and ERS agonist Tunicamycin were administered. Serum creatine kinase-MB (CK-MB), cell viability, lactate dehydrogenase (LDH), malondialdehyde (MDA), superoxide dismutase (SOD), reactive oxygen species (ROS), and cellular ferrous ion concentration were examined. The level of ACSL4, GPX4, ATF4, CHOP, BCL-2, and BAX was detected. Myocardial tissue pathological change was detected by hematoxylin-eosin staining. Cardiac function was monitored by invasive hemodynamic measurements. Evans Blue-triphenyltetrazolium chloride double staining was used to detect the myocardial infarct size. In DM+sham (DS) (or HG) and I/R (or H/R) models, cardiomyocytes were injured accompanied by increased level of ferroptosis and ERS. Moreover, the cell injury was more serious in rat DIR or cell HH/R models. Inhibition of ferroptosis in DIR model could reduce ERS and myocardial injury. Inhibition of ferroptosis in H9c2 cells HG, H/R, and HH/R models could reduce cell injury. Erastin could aggravate ERS and cell injury by stimulating ferroptosis in HH/R cell model. Meanwhile, inhibition of ERS could alleviate ferroptosis and cell injury. Ferroptosis is involved in DIR injury that is related to ERS. Moreover, inhibition of ferroptosis can alleviate DIR injury, which may provide a therapeutic regent for myocardial ischemic disease.
The role of the endoplasmic reticulum stress response following cerebral ischemia
DOI:10.1177/1747493017724584
PMID:28776456
[Cited within: 1]

Background Cornu ammonis 3 (CA3) hippocampal neurons are resistant to global ischemia, whereas cornu ammonis (CA1) 1 neurons are vulnerable. Hamartin expression in CA3 neurons mediates this endogenous resistance via productive autophagy. Neurons lacking hamartin demonstrate exacerbated endoplasmic reticulum stress and increased cell death. We investigated endoplasmic reticulum stress responses in CA1 and CA3 regions following global cerebral ischemia, and whether pharmacological modulation of endoplasmic reticulum stress or autophagy altered neuronal viability. Methods In vivo: male Wistar rats underwent sham or 10 min of transient global cerebral ischemia. CA1 and CA3 areas were microdissected and endoplasmic reticulum stress protein expression quantified at 3 h and 12 h of reperfusion. In vitro: primary neuronal cultures (E18 Wistar rat embryos) were exposed to 2 h of oxygen and glucose deprivation or normoxia in the presence of an endoplasmic reticulum stress inducer (thapsigargin or tunicamycin), an endoplasmic reticulum stress inhibitor (salubrinal or 4-phenylbutyric acid), an autophagy inducer ([4'-(N-diethylamino) butyl]-2-chlorophenoxazine (10-NCP)) or autophagy inhibitor (3-methyladenine). Results In vivo, decreased endoplasmic reticulum stress protein expression (phospho-eIF2α and ATF4) was observed at 3 h of reperfusion in CA3 neurons following ischemia, and increased in CA1 neurons at 12 h of reperfusion. In vitro, endoplasmic reticulum stress inducers and high doses of the endoplasmic reticulum stress inhibitors also increased cell death. Both induction and inhibition of autophagy also increased cell death. Conclusion Endoplasmic reticulum stress is associated with neuronal cell death following ischemia. Neither reduction of endoplasmic reticulum stress nor induction of autophagy demonstrated neuroprotection in vitro, highlighting their complex role in neuronal biology following ischemia.
KDEL receptors are differentially regulated to maintain the ER proteome under calcium deficiency
DOI:S2211-1247(18)31644-9
PMID:30428351
[Cited within: 1]

Retention of critical endoplasmic reticulum (ER) luminal proteins needed to carry out diverse functions (e.g., protein synthesis and folding, lipid metabolism) is mediated through a carboxy-terminal ER retention sequence (ERS) and its interaction with KDEL receptors. Here, we demonstrate that depleting ER calcium causes mass departure of ERS-containing proteins from cells by overwhelming KDEL receptors. In addition, we provide evidence that KDELR2 and KDELR3, but not KDELR1, are unfolded protein response (UPR) genes upregulated as an adaptive response to counteract the loss of ERS-containing proteins, suggesting previously unknown isoform-specific functions of the KDEL receptors. Overall, our findings establish that decreases in ER calcium change the composition of the ER lumina! proteome and secretome, which can impact cellular functions and cell viability. The redistribution of the ER proteome from inside the cell to the outside has implications for dissecting the complex relationship of ER homeostasis with diverse disease pathologies.
Subcutaneous administration of liposomes: a comparison with the intravenous and intraperitoneal routes of injection
PMID:8334142
[Cited within: 1]

