INTRODUCTION
Sepsis is the primary cause of death due to infection and is associated with a high hospital mortality rate worldwide, causing 5.3 million deaths annually.[1,2] The mortality in the hospital was estimated to be 17% for sepsis and 26% for severe sepsis worldwide.[3] The latest version of Sepsis-3 defines sepsis as characterized by life-threatening organ failure and dysfunction due to infection in the host.[4] In the latest version, the definition is established based on the balance between host immune status and pathogens.
The development of sepsis is initiated through a hyper-inflammatory phase, followed by a prolonged immune suppression phase. Severe cytokine storms and low volume perfusion caused by overwhelming inflammation during septic shock could lead to acute organ dysfunction, which contributes to death from sepsis.[5] These two extreme phases are compatible, and a mixture of these phenotypes has been detected in most patients.[6]
Checkpoint regulators are membrane-bound proteins widely expressed on immune cells. They serve as a second signal to direct the immune response of T cells.[7] Programmed death-1 (PD-1) (CD279) and its ligand programmed death-ligand 1 (PD-L1) (CD274) are important checkpoint inhibitors that induce apoptosis, disability and depletion of T cells, causing them to be irresponsible to antigen stimulation.[8] PD-1 or PD-L1 has been proven to be overexpressed among critically ill patients with sepsis. The expression of inhibitory receptors, such as B- and T-lymphocyte attenuator (BTLA), PD-1, cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), and inhibitory ligands (PD-L1, PD-L2, herpesvirus entry mediator [HVEM]), was increased in ICU patients who died of sepsis.[9] PD-1 and PD-L1 on neutrophils could be early predictors for subsequent sepsis.[10] Studies have also revealed that PD-L1 expression on monocytes and natural killer (NK) cells is a promising independent prognostic marker for septic shock or mortality.[7,11]
Antigen-presenting cells (APCs) present antigens and secrete inflammatory cytokines. Increased expression of PD-1 or PD-L1 on the innate myoid cell axis is involved in inhibitory signaling in the APC process and is responsible for immunosuppression in sepsis.[7] However, little is known about the crosstalk between PD-1 and PD-L1 on APCs and inflammation during sepsis. Accordingly, in this study, we aimed to evaluate PD-L1 expression on different APCs and inflammatory cytokines during early sepsis, explore their possible relationships, and test their ability to predict organ failure and prognosis in early sepsis.
METHODS
Study population and data collection
This study was an observational, single-center cohort study conducted in the emergency department (ED) of Beijing Chaoyang Hospital, a tertiary teaching hospital in China. From October 2018 to August 2019, consecutive patients meeting the Sepsis-3 criteria[4] were enrolled in this study from the emergency intensive care unit. Sepsis is defined as an infection-caused change in baseline with a total Sequential Organ Failure Assessment (SOFA) score ≥2 points. Septic shock is identified as a subtype of sepsis with a serum lactate level >2 mmol/L and persisting hypotension, which requires the administration of vasopressors to maintain mean arterial pressure (MAP) ≥65 mmHg (1 mmHg=0.133 kPa) despite adequate volume resuscitation. The inclusion criteria were adult patients who met the Sepsis-3 criteria. Exclusion criteria included patients with any of the following conditions: (1) less than 18 years old; (2) received long-term hormone or immunosuppressive therapy; (3) experienced major surgery or trauma during the past three months; (4) suffered from blood systemic diseases, hepatitis, liver cirrhosis, or end-stage renal disease; (5) suffered from autoimmune disease or immunodeficiency disease; (6) diagnosed with cancer and received radiotherapy or chemotherapy; (7) during the pregnancy period; (8) patients or representatives refused to participate in any stage of the study. All patients received standardized treatment based on the International Guidelines for the Management of Sepsis and Septic Shock (2016).[12] Patients were treated with antibiotics on or before the date of sepsis diagnosis when infection parameters were elevated or radiological imaging indicated infection sites. Patients received mechanical ventilation when they (1) were conscious or had irregular breathing; (2) had airway obstruction; (3) were prone to vomiting and aspiration; and (4) had severe hypoxia or/and carbon dioxide retention. Volunteers who had never experienced the above-mentioned diseases, hypertension, diabetes, or major surgery were regarded as the healthy control group. The control group was matched by age and sex with the sepsis group. There was no significant difference in sex or age. Demographic and clinical data were extracted. Acute Physiology and Chronic Health Evaluation (APACHE) II scores[13] and SOFA scores[14] at day 1 of enrollment were calculated. The 28-day mortality was recorded. The study was approved by the Beijing Chao-yang Hospital Ethics Committee. Written informed consent was obtained from all the subjects or their legally authorized representatives.
Laboratory tests for immune-related antigens in leukocyte subsets
Ethylenediamine tetraacetic acid (EDTA) anticoagulant venous blood was collected within 24 h after sepsis was diagnosed in patients who met the inclusion criteria. Flow cytometry was performed within 3 h of sample collection using 100 μL residual venous blood for each leukocyte subtype. Cytometry was carried out using a Gallios flow cytometer (Beckman Coulter, USA). The data were analyzed using Gallios software version 1.0 (Beckman Coulter, USA). Monoclonal antibodies and their isotype controls were all purchased from BD Biosciences (San Jose, USA). The expression of PD-1 (allophycocyanin, MIH4) and PD-L1 (phycoerythrin, MIH4) was measured on B cells, monocytes, and dendritic cells (DCs). B cells were gated by CD3- CD19+ (anti-CD3: Pacific Blue™, UCHT1; anti-CD19: PE-Cy7, SJ25C1); monocytes were gated by CD14+ (anti-CD14: Pacific Blue™, M5E2); DCs were gated by HLADR+ CD11c+ (anti-CD11c: BV421, B-LY6) for myeloid DCs and HLA-DR+ CD123+ (anti-CD123: PE-Cy7, 7G3) for plasmacytoid DCs. At least 10,000 events were collected in the lymphocyte or mononuclear cell gate for each sample. Fluorescence was compensated using anti-mouse COMP antibody (BD Biosciences, USA). The results are expressed as percentages of positively gated cells or mean fluorescence intensities. Representative plots are shown in supplementary Figure 1.
Evaluation of plasma biomarkers
Residual venous blood (4 mL) taken for routine tests on day 1 of enrollment was used for subsequent determination. The blood was centrifuged, and plasma was collected and stored at -80 °C for plasma biomarker determination.
Cytokine concentrations of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-4 (IL-4), IL-6, IL-10, and IL-17A were measured with a ProcartaPlex Multiplex Luminex assay customized kit (Invitrogen, USA) according to the manufacturer’s protocol.
Statistical analysis
The variables were described as medians (interquartile ranges) for continuous variables that did not conform to the normal distribution or homogeneity of variance test. Discontinuous variables were described as counts or percentages. Categorical data were compared using the Pearson Chi-square test or Fisher’s exact test as appropriate. For comparison of continuous variables, Kruskal-Wallis H-tests or Mann-Whitney U-tests were applied where appropriate. Correlations between two independent parameters were assessed using the Spearman rank correlation test. The results with two-tailed P-values of less than 0.05 were considered significant. All data were analyzed using SPSS Statistics 25.0 software (IBM, USA). The figures were prepared using GraphPad Prism 8.0 software (GraphPad Software, USA).
RESULTS
Baseline characteristics of the enrolled participants
The flow diagram of the present study is shown in supplementary Figure 2. In total, 40 heathy controls and 198 adult patients diagnosed with sepsis according to Sepsis-3, including 111 males and 87 females, were enrolled in the study. Patients were divided into sepsis and septic shock subgroups according to severity. Baseline characteristics are presented in Table 1, Table 2, and supplementary Table 1. There were 121 sepsis patients and 77 septic shock patients based on severity. Alternatively, patients were grouped as 128 survivors and 70 non-survivors according to the outcomes observed at day 28. The cohort was composed of 105 (53.0%) patients with respiratory infection, 47 (23.7%) patients with intra-abdominal infection, 19 (9.6%) patients with cerebral infection, 22 (11.1%) patients with urinary infection, and 5 (2.5%) with other infections. APACHE II scores, SOFA scores and lactate levels were significantly higher in non-survivors than in survivors and in septic shock than in sepsis. No statistically significant differences were detected between the survivor and non-survivor groups or the sepsis and septic shock groups for age, sex, or primary infection sites.
Table 1. Baseline characteristics of the participants
| Characteristics | Healthy control (n=40) | Overall sepsis (n=198) | P-value (control vs. overall) |
|---|---|---|---|
| Age, years | 68 (57-74) | 71 (56-80) | 0.389 |
| Male/Female | 20/20 | 111/87 | 0.121 |
| White blood cell count, ×109 | 5.81 (4.85-6.52) | 12.83 (8.00-19.28) | <0.001 |
| Neutrophil percentage, % | 56.25 (48.03-63.33) | 88.55 (81.98-92.75) | <0.001 |
| Neutrophil count, ×109 | 4.70 (3.62-5.65) | 11.59 (7.08-17.69) | <0.001 |
| Lymphocyte percentage, % | 2.30 (1.70-2.70) | 6.10 (3.30-11.13) | <0.001 |
| Lymphocyte count, ×109 | 2.12 (1.74-2.36) | 0.77 (0.48-1.14) | <0.001 |
| Monocyte count, ×109 | 0.29 (0.27-0.38) | 0.56 (0.28-0.98) | <0.001 |
| Lactate, mmol/L | 1.00 (0.60-1.38) | 1.95 (1.30-3.23) | <0.001 |
| SOFA score | 0 (0-1) | 7 (4-10) | <0.001 |
| APACHE Ⅱ score | 1 (0-2) | 15 (10-21) | <0.001 |
| Comorbidity more than one | 0 | 140 (70.7) | <0.001 |
| Primary infection sites | |||
| Respiratory infection | 105 (53.0) | - | |
| Intra-abdominal infection | 47 (23.7) | - | |
| Cerebral infection | 19 (9.6) | - | |
| Urinary infection | 22 (11.1) | - | |
| Others | 5 (2.5) | - |
Data are expressed as median (interquartile range) or n (percentage). SOFA: Sequential Organ Failure Assessment; APACHE Ⅱ: Acute Physiology and Chronic Health Evaluation Ⅱ.
Table 2. Baseline characteristics of subgroups of sepsis patients on admission
| Characteristics | Sepsis (n=121) | Septic shock (n=77) | P-value |
|---|---|---|---|
| Age, years | 72 (55-82) | 70 (56-79) | 0.412 |
| Male/Female | 71/50 | 40/37 | 0.232 |
| White blood cell count, ×109 | 13.02 (9.02-18.68) | 11.74 (7.59-23.79) | 0.640 |
| Neutrophil percentage, % | 87.90 (81.95-91.50) | 90.30 (81.55-94.05) | 0.087 |
| Neutrophil count, ×109 | 11.80 (7.87-16.93) | 11.41 (6.54-20.90) | 0.915 |
| Lymphocyte percentage, % | 6.40 (3.45-11.05) | 5.00 (3.15-12.70) | 0.384 |
| Lymphocyte count, ×109 | 0.82 (0.52-1.20) | 0.65 (0.39-1.09) | 0.236 |
| Monocyte count, ×109 | 0.67 (0.38-1.00) | 0.45 (0.19-0.74) | 0.004 |
| Lactate, mmol/L | 1.60 (1.15-2.25) | 2.90 (1.72-6.70) | <0.001 |
| SOFA score | 5 (3-7) | 10 (8-12) | <0.001 |
| APACHE Ⅱ score | 13 (8-17) | 19 (14-26) | <0.001 |
| 28-day mortality | 30 (24.8) | 40 (51.9) | <0.001 |
| Comorbidity more than one | 78 (64.5) | 62 (80.5) | 0.853 |
| Primary infection sites | |||
| Respiratory infection | 68 (56.2) | 37 (48.1) | 0.263 |
| Intra-abdominal infection | 27 (22.3) | 20 (26.0) | 0.555 |
| Cerebral infection | 12 (9.9) | 7 (9.1) | 0.847 |
| Urinary infection | 11 (9.1) | 11 (14.3) | 0.257 |
| Others | 3 (2.5) | 2 (2.6) | 0.959 |
Data are expressed as median (interquartile range) or n (percentage). SOFA: Sequential Organ Failure Assessment; APACHE Ⅱ: Acute Physiology and Chronic Health Evaluation Ⅱ.
