INTRODUCTION
Cardiac arrest (CA) is a critical condition that is a concern to healthcare workers. Cardiopulmonary resuscitation (CPR) is widely used in CA of various etiologies and is considered to be the only effective method.[1] Moreover, when the duration of CA exceeds 10 minutes, the survival rate declines rapidly; if the duration of CA exceeds 20 minutes, patients rarely survive.[2]
Extracorporeal membrane oxygenation (ECMO) is a technology that relies on instrumentation to support the basic needs of the human body. Since the 1990s, ECMO combined with CPR technology (extracorporeal cardiopulmonary resuscitation [ECPR]) has been increasingly applied in attempts to improve the success rate of recovery following CA.[3] Several studies have analyzed and summarized relevant cases, and comparatively studied ECPR and conventional cardiopulmonary resuscitation (CCPR) technologies and have indicated that ECPR is superior to CCPR.[4,5,6,7]
However, the current literature mainly comprises retrospective studies or case analyses, and there is a lack of multicenter randomized controlled clinical trials that compare the protective effects of the two resuscitative methods on organs. In particular, there have been no reports on the effects of early oxygenated blood perfusion in relieving post-resuscitation lung injury in experimental animals such as swine.[8,9,10]
In this study, using a swine model of ventricular fibrillation-induced CA, we aim to evaluate whether early application of ECPR has advantages over CCPR in the lung injury and to explore the protective mechanism of ECPR on the post-resuscitation pulmonary injury.
METHODS
Study animals
We used 40-day-old male long-white swine (n=16; weight 35.13±5.57 kg) in this study. The experimental animals were randomly divided into two groups: CCPR group (CCPR after CA, n=8) and ECPR group (ECPR after CA, n=8).
At the beginning, anesthesia was induced using intramuscular injections of ketamine (10 mg/kg) and midazolam (0.5 mg/kg). Then, propofol (2.0 mg/kg) was intravenously administered. Propofol (9.0 mg/[kg·h]) and fentanyl (1.0 µg/[kg·h]) were continuously infused intravenously. Endotracheal intubation was carried out with a 6.5-mm tracheal catheter, and then the animals were placed on mechanical ventilation (Servo 900 c; Siemens, Berlin, Germany) with volume-controlled ventilation (tidal volume 15.0 mL/kg, respiratory rate 12-20 breaths/minute, and oxygen concentration 35%).
Subsequently, a 5-Fr deep venous catheter was inserted into the right external jugular vein of the experimental animal for intravenous infusion and fibrillation access. A 16-Fr (Dragon Laifu Medical Products Co., Ltd., China) intravenous catheter was inserted into the right atrium, and a 14-Fr arterial catheter was inserted into the ascending aorta. These two catheters were connected to the ECMO machine. All catheters were pretreated with normal heparin saline (5.0 U/mL).
Preparation of ECMO
The ECMO equipment consisted of arterial and venous catheters, Levitronix CentriMag control system (Sarns Healthcare/3M, Ann Arbor, USA), a centrifugal force pump (MAQUET Cardiopulmonary AG, Germany), a coated porous biofilm lung (MAQUET Holding B.V. and Co. KG, Germany), and gas mixers (Thoratec Corporation, USA). The catheters and ECMO machine were prewashed with normal heparin saline and filled with heparinized colloid solution (250 U/kg) oxygenated up to 50% oxygen through the oxygenator. An arterial catheter (5 Fr; Terumo, Japan) and Swan-Ganz catheter (7 Fr; Edwards Life Sciences, USA) were placed in the left femoral artery and left femoral vein, respectively, and connected to an HP monitor (M1165; Hewlett-Packard Co., USA) to continuously monitor the heart rate (HR) and the mean arterial pressure (MAP). Furthermore, the thermal dilution method was used to detect extravascular lung water (EVLW), pulmonary vascular permeability index (PVPI), and other relevant data.
Experimental procedure
The swine were placed under observation for 30 minutes. Care was taken to ensure that the animals were in a stable state before the onset of artificial ventricular fibrillation.