The development of long-circulating liposomes containing lipid derivatives of poly(ethylene glycol) (PEG), termed Stealth liposomes, has considerably improved the prospects for therapeutic applications of liposomal drug delivery systems. We have examined the pharmacokinetics and biodistribution of long-circulating, as compared to conventional, liposomes after subcutaneous (sc) administration in mice. Results obtained after subcutaneous administration were compared to those obtained after intravenous (iv) and intraperitoneal (ip) administration. Liposomes, following sc administration, appeared intact in the circulation subsequent to moving down the lymph node chains that drain the site of injection. Liposomes containing PEG-distearoylphosphatidylethanolamine (PEG-DSPE) resulted in the highest levels of small (80-90 nm) liposomes in the blood, with up to 30% of vivo label appearing in the blood at 12 to 24 h post-injection. In the absence PEG-DSPE approx. 4-fold lower levels of liposomes were found in the blood. Small size of the liposomes was critical to their ability to move into the circulation, with liposomes above 110-120 nm not appearing in blood to any significant extent. The presence of PEG-DSPE and cholesterol was important for the in vivo stability of the liposome after sc administration. Although liposome levels were significantly higher in the draining lymph nodes after sc administration, levels associated with other tissues were proportionately reduced relative to the iv and ip routes of administration. Liposomes appeared in blood after ip and sc administration with half-lives of approx. 0.6 and 9 h, respectively, and subsequent to appearing in blood had similar biodistribution, pharmacokinetics and half-lives (20.4 h) to liposomes given by the iv route.
MANF promotes diabetic corneal epithelial wound healing and nerve regeneration by attenuating hyperglycemia-induced endoplasmic reticulum stress
DOI:10.2337/db19-0835
PMID:32312869
[Cited within: 1]

Mesencephalic astrocyte-derived neurotrophic factor (MANF) is a neurotrophic factor widely expressed in mammalian tissues, and it exerts critical protective effects on neurons and other cell types in various disease models, such as those for diabetes. However, to date, the expression and roles of MANF in the cornea, with or without diabetic keratopathy (DK), remain unclear. Here, we demonstrate that MANF is abundantly expressed in normal corneal epithelial cells; however, MANF expression was significantly reduced in both unwounded and wounded corneal epithelium in streptozotocin-induced type 1 diabetic C57BL/6 mice. Recombinant human MANF significantly promoted normal and diabetic corneal epithelial wound healing and nerve regeneration. Furthermore, MANF inhibited hyperglycemia-induced endoplasmic reticulum (ER) stress and ER stress-mediated apoptosis. Attenuation of ER stress with 4-phenylbutyric acid (4-PBA) also ameliorated corneal epithelial closure and nerve regeneration. However, the beneficial effects of MANF and 4-PBA were abolished by an Akt inhibitor and Akt-specific small interfering RNA (siRNA). Finally, we reveal that the subconjunctival injection of MANF-specific siRNA prevents corneal epithelial wound healing and nerve regeneration. Our results provide important evidence that hyperglycemia-suppressed MANF expression may contribute to delayed corneal epithelial wound healing and impaired nerve regeneration by increasing ER stress, and MANF may be a useful therapeutic modality for treating DK.© 2020 by the American Diabetes Association.
Nrf2-mediated neuroprotection by MANF against 6-OHDA-induced cell damage via PI3K/AKT/GSK3β pathway
Raising awareness of acute kidney injury: a global perspective of a silent killer
DOI:10.1038/ki.2013.153
PMID:23636171
[Cited within: 1]

Worldwide, acute kidney injury (AKI) is associated with poor patient outcomes. Over the last few years, collaborative efforts, enabled by a common definition of AKI, have provided a description of the epidemiology, natural history, and outcomes of this disease and improved our understanding of the pathophysiology. There is increased recognition that AKI is encountered in multiple settings and in all age groups, and that its course and outcomes are influenced by the severity and duration of the event. The effect of AKI on an individual patient and the resulting societal burden that ensues from the long-term effects of the disease, including development of chronic kidney disease (CKD) and end-stage renal disease (ESRD), is attracting increasing scrutiny. There is evidence of marked variation in the management of AKI, which is, to a large extent, due to a lack of awareness and an absence of standards for prevention, early recognition, and intervention. These emerging data point to an urgent need for a global effort to highlight that AKI is preventable, its course is modifiable, and its treatment can improve outcomes. In this article, we provide a framework of reference and propose specific strategies to raise awareness of AKI globally, with the goal to ultimately improve outcomes from this devastating disease.
Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury
DOI:10.1038/ki.2011.405
PMID:22157656
[Cited within: 1]