Comparisons of PD-1 and PD-L1 on different APCs according to disease severity and outcomes
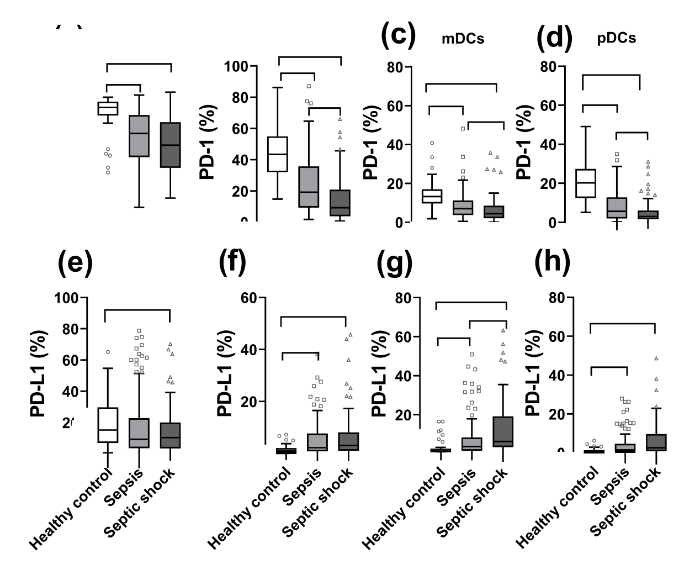
As shown in Figure 1 and supplementary Figure 3, the expression of PD-1 decreased significantly on B cells, monocytes, myeloid DCs (mDCs), and plasmacytoid DCs (pDCs) as the severity of sepsis increased. The expression of PD-1 was also markedly decreased in non-survivors compared with survivors. In contrast, the expression of PD-L1 was markedly higher on mDCs, pDCs, and monocytes in patients with sepsis than in healthy controls and in non-survivors than in survivors.
Figure 1.
Figure 1.
Surface biomarker expression on leukocyte subsets in controls (n=40) and patients with sepsis (n=121) or septic shock (n=77). PD-1 and PD-L1 expression was evaluated on B cells (A, E), monocytes (B, F), myeloid dendritic cells (mDCs) (C, G), and plasmacytoid dendritic cells (pDCs) (D, H). *P<0.05. ns: no significance. PD-L1: programmed death-ligand 1; PD-1: programmed death-1.
Comparisons of cytokines according to disease severity and outcomes
As shown in supplementary Figures 4 and 5, the levels of IL-6 and IL-10 were significantly higher in patients with sepsis than in healthy controls and increased in parallel with the severity of sepsis. The supplementary Figure 5 shows that non-survivors had significantly higher levels of IL-6 and IL-10 than survivors and healthy controls. IL-17A and IL-4 were significantly higher in sepsis patients than in healthy controls, while no significant difference was found between survivors and non-survivors.
Association between cytokines, PD-1 or PD-L1 expression and scoring systems
As shown in supplementary Table 2, associations between cytokines, PD-1 or PD-L1 expression and scoring systems are displayed. PD-1 expression on monocytes showed a weak negative correlation with SOFA score (Spearman’s ρ= -0.310, P<0.001). IL-6 was positively correlated with SOFA score (Spearman’s ρ=0.235, P=0.001) and APACHE Ⅱ score (Spearman’s ρ=0.209, P=0.003). IL-10 was also positively correlated with SOFA score (Spearman’s ρ=0.334, P<0.001) and APACHE Ⅱ score (Spearman’s ρ=0.253, P<0.001). The PD-1 percentage of monocytes (monocyte PD-1%) showed a weak negative correlation with IL-6 (Spearman’s ρ= -0.251, P<0.001) and IL-10 (Spearman’s ρ= -0.266, P<0.001). The PD-L1 percentage (PD-L1%) of B cells showed a weak negative correlation with IL-4 (Spearman’s ρ= -0.214, P=0.002). PD-L1 in mDCs showed a weak positive correlation with IFN-γ (Spearman’s ρ=0.271, P<0.001), IL-4 (Spearman’s ρ=0.243, P=0.001), IL-6 (Spearman’s ρ=0.218, P=0.002), TNF-α (Spearman’s ρ=0.280, P<0.001), and IL-17A (Spearman’s ρ=0.239, P=0.001).
Independent predictors for 28-day mortality during sepsis
Univariate analysis showed that parameters, including IL-6, IL-10, monocyte PD-1%, and PD-L1% of mDCs (mDC PD-L1%), were significantly different between survivors and non-survivors. The above parameters were included in the binary logistic regression model. The results are shown in supplementary Table 3. Monocyte PD-1% was an independent risk factor for 28-day mortality (risk ratio 0.971, 95% confidence interval [95% CI] 0.951-0.991; P=0.005).
Predictive performance of PD-1 and PD-L1 on APCs, clinical severity, and inflammatory cytokines alone and in combination
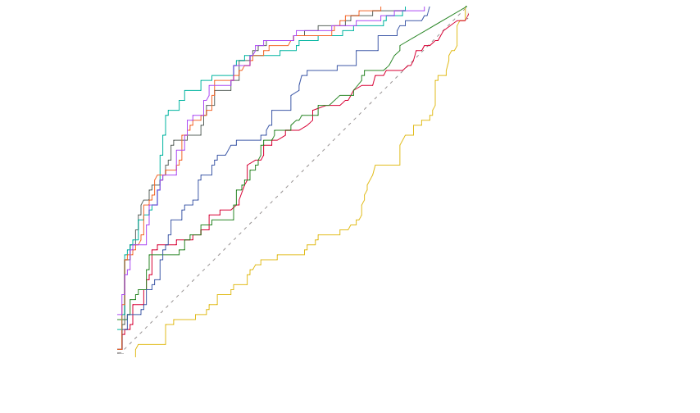
The area under the receiver operating characteristic curve (AUC) of the biomarkers in predicting 28-day mortality of sepsis was 0.811 (SOFA score), followed by 0.809 (APACHE Ⅱ score), 0.675 (IL-10), 0.607 (IL-6), 0.591 (mDC PD-L1%), and 0.575 (pDC PD-L1%), as presented in Figure 2 and supplementary Table 4. We also compared the AUC of the parameters in combination. The AUC of the monocyte PD-1%+ APACHE Ⅱ model (0.823) was higher than that of the monocyte PD-1%+SOFA model (0.816), followed by that of the mDC PD-L1%+SOFA model (0.813). No statistically significant difference was found between the combined model and the other isolated indicators for mortality prediction.
Figure 2.
Figure 2.
The receiver operating characteristic curve of PD-1 or PD-L1 expression of antigen-presenting cells, scores, cytokines alone or in combination in predicting 28-day outcome.PD-1: programmed death-1; PD-L1: programmed death-ligand 1; mDC: myeloid dendritic cell; IL-6: interleukin-6; IL-10: interleukin-10; SOFA: Sequential Organ Failure Assessment; APACHE II: Acute Physiology and Chronic Health Evaluation II.
DISCUSSION
In the present study, PD-L1 was up-regulated on APCs and correlated with SOFA and APACHE Ⅱ scores during early sepsis. The PD-1 or PD-L1 axis plays an important role in immunosuppressive mechanisms during sepsis.[15] It was reported that the induction of an adaptive response was impaired in patients with severe endotoxin tolerance status, which was dependent on the PD-L1 or PD-1 pathway;[16] PD-1-deficient mice showed increased survival and maintained macrophage function, demonstrating improved bacterial clearance and reduced inflammation.[17,18] In line with these reports, we found that monocyte PD-1% and lactate were independent risk factors for 28-day mortality. Monocyte PD-1% combined with the APACHE II or SOFA model had higher prognostic value than other parameters. It was observed that in septic newborns with complications, there was a higher percentage of intermediate monocytes with PD-1 expression. PD-1 might indicate the immunosuppressive phase of sepsis in prematurely born children with sepsis.[19] PD-1+ monocytes presented a preference toward M2 polarization and had a deficiency in supporting CD8 T cells in hepatocellular carcinoma.[20] However, as this was an observational, single-center clinical study, this result will eventually need to be validated by studies with larger scales. Further basic research is needed to interpret the potential function of this subpopulation.
Prior observational clinical studies have shown that critically ill patients who developed severe septic shock expressed markedly elevated levels of PD-1 or PD-L1 on various leukocyte subsets, commonly T cells.[6,7,21] The novelty of the present study was that PD-L1 on APCs significantly increased as the severity of sepsis increased. In line with previous studies, PD-L1 on APCs has been demonstrated to be an important indicator reflecting prognosis during sepsis. Our previous research showed that the increased expression of PD-L1 on the monocyte surface was an independent risk factor for risk stratification and prognosis at 3-4 d of sepsis.[11] The combination of monocyte PD-L1% and plasma infection biomarker presepsin or procalcitonin (PCT) can also help to improve the prognostic value of sepsis.[22] An increased percentage of PD-L1+ natural killer (NK) cells has also been suggested as a novel prognostic biomarker in predicting sepsis.[23]
Classical studies have suggested that PD-L1 on APCs binds to PD-1 on effector T cells, leading to T-cell apoptosis, anergy, and exhaustion, and plays a critical role in T-cell tolerance.[15,24,25] In contrast, PD-L1 binds to PD-1 on inhibitory Tregs and promotes proliferation.[26] Few studies have focused on the effects of PD-L1 activation on APCs themselves. It was reported that reduced monocyte phagocytic function correlated with higher expression of PD-L1 on total monocytes in sepsis. In addition, ex vivo incubation of whole blood with anti-PD-L1 and anti-PD-1 mAbs was able to increase the phagocytosis function of monocytes.[6] Recent tumor studies have shown that incubation with soluble CD80 or PD-1 increased the proliferation, survival and activation of tumor-associated macrophages (TAMs). Anti-PD-L1 antibody treatment induced the transformation of TAMs to a pro-inflammatory phenotype (M1-like macrophages) and increased the secretion of pro-inflammatory cytokines.[27,28] The role of APC-expressed PD-L1 in the pathogenesis of sepsis remains to be elucidated. Therefore, we focused on PD-L1 expression on APCs, the first barrier in fighting against infection, and found their association with inflammation, organ failure, and mortality during early sepsis.
The regulation of nuclear factor-kappa B (NF-κB), the key factor involved in inflammation, might account for the correlation observed in our study. Peripheral blood mononuclear cells (PBMCs) from non-survivors of sepsis had increased levels of NF-κB activation. Increased NF-κB activation was also strongly correlated with the severity of illness (APACHE II score) and associated with higher mortality.[29,30] The association between PD-L1 and NF-κB, however, has not been thoroughly studied in sepsis. A study showed that TNF-α induced the NF-κB pathway and promoted demethylated PD-L1 promoter expression in non-small cell lung carcinoma. Inhibition of NF-κB resulted in the abolition of PD-L1 expression.[31] Other studies confirmed that NF-κB occupied the CD274 promoter and acted as a regulator of PD-L1 mRNA expression in various cancer types.[32,33] However, the underlying regulatory mechanism during sepsis still requires future research.
There are some limitations to our study. First, this was a single-center, observational study, and the sample size was relatively small. Further large-scale studies should be conducted to validate our findings. Second, the observation period was relatively short. We only traced the patients for 28 d and did not evaluate long-term changes in the immune system. Third, we only picked a single time point to observe the immune condition, which could not reflect the dynamic changes during the sepsis course. Patients should be stratified and discussed to avoid bias. Fourth, only a correlation between PD-L1 on APCs and cytokines was observed. The possible role of inflammation master molecules, such as NF-κB, should be elucidated to explain the mechanism of our findings. Finally, the flow cytometry results may have differed due to different protocols and processing software. Thus, the results of our study could only reflect trends in relative expression levels during sepsis.
CONCLUSIONS
PD-L1 was over-expressed on APCs during early sepsis and correlated with the severity of sepsis. PD-L1 on APCs (monocytes and DCs) was weakly correlated with inflammation and organ dysfunction during early sepsis. The combination of SOFA or APACHE II scores with monocyte PD-1% could improve the ability for mortality prediction.
Funding: None.
Ethical approval: All operations were in compliance with the ethics standards of Beijing Chaoyang Hospital. Informed consents for participation were obtained from patients or their legally authorized representatives. Ethical approval was acquired from Beijing Chaoyang Hospital Medical Ethics Committee (Ethical number: 2013-KE-1). The study conformed to the Declaration of Helsinki.
Conflicts of interest: The authors do not have a financial interest or relationship to disclose regarding this research project.
Contributors: CSL contributed to the study conception and design, revised the manuscript, and provided research funding. JBL, MLD, YNY, CCH, ZRT, and MRX were involved in sample collection and material preparation, data collection and analysis during the experimental process. JBL collected data, drafted the manuscript, performed statistical analysis, and critically revised the manuscript. All authors commented on previous versions of the manuscript. All authors consented to the publication of the manuscript and agreed to be responsible for the manuscript.
All the supplementary files in this paper are available at http://wjem.com.cn.
Reference
Effects of viral infection and microbial diversity on patients with sepsis: A retrospective study based on metagenomic next-generation sequencing
DOI:10.5847/wjem.j.1920-8642.2021.01.005 URL [Cited within: 1]
Prognosis-related classification and dynamic monitoring of immune status in patients with sepsis: A prospective observational study
DOI:10.5847/wjem.j.1920-8642.2021.03.004
PMID:34141032
[Cited within: 1]