A programmed medical stimulator (GY-600A; Kaifeng Huanan Instrument Co., China) was used to induce fibrillation (parameters: mode, S1/S2 [300/200 ms]; output voltage, 40 V; 8:1 ratio; step-size, -10 ms continuous program-controlled stimulation until ventricular fibrillation). The success of the ventricular fibrillation was determined by the rapid decrease in arterial pressure and the observance of ventricular fibrillation on the ECG monitor. Both the CCPR and ECPR groups were observed for 12 minutes, during which no treatment or intervention was administered in either group. Thereafter, CPR without any defibrillation treatment was conducted in both groups. Fourteen minutes after shock, the ECPR group was treated with ECMO using continuous CPR.
Both the CCPR group and ECPR group received their first defibrillation at 18 minutes after shock. The defibrillator was applied with a two-way wave of 4.0 J/kg for defibrillation. Subsequent compressions, defibrillation, and medication use were in accordance with the 2015 guidelines for CPR.[11]
The standard criteria for the return of spontaneous circulation (ROSC) are as follows: MAP greater than 60 mmHg (1 mmHg=0.133 kPa) or systolic pressure greater than 80 mmHg for more than 20 minutes.[12]
The endpoints were the status of the animal at 6 hours after ROSC, or the death of the animals. After 6 hours of ROSC, the swine were intravenously injected with 60 mg propofol before administered with 20 mL of 10% potassium chloride and sacrificed.
Data collection and testing
Hemodynamic parameters including HR, MAP, and end-expiratory carbon dioxide were monitored continuously. EVLW and PVPI were measured by the thermal dilution method at baseline in both groups. These were measured in the CCPR group at 6 hours after ROSC, and in the ECPR group the ECMO machine was stopped at 6 hours after ROSC.
Blood samples were collected at baseline, ROSC, and 1, 2, 4, and 6 hours after ROSC. The samples were tested by enzyme-linked immunosorbent assay (ELISA) using ELISA kits (Shanghai Sangon Biotechnology Company, Ltd., China) for pulmonary surfactant protein A (SP-A), pulmonary surfactant protein D (SP-D), Clara cell protein 16 (CC16), malondialdehyde (MDA), and superoxide dismutase (SOD).
Lung tissue samples (lower lobe of the right lung) were collected immediately after the swine were euthanized. The samples were also tested for myeloperoxidase (MPO), MDA, SOD, SP-A, and SP-D using ELISA (Shanghai Sangon Biotechnology Company, Ltd., China).
The ultrastructure of the tissue was observed using electron microscopy (JEM-1010; JEOL, Japan).
Statistical analysis
All the data were statistically analyzed using SPSS 22.0 (IBM, NY, USA). Continuous variables were expressed as mean±standard deviation (SD), and the differences between CCPR and ECPR groups were determined by the Student’s t-test. Survival rates between the two groups were estimated by Kaplan-Meier analysis. The P-value <0.05 was considered statistically significant.
RESULTS
Comparison of survival rates
All swine were successfully induced into ventricular fibrillation. In the CCPR group, two swine died at 3.7 and 5.3 hours after ROSC, while the remaining survived up to 6 hours (6/8, 67.5%). All animals in the ECPR group successfully survived to ROSC for 6 hours (8/8, 100%). The survival rates between the two groups were not statistically significant (P>0.05).
Comparison of blood and tissue biomarkers
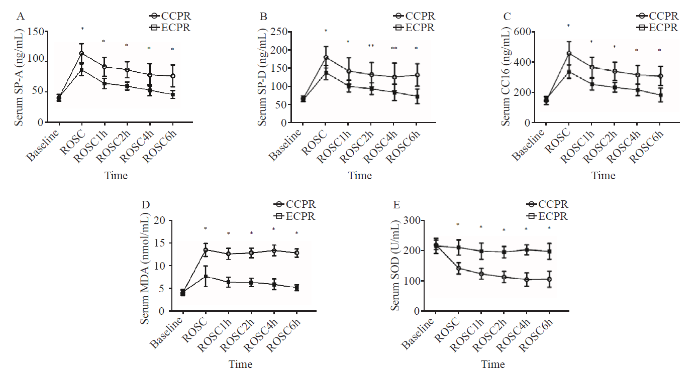
There were no significant differences in serum SP-A, SP-D, CC16, MDA, and SOD at baseline between the two groups (P>0.05). Serum SP-A, SP-D, CC16, and MDA were found in the ROSC and 1, 2, 4, 6 hours after ROSC. The levels of the biomarkers mentioned at the above time points were statistically higher in the CCPR group than in the ECPR group (P<0.05), whereas serum SOD at the above mentioned five time points in the CCPR group was lower than that of the ECPR group (P<0.01) (Figures 1A-E). The comparison of tissues from the two groups showed that the levels of MDA (11.05±1.07 nmol/mL vs. 5.67±1.62 nmol/mL, P<0.01) and MPO (634.66±54.62 ng/mL vs. 274.08±99.78 ng/mL, P<0.01) were significantly higher in the CCPR group than in the ECPR group, whereas the levels of SP-A (32.06±4.13 ng/mL vs. 71.87±18.88 ng/mL, P<0.01), SP-D (45.08±3.40 ng/mL vs. 131.29±22.80 ng/mL, P<0.01), and SOD (82.92±32.02 U/mL vs. 158.65±20.68 U/mL, P<0.01) were significantly lower in the CCPR group.