Acute kidney injury increases mortality risk among those with established chronic kidney disease. In this study we used a propensity score-matched cohort method to retrospectively evaluate the risks of death and de novo chronic kidney disease after reversible, hospital-associated acute kidney injury among patients with normal pre-hospitalization kidney function. Of 30,207 discharged patients alive at 90 days, 1610 with reversible acute kidney injury that resolved within the 90 days were successfully matched across multiple parameters with 3652 control patients who had not experienced acute kidney injury. Median follow-up was 3.3 and 3.4 years (injured and control groups, respectively). In Cox proportional hazard models, the risk of death associated with reversible acute kidney injury was significant (hazard ratio 1.50); however, adjustment for the development of chronic kidney injury during follow-up attenuated this risk (hazard ratio 1.18). Reversible acute kidney injury was associated with a significant risk of de novo chronic kidney disease (hazard ratio 1.91). Thus, a resolved episode of hospital-associated acute kidney injury has important implications for the longitudinal surveillance of patients without preexisting, clinically evident kidney disease.
Fluorescence microangiography for quantitative assessment of peritubular capillary changes after AKI in mice
DOI:10.1681/ASN.2013101121
PMID:24652794
[Cited within: 1]

AKI predicts the future development of CKD, and one proposed mechanism for this epidemiologic link is loss of peritubular capillaries triggering chronic hypoxia. A precise definition of changes in peritubular perfusion would help test this hypothesis by more accurately correlating these changes with future loss of kidney function. Here, we have adapted and validated a fluorescence microangiography approach for use with mice to visualize, analyze, and quantitate peritubular capillary dynamics after AKI. A novel software-based approach enabled rapid and automated quantitation of capillary number, individual area, and perimeter. After validating perfusion in mice with genetically labeled endothelia, we compared peritubular capillary number and size after moderate AKI, characterized by complete renal recovery, and after severe AKI, characterized by development of interstitial fibrosis and CKD. Eight weeks after severe AKI, we measured a 40%±7.4% reduction in peritubular capillary number (P<0.05) and a 36%±4% decrease in individual capillary cross-sectional area (P<0.001) for a 62%±2.2% reduction in total peritubular perfusion (P<0.01). Whereas total peritubular perfusion and number of capillaries did not change, we detected a significant change of single capillary size following moderate AKI. The loss of peritubular capillary density and caliber at week 8 closely correlated with severity of kidney injury at day 1, suggesting irreparable microvascular damage. These findings emphasize a direct link between severity of acute injury and future loss of peritubular perfusion, demonstrate that reduced capillary caliber is an unappreciated long-term consequence of AKI, and offer a new quantitative imaging tool for understanding how AKI leads to future CKD in mouse models. Copyright © 2014 by the American Society of Nephrology.
Immune cells and inflammation in AKI to CKD progression
DOI:10.1152/ajprenal.00195.2018
URL
[Cited within: 1]

Acute kidney injury (AKI) is a common clinical state resulting from pathogenic conditions such as ischemic and toxic insults. The pathophysiology of AKI shares common pathogenic denominators including cell death/injury, inflammation, and fibrosis, regardless of the initiating insults. Recent clinical studies have shown that a single episode of AKI can lead to subsequent chronic kidney disease (CKD). Although the involvement of multiple types of cells in the pathophysiology of AKI is becoming increasingly clear, the precise mechanisms for this “AKI to CKD progression” are still unknown, and no drug has been shown to halt this progression. An increasing number of epidemiological studies have also revealed that the presence of aging greatly increases the risk of AKI to CKD progression, and chronic inflammation is increasingly recognized as an important determinant factor for this progression. In this review article, we first describe the current understanding of the pathophysiology of AKI to CKD progression based on multiple types of cells. In particular, we will highlight the recent findings in regard to the mechanisms for chronic inflammation after AKI. Subsequently, we will focus on the mechanisms responsible for the increased risk of AKI to CKD progression in the elderly. Finally, we highlight our recent finding of age-dependent tertiary lymphoid tissue formation and its roles in AKI to CKD progression and speculate on the potential therapeutic opportunities that come from targeting aberrant inflammation after AKI.
Pathophysiology of ischemic acute kidney injury
DOI:10.1038/nrneph.2011.16
PMID:21364518
[Cited within: 1]

Acute kidney injury (AKI) as a consequence of ischemia is a common clinical event leading to unacceptably high morbidity and mortality, development of chronic kidney disease (CKD), and transition from pre-existing CKD to end-stage renal disease. Data indicate a close interaction between the many cell types involved in the pathophysiology of ischemic AKI, which has critical implications for the treatment of this condition. Inflammation seems to be the common factor that links the various cell types involved in this process. In this Review, we describe the interactions between these cells and their response to injury following ischemia. We relate these events to patients who are at high risk of AKI, and highlight the characteristics that might predispose these patients to injury. We also discuss how therapy targeting specific cell types can minimize the initial and subsequent injury following ischemia, thereby limiting the extent of acute changes and, hopefully, long-term structural and functional alterations to the kidney.