The dynamic monitoring of immune status is crucial to the precise and individualized treatment of sepsis. In this study, we aim to introduce a model to describe and monitor the immune status of sepsis and to explore its prognostic value.A prospective observational study was carried out in Zhongshan Hospital, Fudan University, enrolling septic patients admitted between July 2016 and December 2018. Blood samples were collected at days 1 and 3. Serum cytokine levels (e.g., tumor necrosis factor-α [TNF-α], interleukin-10 [IL-10]) and CD14 monocyte human leukocyte antigen-D-related (HLA-DR) expression were measured to serve as immune markers. Classification of each immune status, namely systemic inflammatory response syndrome (SIRS), compensatory anti-inflammatory response syndrome (CARS), and mixed antagonistic response syndrome (MARS), was defined based on levels of immune markers. Changes of immune status were classified into four groups which were stabilization (SB), deterioration (DT), remission (RM), and non-remission (NR).A total of 174 septic patients were enrolled including 50 non-survivors. Multivariate analysis discovered that IL-10 and HLA-DR expression levels at day 3 were independent prognostic factors. Patients with MARS had the highest mortality rate. Immune status of 46.1% patients changed from day 1 to day 3. Among four groups of immune status changes, DT had the highest mortality rate, followed by NR, RM, and SB with mortality rates of 64.7%, 42.9%, and 11.2%, respectively.Severe immune disorder defined as MARS or deterioration of immune status defined as DT lead to the worst outcomes. The preliminary model of the classification and dynamic monitoring of immune status based on immune markers has prognostic values and is worthy of further investigation.Copyright: © World Journal of Emergency Medicine.
Assessment of global incidence and mortality of hospital-treated Sepsis. current estimates and limitations
DOI:10.1164/rccm.201504-0781OC URL [Cited within: 1]
The third international consensus definitions for sepsis and septic shock (Sepsis-3)
DOI:10.1001/jama.2016.0287
PMID:26903338
[Cited within: 2]