Figure 1.
Figure 1.
Comparison of serum markers at each time point between the two groups. Data were expressed as mean±standard deviation (n=8); CCPR: conventional cardiopulmonary resuscitation; ECPR: extracorporeal cardiopulmonary resuscitation; SP-A: surfactant protein A; SP-D: surfactant protein D; CC16: Clara cell protein 16; MDA: malondialdehyde; SOD: superoxide dismutase; ROSC: return of spontaneous circulation; ROSC1h/2h/4h/6h: 1, 2, 4, and 6 hours after ROSC; compared with baseline, *P<0.01, **P <0.05.
Significant differences in EVLW and PVPI between the two groups
Table 1 compares the EVLW and PVPI between the two groups. At baseline, there were no significant differences between the two groups (P>0.05). The EVLW at 6 hours after ROSC (ROSC6h) and the PVPI values at ROSC6h in both groups showed statistically significant differences (P<0.01). Moreover, the EVLW values at ROSC6h compared with the baseline in both groups also showed statistically significant differences (P<0.05). The PVPI at ROSC6h in the CCPR group was statistically higher than that at baseline (P<0.01), while there was no difference for the ECPR group (P>0.05).
Table 1 Comparison of EVLW and PVPI between the two groups
| Variables | Baseline | ROSC6h | P-value |
|---|---|---|---|
| EVLW (mL/kg) | |||
| CCPR group | 9.61±1.37 | 21.85±3.92 | <0.001 |
| ECPR group | 9.39±1.70 | 11.78±1.82* | 0.017 |
| PVPI | |||
| CCPR group | 2.24±0.50 | 5.97±1.39 | 0.001 |
| ECPR group | 2.15±0.43 | 2.06±0.91* | 0.812 |
Data were expressed as mean±standard deviation (n=8); EVLW: extravascular lung water; PVPI: pulmonary vascular permeability index; CCPR: conventional cardiopulmonary resuscitation; ECPR: extracorporeal cardiopulmonary resuscitation; ROSC6h: 6 hours after return of spontaneous circulation; compared with CCPR group, *P<0.001.
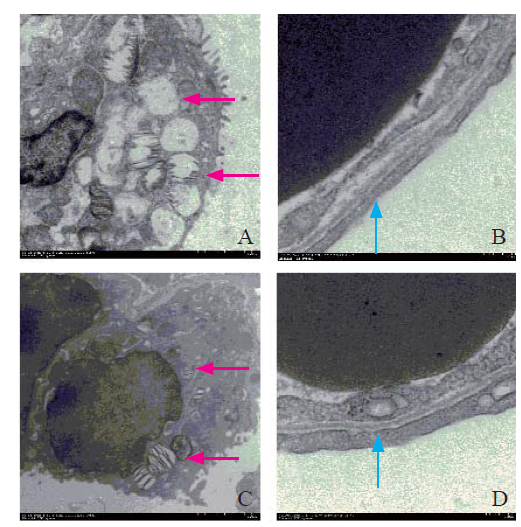
Electron microscopy results between the two groups
In the CCPR group, the broadening of the blood-gas barrier was observed. Furthermore, in the epithelial cells, we observed empty vacuoles in type II lamellar bodies. In the ECPR group, the blood-gas barrier was clear without any broadening, and the epithelial cells showed non-empty type II lamellar bodies (Figure 2).
Figure 2.
Figure 2.