Definitions of sepsis and septic shock were last revised in 2001. Considerable advances have since been made into the pathobiology (changes in organ function, morphology, cell biology, biochemistry, immunology, and circulation), management, and epidemiology of sepsis, suggesting the need for reexamination.To evaluate and, as needed, update definitions for sepsis and septic shock.A task force (n = 19) with expertise in sepsis pathobiology, clinical trials, and epidemiology was convened by the Society of Critical Care Medicine and the European Society of Intensive Care Medicine. Definitions and clinical criteria were generated through meetings, Delphi processes, analysis of electronic health record databases, and voting, followed by circulation to international professional societies, requesting peer review and endorsement (by 31 societies listed in the Acknowledgment).Limitations of previous definitions included an excessive focus on inflammation, the misleading model that sepsis follows a continuum through severe sepsis to shock, and inadequate specificity and sensitivity of the systemic inflammatory response syndrome (SIRS) criteria. Multiple definitions and terminologies are currently in use for sepsis, septic shock, and organ dysfunction, leading to discrepancies in reported incidence and observed mortality. The task force concluded the term severe sepsis was redundant.Sepsis should be defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. For clinical operationalization, organ dysfunction can be represented by an increase in the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score of 2 points or more, which is associated with an in-hospital mortality greater than 10%. Septic shock should be defined as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone. Patients with septic shock can be clinically identified by a vasopressor requirement to maintain a mean arterial pressure of 65 mm Hg or greater and serum lactate level greater than 2 mmol/L (>18 mg/dL) in the absence of hypovolemia. This combination is associated with hospital mortality rates greater than 40%. In out-of-hospital, emergency department, or general hospital ward settings, adult patients with suspected infection can be rapidly identified as being more likely to have poor outcomes typical of sepsis if they have at least 2 of the following clinical criteria that together constitute a new bedside clinical score termed quickSOFA (qSOFA): respiratory rate of 22/min or greater, altered mentation, or systolic blood pressure of 100 mm Hg or less.These updated definitions and clinical criteria should replace previous definitions, offer greater consistency for epidemiologic studies and clinical trials, and facilitate earlier recognition and more timely management of patients with sepsis or at risk of developing sepsis.
Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy
DOI:10.1038/nri3552
PMID:24232462
[Cited within: 1]

Sepsis - which is a severe life-threatening infection with organ dysfunction - initiates a complex interplay of host pro-inflammatory and anti-inflammatory processes. Sepsis can be considered a race to the death between the pathogens and the host immune system, and it is the proper balance between the often competing pro- and anti-inflammatory pathways that determines the fate of the individual. Although the field of sepsis research has witnessed the failure of many highly touted clinical trials, a better understanding of the pathophysiological basis of the disorder and the mechanisms responsible for the associated pro- and anti-inflammatory responses provides a novel approach for treating this highly lethal condition. Biomarker-guided immunotherapy that is administered to patients at the proper immune phase of sepsis is potentially a major advance in the treatment of sepsis and in the field of infectious disease.
Frontline science: defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1
DOI:10.1189/jlb.4HI0616-255R
URL
[Cited within: 3]

Sepsis is a heterogeneous syndrome comprising a highly diverse and dynamic mixture of hyperinflammatory and compensatory anti-inflammatory immune responses. This immune phenotypic diversity highlights the importance of proper patient selection for treatment with the immunomodulatory drugs that are entering clinical trials. To better understand the serial changes in immunity of critically ill patients and to evaluate the potential efficacy of blocking key inhibitory pathways in sepsis, we undertook a broad phenotypic and functional analysis of innate and acquired immunity in the same aliquot of blood from septic, critically ill nonseptic, and healthy donors. We also tested the ability of blocking the checkpoint inhibitors programmed death receptor-1 (PD-1) and its ligand (PD-L1) to restore the function of innate and acquired immune cells. Neutrophil and monocyte function (phagocytosis, CD163, cytokine expression) were progressively diminished as sepsis persisted. An increasing frequency in PD-L1+-suppressor phenotype neutrophils [low-density neutrophils (LDNs)] was also noted. PD-L1+ LDNs and defective neutrophil function correlated with disease severity, consistent with the potential importance of suppressive neutrophil populations in sepsis. Reduced neutrophil and monocyte function correlated both with their own PD-L1 expression and with PD-1 expression on CD8+ T cells and NK cells. Conversely, reduced CD8+ T cell and NK cell functions (IFN-γ production, granzyme B, and CD107a expression) correlated with elevated PD-L1+ LDNs. Importantly, addition of antibodies against PD-1 or PD-L1 restored function in neutrophil, monocyte, T cells, and NK cells, underlining the impact of the PD-1:PD-L1 axis in sepsis-immune suppression and the ability to treat multiple deficits with a single immunomodulatory agent.
Check point inhibitors and their role in immunosuppression in sepsis
DOI:S0749-0704(19)30069-7
PMID:31733683
[Cited within: 4]

Checkpoint regulators are a group of membrane-bound receptors or ligands expressed on immune cells to regulate the immune cell response to antigen presentation and other immune stimuli, such as cytokines, chemokines, and complement. In the context of profound immune activation, such as sepsis, the immune system can be rendered anergic by these receptors to prevent excessive inflammation and tissue damage. If this septic immunosuppression is prolonged, the host is unable to mount the appropriate immune response to a secondary insult or infection. This article describes the manner in which major regulators in the B7-CD28 family and their ligands mediate immunosuppression in sepsis.Copyright © 2019 Elsevier Inc. All rights reserved.
Effects of Maxingloushi decoction on immune inflammation and programmed death markers in mice with chronic obstructive pulmonary disease
DOI:10.5847/wjem.j.1920-8642.2022.023
PMID:35003414
[Cited within: 1]

To investigate effects of Maxingloushi decoction on lung inflammation and programmed death markers (programmed death-1 [PD-1], programmed death-ligand 1 [PD-L1]) in the lung tissue, peripheral blood, and bronchoalveolar lavage fluid (BLF) in a mouse model of chronic obstructive pulmonary disease (COPD).Thirty-six mature male BALB/C mice were randomly divided into normal group (group A, =6), COPD model group (group B, =10), Maxingloushi decoction + COPD group (group C, =10), and PD-1 inhibitor + COPD group (group D, =10). The COPD model was established by smoke inhalation combined with lipopolysaccharide (LPS). Levels of PD-1 and PD-L1 in plasma and BLF were measured by enzyme-linked immunosorbent assay (ELISA). Histopathological techniques were used to semi-quantitatively analyze the immuno-fluorescence optical density (IOD) value of the lung tissue.In plasma and BLF, the expression of PD-1 in the group B was higher than that in the group A, and the expression of PD-L1 was lower than that in the group A. The expression of PD-1 and PD-L1 in the lung tissue was normalized in the group C in comparison with the group B (<0.05) and the group D (<0.05), and inflammatory cell infiltration in the lung tissue was also improved.These findings reveal that COPD causes an immune imbalance in the peripheral blood and lung tissue, and that both Maxingloushi decoction and PD-1 inhibitor treatment can mitigate lung inflammation in COPD by reducing PD-1 expression and increasing PD-L1 expression. The treatment effect of Maxingloushi decoction may be superior to that of PD-1 inhibitor.Copyright: © World Journal of Emergency Medicine.
Immunosuppression in patients who die of sepsis and multiple organ failure
DOI:10.1001/jama.2011.1829
PMID:22187279
[Cited within: 1]

Severe sepsis is typically characterized by initial cytokine-mediated hyperinflammation. Whether this hyperinflammatory phase is followed by immunosuppression is controversial. Animal studies suggest that multiple immune defects occur in sepsis, but data from humans remain conflicting.To determine the association of sepsis with changes in host innate and adaptive immunity and to examine potential mechanisms for putative immunosuppression.Rapid postmortem spleen and lung tissue harvest was performed at the bedsides of 40 patients who died in intensive care units (ICUs) of academic medical centers with active severe sepsis to characterize their immune status at the time of death (2009-2011). Control spleens (n = 29) were obtained from patients who were declared brain-dead or had emergent splenectomy due to trauma; control lungs (n = 20) were obtained from transplant donors or from lung cancer resections.Cytokine secretion assays and immunophenotyping of cell surface receptor-ligand expression profiles were performed to identify potential mechanisms of immune dysfunction. Immunohistochemical staining was performed to evaluate the loss of immune effector cells.The mean ages of patients with sepsis and controls were 71.7 (SD, 15.9) and 52.7 (SD, 15.0) years, respectively. The median number of ICU days for patients with sepsis was 8 (range, 1-195 days), while control patients were in ICUs for 4 or fewer days. The median duration of sepsis was 4 days (range, 1-40 days). Compared with controls, anti-CD3/anti-CD28-stimulated splenocytes from sepsis patients had significant reductions in cytokine secretion at 5 hours: tumor necrosis factor, 5361 (95% CI, 3327-7485) pg/mL vs 418 (95% CI, 98-738) pg/mL; interferon γ, 1374 (95% CI, 550-2197) pg/mL vs 37.5 (95% CI, -5 to 80) pg/mL; interleukin 6, 3691 (95% CI, 2313-5070) vs 365 (95% CI, 87-642) pg/mL; and interleukin 10, 633 (95% CI, -269 to 1534) vs 58 (95% CI, -39 to 156) pg/mL; (P <.001 for all). There were similar reductions in 5-hour lipopolysaccharide-stimulated cytokine secretion. Cytokine secretion in sepsis patients was generally less than 10% that in controls, independent of age, duration of sepsis, corticosteroid use, and nutritional status. Although differences existed between spleen and lung, flow cytometric analysis showed increased expression of selected inhibitory receptors and ligands and expansion of suppressor cell populations in both organs. Unique differences in cellular inhibitory molecule expression existed in immune cells isolated from lungs of sepsis patients vs cancer patients and vs transplant donors. Immunohistochemical staining showed extensive depletion of splenic CD4, CD8, and HLA-DR cells and expression of ligands for inhibitory receptors on lung epithelial cells.Patients who die in the ICU following sepsis compared with patients who die of nonsepsis etiologies have biochemical, flow cytometric, and immunohistochemical findings consistent with immunosuppression. Targeted immune-enhancing therapy may be a valid approach in selected patients with sepsis.
Early PREdiction of sepsis using leukocyte surface biomarkers: the ExPRES-sepsis cohort study
DOI:10.1007/s00134-018-5389-0
PMID:30291379
[Cited within: 1]

Reliable biomarkers for predicting subsequent sepsis among patients with suspected acute infection are lacking. In patients presenting to emergency departments (EDs) with suspected acute infection, we aimed to evaluate the reliability and discriminant ability of 47 leukocyte biomarkers as predictors of sepsis (Sequential Organ Failure Assessment score ≥ 2 at 24 h and/or 72 h following ED presentation).In a multi-centre cohort study in four EDs and intensive care units (ICUs), we standardised flow-cytometric leukocyte biomarker measurement and compared patients with suspected acute infection (cohort-1) with two comparator cohorts: ICU patients with established sepsis (cohort-2), and ED patients without infection or systemic inflammation but requiring hospitalization (cohort-3).Between January 2014 and February 2016, we recruited 272, 59 and 75 patients to cohorts 1, 2, and 3, respectively. Of 47 leukocyte biomarkers, 14 were non-reliable, and 17 did not discriminate between the three cohorts. Discriminant analyses for predicting sepsis within cohort-1 were undertaken for eight neutrophil (cluster of differentiation antigens (CD) CD15; CD24; CD35; CD64; CD312; CD11b; CD274; CD279), seven monocyte (CD35; CD64; CD312; CD11b; HLA-DR; CD274; CD279) and a CD8 T-lymphocyte biomarker (CD279). Individually, only higher neutrophil CD279 [OR 1.78 (95% CI 1.23-2.57); P = 0.002], higher monocyte CD279 [1.32 (1.03-1.70); P = 0.03], and lower monocyte HLA-DR [0.73 (0.55-0.97); P = 0.03] expression were associated with subsequent sepsis. With logistic regression the optimum biomarker combination was increased neutrophil CD24 and neutrophil CD279, and reduced monocyte HLA-DR expression, but no combination had clinically relevant predictive validity.From a large panel of leukocyte biomarkers, immunosuppression biomarkers were associated with subsequent sepsis in ED patients with suspected acute infection.NCT02188992.
Monocyte programmed death ligand-1 expression after 3-4 days of sepsis is associated with risk stratification and mortality in septic patients: a prospective cohort study
DOI:10.1186/s13054-016-1301-x URL [Cited within: 2]
Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016
DOI:10.1007/s00134-017-4683-6
PMID:28101605
[Cited within: 1]