Organizational structure on electron microscopy. Red arrow: type II lamellar (×2,500); blue arrow: blood-gas barrier (×12,000); A, B: CCPR group; C, D: ECPR group; CCPR: conventional cardiopulmonary resuscitation; ECPR: extracorporeal cardiopulmonary resuscitation.
DISCUSSION
This study showed that ECPR had a better pulmonary protective effect than CCPR. Compared with the ECPR group, the CCPR group experienced more severe oxidative stress injury, and had a worse scavenging ability for oxygen free radicals. In the ECPR group, the more protective active proteins were present on the alveolar surface, the blood-gas barrier was intact, and there was greater abundance of the alveolar surface-active protein in the lamellar body and less pulmonary edema.
SP-A and SP-D can minimize lung injury by reducing the production of inflammatory factors, and can clear various pathogens.[13] Meanwhile, SP-A and SP-D have strong antioxidant functions, and these two proteins can alleviate and prevent the oxidative stress response of the lung induced by various etiologies.[14] Furthermore, SP-A can control apoptosis and stabilize alveolar epithelial cells.[15] Low SP-A expression in lung tissues will lead to decreased stability of alveolar epithelial cells and increase the possibility of pulmonary edema, pulmonary infection, and pulmonary injury.[16] The SP-D secretion in lung tissues has a clear protective effect on lungs against infection and maintains the stability of alveolar epithelial cells.[17,18] Lung injury will lead to the reduction of SP-A and SP-D in lung tissue.[13,19] The injury to the blood-gas barrier caused by lung injury will lead to the increased secretion of serum SP-A and SP-D.[20] Serum SP-D concentration is positively correlated with the degree of lung injury[21] as well as mortality.[22] When Clara cells are damaged or there are changes in alveolar epithelial permeability, the synthesis and secretion of CC16 will change, which is of great value in the diagnosis of lung injury.[21] Increased serum concentrations of CC16 can clearly indicate the lung injury, and are positively correlated with the degree of lung injury.[23-25] SOD is a key enzyme for scavenging oxygen free radicals. Cells are protected from superoxide damage by catalysis, competitive binding of superoxide radicals, and increased bioavailability of nitric oxide (NO).[26,27] MDA is the final product of the lipid peroxidation of the main structure of the cell membrane and occurs because of the degradation of polyunsaturated lipids. Lipid peroxidation is a mature mechanism of cell damage in plants and animals, and it is used as an indicator of oxidative stress in cells and tissues.[28] Several experiments have shown that serum examination of MDA and SOD can reflect the degree of oxidative damage to tissues.[29,30] MPO is an important enzyme released by neutrophils, and promotes the formation of hypochlorous acid, a powerful oxidant associated with bactericidal effects and tissue destruction through induction of necrosis and apoptosis.[31,32] The increased tissue MPO has been associated with aggravated oxidative damage of tissues.[29,33] Oxidative damage leads to the destruction of lipid structure of tissue cells, and subsequent increase of MDA in tissues.[30,33] This process will excessively consume SOD, resulting in a sharp decline in tissue SOD levels.[33]
The levels of serum and tissue SOD in the CCPR group decreased significantly, however, the levels of serum SP-A, SP-D, CC16, and MDA, and the levels of tissue MDA and MPO were significantly increased, suggesting more severe lung injury and more severe damage from oxidative free radicals. The differences between the two groups after ROSC indicated that ECMO began to protect the lungs during the resuscitation process. Lung injury occurred in both groups and gradually decreased after resuscitation, but there was less injury and damage from oxidative free radicals in the ECPR group.
From the EVLW and PVPI results, we found that the pulmonary edema in the CCPR group was more serious when comparing the changes of EVLW and baseline values in the CCPR group and the ECPR group at ROSC6h. The PVPI value of the CCPR group showed an abnormal increase at ROSC6h, whereas the PVPI value of the ECPR group remained in the normal range and did not differ from the baseline PVPI value. The increase in PVPI is attributable to permeable pulmonary edema, and the increased EVLW in the CCPR group was found to be mainly caused by permeable pulmonary edema. The serum and tissue SP-A and SP-D levels showed that the loss of SP-A and SP-D was serious in the CCPR group because of the release of more surface-active materials through damage to the blood-gas barrier rather than by secretion into the alveoli, which subsequently led to changes in the lung microstructure, increased alveolar tension, and abnormal changes of permeability, and contributed to the severity of the permeable pulmonary edema. Through this between-group comparison, ECMO was found to have a positive effect on reducing pulmonary fluid extravasation and also on stabilizing the vascular permeability of the lungs. Moreover, it indicated that the ECPR group had significantly less pulmonary edema compared with the CCPR group, which indicated the protection of lung function.