To provide an update to "Surviving Sepsis Campaign Guidelines for Management of Sepsis and Septic Shock: 2012".A consensus committee of 55 international experts representing 25 international organizations was convened. Nominal groups were assembled at key international meetings (for those committee members attending the conference). A formal conflict-of-interest (COI) policy was developed at the onset of the process and enforced throughout. A stand-alone meeting was held for all panel members in December 2015. Teleconferences and electronic-based discussion among subgroups and among the entire committee served as an integral part of the development.The panel consisted of five sections: hemodynamics, infection, adjunctive therapies, metabolic, and ventilation. Population, intervention, comparison, and outcomes (PICO) questions were reviewed and updated as needed, and evidence profiles were generated. Each subgroup generated a list of questions, searched for best available evidence, and then followed the principles of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system to assess the quality of evidence from high to very low, and to formulate recommendations as strong or weak, or best practice statement when applicable.The Surviving Sepsis Guideline panel provided 93 statements on early management and resuscitation of patients with sepsis or septic shock. Overall, 32 were strong recommendations, 39 were weak recommendations, and 18 were best-practice statements. No recommendation was provided for four questions.Substantial agreement exists among a large cohort of international experts regarding many strong recommendations for the best care of patients with sepsis. Although a significant number of aspects of care have relatively weak support, evidence-based recommendations regarding the acute management of sepsis and septic shock are the foundation of improved outcomes for these critically ill patients with high mortality.
The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults
DOI:10.1378/chest.100.6.1619
PMID:1959406
[Cited within: 1]

The objective of this study was to refine the APACHE (Acute Physiology, Age, Chronic Health Evaluation) methodology in order to more accurately predict hospital mortality risk for critically ill hospitalized adults. We prospectively collected data on 17,440 unselected adult medical/surgical intensive care unit (ICU) admissions at 40 US hospitals (14 volunteer tertiary-care institutions and 26 hospitals randomly chosen to represent intensive care services nationwide). We analyzed the relationship between the patient's likelihood of surviving to hospital discharge and the following predictive variables: major medical and surgical disease categories, acute physiologic abnormalities, age, preexisting functional limitations, major comorbidities, and treatment location immediately prior to ICU admission. The APACHE III prognostic system consists of two options: (1) an APACHE III score, which can provide initial risk stratification for severely ill hospitalized patients within independently defined patient groups; and (2) an APACHE III predictive equation, which uses APACHE III score and reference data on major disease categories and treatment location immediately prior to ICU admission to provide risk estimates for hospital mortality for individual ICU patients. A five-point increase in APACHE III score (range, 0 to 299) is independently associated with a statistically significant increase in the relative risk of hospital death (odds ratio, 1.10 to 1.78) within each of 78 major medical and surgical disease categories. The overall predictive accuracy of the first-day APACHE III equation was such that, within 24 h of ICU admission, 95 percent of ICU admissions could be given a risk estimate for hospital death that was within 3 percent of that actually observed (r2 = 0.41; receiver operating characteristic = 0.90). Recording changes in the APACHE III score on each subsequent day of ICU therapy provided daily updates in these risk estimates. When applied across the individual ICUs, the first-day APACHE III equation accounted for the majority of variation in observed death rates (r2 = 0.90, p less than 0.0001).
The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure
DOI:10.1007/BF01709751 URL [Cited within: 1]
Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation
DOI:10.1084/jem.192.7.1027
PMID:11015443
[Cited within: 2]

PD-1 is an immunoinhibitory receptor expressed by activated T cells, B cells, and myeloid cells. Mice deficient in PD-1 exhibit a breakdown of peripheral tolerance and demonstrate multiple autoimmune features. We report here that the ligand of PD-1 (PD-L1) is a member of the B7 gene family. Engagement of PD-1 by PD-L1 leads to the inhibition of T cell receptor-mediated lymphocyte proliferation and cytokine secretion. In addition, PD-1 signaling can inhibit at least suboptimal levels of CD28-mediated costimulation. PD-L1 is expressed by antigen-presenting cells, including human peripheral blood monocytes stimulated with interferon gamma, and activated human and murine dendritic cells. In addition, PD-L1 is expressed in nonlymphoid tissues such as heart and lung. The relative levels of inhibitory PD-L1 and costimulatory B7-1/B7-2 signals on antigen-presenting cells may determine the extent of T cell activation and consequently the threshold between tolerance and autoimmunity. PD-L1 expression on nonlymphoid tissues and its potential interaction with PD-1 may subsequently determine the extent of immune responses at sites of inflammation.
PD-L1 overexpression during endotoxin tolerance impairs the adaptive immune response in septic patients via HIF1α
DOI:10.1093/infdis/jix279
PMID:28973671
[Cited within: 1]

Sepsis, among other pathologies, is an endotoxin tolerance (ET)-related disease. On admission, we classified 48 patients with sepsis into 3 subgroups according to the ex vivo response to lipopolysaccharide. This response correlates with the Acute Physiology and Chronic Health Evaluation (APACHE) II score and the ET degree. Moreover, the ET-related classification determines the outcome of these patients. Programmed cell death-ligand 1 (PD-L1) expression on septic monocytes is also linked with ET status. In addition to the regulation of cytokine production, one of the hallmarks of ET that significantly affects patients with sepsis is T-cell proliferation impairment or a poor switch to the adaptive response. PD-L1/programmed cell death-1 (PD-1) blocking and knockdown assays on tolerant monocytes from both patients with sepsis and the in vitro model reverted the impaired adaptive response. Mechanistically, the transcription factor hypoxia-inducible factor-1α (HIF1α) has been translocated into the nucleus and drives PD-L1 expression during ET in human monocytes. This fact, together with patient classification according to the ex vivo lipopolysaccharide response, opens an interesting field of study and potential personalized clinical applications, not only for sepsis but also for all ET-associated pathologies.© The Author(s) 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Improved survival after induction of sepsis by cecal slurry in PD-1 knockout murine neonates
DOI:10.1016/j.surg.2016.11.008 URL [Cited within: 1]
PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis
DOI:10.1073/pnas.0809422106
URL
[Cited within: 1]

\n Sepsis, a leading cause of death worldwide, involves concomitant expression of an overzealous inflammatory response and inefficient bacterial clearance. Macrophage function is pivotal to the development of these two aspects during sepsis; however, the mechanisms underlying these changes remain unclear. Here we report that the PD-1:PD-L pathway appears to be a determining factor of the outcome of sepsis, regulating the delicate balance between effectiveness and damage by the antimicrobial immune response. To this end we observed that PD-1\n −/−\n mice were markedly protected from the lethality of sepsis, accompanied by a decreased bacterial burden and suppressed inflammatory cytokine response. To the extent that this is a macrophage-specific aspect of the effects of PD-1, we found the following: first, peritoneal macrophages expressed significantly higher levels of PD-1 during sepsis, which was associated with their development of cellular dysfunction; second, when peritoneal macrophages were depleted (using clodronate liposomes) from PD-1\n −/−\n mice, the animals' bactericidal capacity was lowered, their inflammatory cytokine levels were elevated, and protection from septic lethality was diminished; and third, blood monocytes from both septic mice and patients with septic shock shared markedly increased PD-1 levels. Together, these data suggest that PD-1 may not only be a dysfunctional marker/effector of macrophages/monocytes, but may also be a potential therapeutic target for designing measures to modulate the innate immune response, thereby preventing the detrimental effects of sepsis.\n
Analysis of PD-1 expression in the monocyte subsets from non-septic and septic preterm neonates
PD-1 expression is elevated in monocytes from hepatocellular carcinoma patients and contributes to CD 8 T cell suppression
DOI:10.1007/s12026-020-09155-3 [Cited within: 1]
Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients
DOI:10.1186/cc10112 URL [Cited within: 1]
Association between elevation of plasma biomarkers and monocyte dysfunction and their combination in predicting sepsis: an observational single-centre cohort study
DOI:10.1177/1753425920926602
URL
[Cited within: 1]

This study aimed to investigate the possible relationship between the two biomarkers presepsin and procalcitonin (PCT) and monocyte immune function, and to explore their combination in mortality prediction in the early stage of sepsis. A total of 198 patients with bacterial infection and diagnosed with sepsis and 40 healthy control subjects were included. Blood samples were collected on admission within 24 h. Plasma concentrations of presepsin and PCT were measured. Expression of monocyte surface CD14, programmed cell death receptor ligand-1 (PD-L1) and human leucocyte Ag (HLA)-DR were determined using flow cytometry. Levels of plasma presepsin and PCT were significantly higher under septic conditions, and increased with the progression of sepsis. Monocyte CD14 and HLA-DR expression were decreased, while PD-L1 was overexpressed in sepsis compared to control. Presepsin and PCT concentrations were positively correlated with Sequential Organ Failure Assessment score, Acute Physiology and Chronic Health Evaluation System II score and PD-L1, while they were negatively correlated with CD14 and HLA-DR. Presepsin plus monocyte HLA-DR mean fluorescence intensity had the highest prognostic value over other parameters alone or in combination. Presepsin and PCT had a weak correlation with monocyte dysfunction during early sepsis. The combination of presepsin and monocyte HLA-DR could help improve prognostic value.
Increased percentage of PD-L1+ natural killer cells predicts poor prognosis in sepsis patients: a prospective observational cohort study
DOI:10.1186/s13054-020-03329-z
[Cited within: 1]

Natural killer (NK) cells play a major role in immune tolerance after sepsis, and the programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) system mediates evasion of host immunity. The correlation between PD-L1 levels in NK cells and the prognosis of patients with sepsis, however, has not been elucidated. Thus, it was hypothesized that PD-L1 in NK cells could be a novel biomarker of the mortality for sepsis patients.
Tissue expression of PD-L1 mediates peripheral T cell tolerance
DOI:10.1084/jem.20051776
PMID:16606670
[Cited within: 1]

Programmed death 1 (PD-1), an inhibitory receptor expressed on activated lymphocytes, regulates tolerance and autoimmunity. PD-1 has two ligands: PD-1 ligand 1 (PD-L1), which is expressed broadly on hematopoietic and parenchymal cells, including pancreatic islet cells; and PD-L2, which is restricted to macrophages and dendritic cells. To investigate whether PD-L1 and PD-L2 have synergistic or unique roles in regulating T cell activation and tolerance, we generated mice lacking PD-L1 and PD-L2 (PD-L1/PD-L2(-/-) mice) and compared them to mice lacking either PD-L. PD-L1 and PD-L2 have overlapping functions in inhibiting interleukin-2 and interferon-gamma production during T cell activation. However, PD-L1 has a unique and critical role in controlling self-reactive T cells in the pancreas. Our studies with bone marrow chimeras demonstrate that PD-L1/PD-L2 expression only on antigen-presenting cells is insufficient to prevent the early onset diabetes that develops in PD-L1/PD-L2(-/-) non-obese diabetic mice. PD-L1 expression in islets protects against immunopathology after transplantation of syngeneic islets into diabetic recipients. PD-L1 inhibits pathogenic self-reactive CD4+ T cell-mediated tissue destruction and effector cytokine production. These data provide evidence that PD-L1 expression on parenchymal cells rather than hematopoietic cells protects against autoimmune diabetes and point to a novel role for PD-1-PD-L1 interactions in mediating tissue tolerance.
PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells
DOI:10.1073/pnas.0307252101
PMID:15249675
[Cited within: 1]

Both positive and negative regulatory roles have been suggested for the B7 family member PD-L1(B7-H1). PD-L1 is expressed on antigen-presenting cells (APCs), activated T cells, and a variety of tissues, but the functional significance of PD-L1 on each cell type is not yet clear. To dissect the functions of PD-L1 in vivo, we generated PD-L1-deficient (PD-L1(-/-)) mice. CD4(+) and CD8(+) T cell responses were markedly enhanced in PD-L1(-/-) mice compared with wild-type mice in vitro and in vivo. PD-L1(-/-) dendritic cells stimulated greater wild-type CD4(+) T cell responses than wild-type dendritic cells, and PD-L1(-/-) CD4(+) T cells produced more cytokines than wild-type CD4(+) T cells in vitro, demonstrating an inhibitory role for PD-L1 on APCs and T cells. In vivo CD8(+) T cell responses also were significantly enhanced, indicating that PD-L1 has a role in limiting the expansion or survival of CD8(+) T cells. Studies using the myelin oligodendrocyte model of experimental autoimmune encephalomyelitis showed that PD-L1 on T cells and in host tissues limits responses of self-reactive CD4(+) T cells in vivo. PD-L1 deficiency converted the 129S4/SvJae strain from a resistant to experimental autoimmune encephalomyelitis-susceptible strain. Transfer of encephalitogenic T cells from wild-type mice into PD-L1(-/-) recipients led to exacerbated disease. Disease was even more severe in PD-L1(-/-) recipients of PD-L1(-/-) T cells. These results demonstrate that PD-L1 on T cells, APCs, and host tissue inhibits naïve and effector T cell responses and plays a critical role in T cell tolerance.
Molecular mechanisms of T cell co-stimulation and co-inhibition
Anti-PD-L1 treatment results in functional remodeling of the macrophage compartment
DOI:10.1158/0008-5472.CAN-18-3208
PMID:30679180
[Cited within: 1]

Checkpoint inhibitors like anti-PD1/PD-L1 have demonstrated significant therapeutic efficacy in a subset of patients partly through reinvigoration of CD8 T cells. However, their impact on myeloid cells remains largely unknown. Here, we report that anti-PD-L1 treatment favorably impacts the phenotype and function of tumor macrophages by polarizing the macrophage compartment toward a more proinflammatory phenotype. This phenotype was characterized by a decrease in Arginase-I (ARG1) expression and an increase in iNOS, MHCII, and CD40 expression. Whole-transcriptome profiling further confirmed extensive polarization of both tumor monocytes and macrophages from a suppressive to a proinflammatory, immunostimulatory phenotype. This polarization was driven mainly through IFNγ and was associated with enhanced T-cell activity. Transfer of monocytes into anti-PD-L1-treated tumor-bearing mice led to macrophage differentiation into a more proinflammatory phenotype, with an increase in CD8 T cells expressing granzyme-B and an increase in the CD8/Treg ratio compared with control-treated mice. Although in responsive tumor models, anti-PD-L1 treatment remodeled the macrophage compartment with beneficial effects on T cells, both macrophage reprogramming and depletion were needed to maximize anti-PD-L1 responses in a tumor immune contexture with high macrophage burden. Our results demonstrate that anti-PD-L1 treatment can favorably remodel the macrophage compartment in responsive tumor models toward a more proinflammatory phenotype, mainly through increased IFNγ levels. They also suggest that directly targeting these cells with reprogramming and depleting agents may further augment the breadth and depth of response to anti-PD-L1 treatment in less responsive or more macrophage-dense tumor microenvironments. SIGNIFICANCE: This work demonstrates that increased IFNγ signaling following anti-PD-L1 treatment can remodel the macrophage compartment to enhance T-cell responses. http://cancerres.aacrjournals.org/content/canres/79/7/1493/F1.large.jpg.©2019 American Association for Cancer Research.
Blockade of PD-L 1 enhances cancer immunotherapy by regulating dendritic cell maturation and macrophage polarization
DOI:10.3390/cancers11091400
URL
[Cited within: 1]