Furthermore, histology with electron microscopic examination showed that the CCPR group had severe alveolar type II lamellar body cell loss and inadequate storage of alveolar surface-active protein, which increased the damage to the blood-gas barrier, indicating an inability to guarantee the stability of the alveolar membrane permeability and increased permeability leading to pulmonary edema. However, the ECPR group retained better organized microstructure and showed alveolar type II cells with plenty of stored alveolar surface-active materials, which can be secreted as needed into the alveolar space. There was no serious damage to the blood-gas barrier, and therefore the optimal functioning of the barrier was maintained. Based on the histological changes between the two groups, it is reasonable to assume that the ECPR group can have greater resistance to resuscitated pulmonary exogenous infection than the CCPR group when the duration of treatment is prolonged.
The limitations of our experimental study are as follows. First, it is nearly impossible to carry out large-scale animal experiments; therefore, the sample size was small and there existed bias. Second, in order to enable ECMO to be added to the previous treatment process of ROSC, the catheterization for ECMO was completed well in advance. The timely placement and operation of ECMO are extremely difficult to be completed quickly in practical circumstances.
CONCLUSIONS
Early application of ECPR may have a better protective effect on post-resuscitation lung injury than CCPR. Its mechanism may be partly related to the regulation of the alveolar surface-active proteins and the improvement of oxidative stress response after resuscitation.
Funding: There was no funding support for this study.
Ethical approval: This study was approved by the Animal Care Committee of Capital Medical University Institutional and the Animal Care and Use Committee of Beijing Chaoyang Hospital, Capital Medical University.
Conflicts of interests: The authors declare that they have no conflicts of interest regarding this article.
Contributors: JYL proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
Reference
Cardiopulmonary resuscitation for hospital inpatients in Taiwan: an 8-year nationwide survey
DOI:10.1016/j.resuscitation.2011.09.006 URL [Cited within: 1]
Impact of cardiopulmonary resuscitation duration on survival from paramedic witnessed out-of-hospital cardiac arrests: an observational study
DOI:10.1016/j.resuscitation.2015.12.011 URL [Cited within: 1]
Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation
Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis
DOI:10.1016/S0140-6736(08)60958-7 URL [Cited within: 1]
Extracorporeal resuscitation for refractory out-of-hospital cardiac arrest in adults: a systematic review of international practices and outcomes
DOI:10.1016/j.resuscitation.2016.01.018
PMID:26836946
[Cited within: 1]

Extracorporeal resuscitation during cardiopulmonary resuscitation (ECPR) deploys rapid cardiopulmonary bypass to sustain oxygenated circulation until the return of spontaneous circulation (ROSC). The purpose of this systematic review is to address the defining elements and outcomes (quality survival and organ donation) of currently active protocols for ECPR in refractory out-of-hospital cardiac arrest (OHCA) of cardiac origin in adult patients. The results may inform policy and practices for ECPR and help clarify the corrresponding intersection with deceased organ donation.We searched Medline, Embase, Cochrane and seven other electronic databases from 2005 to 2015, with no language restrictions. Internal validity and the quality of the studies reporting outcomes and guidelines were assessed. The review was included in the international prospective register of systematic reviews (Prospero, CRD42014015259).One guideline and 20 outcome studies were analyzed. Half of the studies were prospective observational studies assessed to be of fair to good methodological quality. The remainder were retrospective cohorts, case series, and case studies. Ages ranged from 16 to 75 years and initial shockable cardiac rhythms, witnessed events, and a reversible primary cause of cardiac arrest were considered favorable prognostic factors. CPR duration and time to hospital cannulation varied considerably. Coronary revascularization, hemodynamic interventions and targeted temperature management neuroprotection were variable. A total of 833 patients receiving this ECPR approach had an overall reported survival rate of 22%, including 13% with good neurological recovery. Additionally, 88 potential and 17 actual deceased organ donors were identified among the non-survivor population in 8 out of 20 included studies. Study heterogeneity precluded a meta-analysis preventing any meaningful comparison between protocols, interventions and outcomes.ECPR is feasible for refractory OHCA of cardiac origin in adult patients. It may enable neurologically good survival in selected patients, who practically have no other alternative in order to save their lives with quality of life, and contribute to organ donation in those who die. Large, prospective studies are required to clarify patient selection, modifiable outcome variables, risk-benefit and cost-effectiveness.Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Comparing extracorporeal cardiopulmonary resuscitation with conventional cardiopulmonary resuscitation: a meta-analysis
DOI:10.1016/j.resuscitation.2016.01.019 URL [Cited within: 1]
Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children
PMID:17893278
[Cited within: 1]

Extracorporeal membrane oxygenation (ECMO) has been used to support cardiorespiratory function during pediatric cardiopulmonary resuscitation (CPR). We report on outcomes and predictors of in-hospital mortality after ECMO used to support CPR (E-CPR).Outcomes for patients aged <18 years using E-CPR were analyzed with data from the Extracorporeal Life Support Organization, and predictors of in-hospital mortality were determined. Of 26,242 ECMO uses reported, 695 (2.6%) were for E-CPR (n=682 patients). Survival to hospital discharge was 38%. In a multivariable model, pre-ECMO factors such as cardiac disease (odds ratio [OR] 0.51, 95% confidence interval [CI] 0.31 to 0.82) and neonatal respiratory disease (OR 0.28, 95% CI 0.12 to 0.66), white race (OR 0.65, 95% CI 0.45 to 0.94), and pre-ECMO arterial blood pH >7.17 (OR 0.50, 95% CI 0.30 to 0.84) were associated with decreased odds of mortality. During ECMO, renal dysfunction (OR 1.89, 95% CI 1.17 to 3.03), pulmonary hemorrhage (OR 2.23, 95% CI 1.11 to 4.50), neurological injury (OR 2.79, 95% CI 1.55 to 5.02), CPR during ECMO (OR 3.06, 95% CI 1.42 to 6.58), and arterial blood pH <7.2 (OR 2.23, 95% CI 1.23 to 4.06) were associated with increased odds of mortality.ECMO used to support CPR rescued one third of patients in whom death was otherwise certain. Patient diagnosis, absence of severe metabolic acidosis before ECMO support, and uncomplicated ECMO course were associated with improved survival.
Death and do-not-resuscitate order in the emergency department: A single-center three-year retrospective study in the Chinese mainland
DOI:10.5847/wjem.j.1920-8642.2020.04.005 URL [Cited within: 1]
Feasibility of initiating extracorporeal life support during mechanical chest compression CPR: a porcine pilot study
DOI:10.1016/j.resuscitation.2011.07.030
PMID:21835144
[Cited within: 1]

Recently, portable extracorporeal membrane oxygenation (ECMO) machines have become commercially available. This creates the potential to utilize extracorporeal life support (ECLS) for the treatment of sudden cardiac arrest in the emergency department, and potentially in the out-of-hospital setting.We sought to determine the feasibility of installing the ECMO circuit during delivery of mechanical chest compression CPR.We used 5 mixed-breed domestic swine with a mean mass of 26.0 kg. After induction of anesthesia, animals were instrumented with micromanometer-tipped transducers placed in the aorta and right atrium via the left femoral artery and vein. Ventricular fibrillation (VF) was induced electrically with a transthoracic shock and left untreated for 8 min. Then, mechanical chest compressions were begun (LUCAS, Jolife, Lund, Sweden) and manual ventilations were performed to maintain ETCO(2) between 35 and 45Torr. Compressions continued until ECMO flow was started. Ten minutes after induction of VF, drugs were given (epinephrine, vasopressin, and propranolol). ECMO installation was started via cutdown on the right external jugular vein and right femoral artery for placement of venous and arterial catheters while chest compressions continued. ECMO installation start time varied from 17 to 30 min after start of compressions and continued until ECG indicated a shockable rhythm. First rescue shocks were given at 22, 32, 35, 44, and 65 min.ECMO was successfully installed in all five animals without incident. It was necessary to briefly discontinue chest compressions during the most delicate part of inserting the catheters into the vessels. ECMO also allowed for very rapid cooling of the animals and facilitated post-resuscitation hemodynamic support. Only the 65-min animal did not attain return of spontaneous circulation (ROSC).Mechanical chest compression may be a suitable therapeutic bridge to the installation of ECMO and does not interfere with ECMO catheter placement.Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial)