The immuno-inhibitory checkpoint PD-L1, regulated by tumor cells and antigen-presenting cells (APCs), dampened the activation of T cells from the PD-1/PD-L1 axis. PD-L1-expressing APCs rather than tumor cells demonstrated the essential anti-tumor effects of anti-PD-L1 monotherapy in preclinical tumor models. Using the murine tumor model, we investigated whether anti-PD-L1 antibody increased the antigen-specific immune response and anti-tumor effects induced by the antigen-specific protein vaccine, as well as the possible mechanisms regarding activation of APCs. Anti-PD-L1 antibody combined with the PEK protein vaccine generated more potent E7-specific immunity (including the number and cytotoxic activity of E7-specific cytotoxic CD8+ T lymphocytes) and anti-tumor effects than protein vaccine alone. Anti-PD-L1 antibody enhanced the maturation of dendritic cells and the proportion of M1-like macrophages in tumor-draining lymph nodes and tumors in tumor-bearing mice treated with combinatorial therapy. PD-L1 blockade overturned the immunosuppressive status of the tumor microenvironment and then enhanced the E7 tumor-specific antigen-specific immunity and anti-tumor effects generated by an E7-specific protein vaccine through modulation of APCs in an E7-expressing small tumor model. Tumor-specific antigen (like HPV E7 antigen)-specific immunotherapy combined with APC-targeting modality by PD-L1 blockade has a high translational potential in E7-specific cancer therapy.
Predictive value of nuclear factor kappa B activity and plasma cytokine levels in patients with sepsis
DOI:10.1128/IAI.68.4.1942-1945.2000
PMID:10722586
[Cited within: 1]

The relationship between fluctuating cytokine concentrations in plasma and the outcome of sepsis is complex. We postulated that early measurement of the activation of nuclear factor kappaB (NF-kappaB), a transcriptional regulatory protein involved in proinflammatory cytokine expression, may help to predict the outcome of sepsis. We determined NF-kappaB activation in peripheral blood mononuclear cells of 34 patients with severe sepsis (23 survivors and 11 nonsurvivors) and serial concentrations of inflammatory cytokines (interleukin-6, interleukin-1, and tumor necrosis factor) and various endogenous antagonists in plasma. NF-kappaB activity was significantly higher in nonsurvivors and correlated strongly with the severity of illness (APACHE II score), although neither was related to the cytokine levels. Apart from NF-kappaB activity, the interleukin-1 receptor antagonist was the only cytokine tested whose level in plasma was of value in predicting mortality by logistic regression analysis. These results underscore the prognostic value of early measurement of NF-kappaB activity in patients with severe sepsis.
Role of NFkappa B in the mortality of sepsis
DOI:10.1172/JCI119648
PMID:9276714
[Cited within: 1]

Binding activity for nuclear factor kappa B (NFkappaB) consensus probes was studied in nuclear extracts from peripheral blood mononuclear cells of 15 septic patients (10 surviving and 5 not surviving). Nonsurvivors could be distinguished from survivors by an increase in NFkappaB binding activity during the observation period (P < 0.001). The increase in NFkappaB binding activity was comparable to the APACHE-II score as a predictor of outcome. Intravenous somatic gene transfer with an expression plasmid coding for IkappaBalpha was used to investigate the role of members of the NFkappaB family in a mouse model of endotoxemia. In this model, increased NFkappaB binding activity was present after injection of LPS. Intravenous somatic gene transfer with IkappaBalpha given before LPS attenuated renal NFkappaB binding activity and increased survival. Endothelial cells and monocytes/macrophages were the major target cells for somatic gene transfer, transfected with an average transfection efficiency of 20-35%. Tissue factor, a gene under regulatory control of NFkappaB, was induced by LPS. Somatic gene transfer with a reporter plasmid containing the functional tissue factor promoter demonstrated NFkappaB-dependent stimulation by LPS. Intravenous somatic gene transfer with IkappaBalpha reduced LPS-induced renal tissue factor expression, activation of the plasmatic coagulation system (decrease of thrombin-antithrombin III complexes) and renal fibrin/fibrinogen deposition. Somatic gene transfer with an expression plasmid with tissue factor cDNA in the antisense direction (in contrast to sense or vector alone) also increased survival. Furthermore, antisense tissue factor decreased renal tissue factor expression and the activation of the plasmatic coagulation system.
PD-L1 expression is regulated by both DNA methylation and NF-κB during EMT signaling in non-small cell lung carcinoma
MUC1-C integrates PD-L 1 induction with repression of immune effectors in non-small-cell lung cancer
DOI:10.1038/onc.2017.47
PMID:28288138
[Cited within: 1]

Immunotherapeutic approaches, particularly programmed death 1/programmed death ligand 1 (PD-1/PD-L1) blockade, have improved the treatment of non-small-cell lung cancer (NSCLC), supporting the premise that evasion of immune destruction is of importance for NSCLC progression. However, the signals responsible for upregulation of PD-L1 in NSCLC cells and whether they are integrated with the regulation of other immune-related genes are not known. Mucin 1 (MUC1) is aberrantly overexpressed in NSCLC, activates the nuclear factor-κB (NF-κB) p65→︀ZEB1 pathway and confers a poor prognosis. The present studies demonstrate that MUC1-C activates PD-L1 expression in NSCLC cells. We show that MUC1-C increases NF-κB p65 occupancy on the CD274/PD-L1 promoter and thereby drives CD274 transcription. Moreover, we demonstrate that MUC1-C-induced activation of NF-κB→︀ZEB1 signaling represses the TLR9 (toll-like receptor 9), IFNG, MCP-1 (monocyte chemoattractant protein-1) and GM-CSF genes, and that this signature is associated with decreases in overall survival. In concert with these results, targeting MUC1-C in NSCLC tumors suppresses PD-L1 and induces these effectors of innate and adaptive immunity. These findings support a previously unrecognized central role for MUC1-C in integrating PD-L1 activation with suppression of immune effectors and poor clinical outcome.
Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κB to foster an immunosuppressive tumor microenvironment in ovarian cancer
DOI:10.1158/0008-5472.CAN-14-3098
PMID:26573793
[Cited within: 1]

Emerging evidence has highlighted the host immune system in modulating the patient response to chemotherapy, but the mechanism of this modulation remains unclear. The aim of this study was to analyze the effect of chemotherapy on antitumor immunity in the tumor microenvironment of ovarian cancer. Treatment of ovarian cancer cell lines with various chemotherapeutic agents resulted in upregulated expression of MHC class I and programmed cell death 1 ligand 1 (PD-L1) in a NF-κB-dependent manner and suppression of antigen-specific T-cell function in vitro. In a mouse model of ovarian cancer, treatment with paclitaxel increased CD8(+) T-cell infiltration into the tumor site, upregulated PD-L1 expression, and activated NF-κB signaling. In particular, tumor-bearing mice treated with a combination of paclitaxel and a PD-L1/PD-1 signal blockade survived longer than mice treated with paclitaxel alone. In summary, we found that chemotherapy induces local immune suppression in ovarian cancer through NF-κB-mediated PD-L1 upregulation. Thus, a combination of chemotherapy and immunotherapy targeting the PD-L1/PD-1 signaling axis may improve the antitumor response and offers a promising new treatment modality against ovarian cancer.©2015 American Association for Cancer Research.