DOI:10.1016/j.resuscitation.2014.09.010 URL [Cited within: 1]
Part 7: adult advanced cardiovascular life support 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care
Part 8: post-cardiac arrest care 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care
Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa
DOI:10.1165/rcmb.2005-0461OC URL [Cited within: 2]
Pulmonary surfactant proteins A and D are potent endogenous inhibitors of lipid peroxidation and oxidative cellular injury
PMID:10969075
[Cited within: 1]

The lung is composed of a series of branching conducting airways that terminate in grape-like clusters of delicate gas-exchanging airspaces called pulmonary alveoli. Maintenance of alveolar patency at end expiration requires pulmonary surfactant, a mixture of phospholipids and proteins that coats the epithelial surface and reduces surface tension. The surfactant lining is exposed to the highest ambient oxygen tension of any internal interface and encounters a variety of oxidizing toxicants including ozone and trace metals contained within the 10 kl of air that is respired daily. The pathophysiological consequences of surfactant oxidation in humans and experimental animals include airspace collapse, reduced lung compliance, and impaired gas exchange. We now report that the hydrophilic surfactant proteins A (SP-A) and D (SP-D) directly protect surfactant phospholipids and macrophages from oxidative damage. Both proteins block accumulation of thiobarbituric acid-reactive substances and conjugated dienes during copper-induced oxidation of surfactant lipids or low density lipoprotein particles by a mechanism that does not involve metal chelation or oxidative modification of the proteins. Low density lipoprotein oxidation is instantaneously arrested upon SP-A or SP-D addition, suggesting direct interference with free radical formation or propagation. The antioxidant activity of SP-A maps to the carboxyl-terminal domain of the protein, which, like SP-D, contains a C-type lectin carbohydrate recognition domain. These results indicate that SP-A and SP-D, which are ubiquitous among air breathing organisms, could contribute to the protection of the lung from oxidative stresses due to atmospheric or supplemental oxygen, air pollutants, and lung inflammation.
The role of surfactant protein A in bleomycin-induced acute lung injury
DOI:10.1164/rccm.200907-1002OC URL [Cited within: 1]
Influence of donor lung surfactant-A and -B protein expression on the development of primary graft dysfunction after lung transplantation: a pilot study
DOI:10.12659/AOT.903313 URL [Cited within: 1]
A pulmonary source of infection in patients with sepsis-associated acute kidney injury leads to a worse outcome and poor recovery of kidney function
DOI:10.5847/wjem.j.1920-8642.2020.01.003 URL [Cited within: 1]
Surfactant protein D inhibits lung inflammation caused by ventilation in premature newborn lambs
DOI:10.1164/rccm.200912-1818OC URL [Cited within: 1]
Restoration of lung surfactant protein D by IL-6 protects against secondary pneumonia following hemorrhagic shock
DOI:10.1016/j.jinf.2013.11.010 URL [Cited within: 1]
The sitting position during neurosurgical procedures does not influence serum biomarkers of pulmonary parenchymal injury
DOI:10.1186/1471-2482-12-24 URL [Cited within: 1]
Investigation of surfactant protein-D and interleukin-6 levels in patients with blunt chest trauma with multiple rib fractures and pulmonary contusions: a cross-sectional study in Black Sea Region of Turkey
DOI:10.1136/bmjopen-2016-011797 URL [Cited within: 2]
Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury
DOI:10.1378/chest.09-1484 URL [Cited within: 1]
Clinical study on the changes of lung-specific proteins: CC16 after lung contusion
DOI:10.3892/etm.2017.4842 URL [Cited within: 1]
Circulating levels of Clara cell protein 16 but not surfactant protein D identify and quantify lung damage in patients with multiple injuries
Plasma CC16 levels are associated with development of ALI/ARDS in patients with ventilator-associated pneumonia: a retrospective observational study
DOI:10.1186/1471-2466-9-1 URL [Cited within: 1]
Cu/Zn superoxide dismutase plays important role in immune response
PMID:12626552
[Cited within: 1]

Activation of macrophages leads to the secretion of cytokines and enzymes that shape the inflammatory response and increase metabolic processes. This, in turn, results in increased production of reactive oxygen species. The role of Cu/Zn superoxide dismutase (SOD-1), an important enzyme in cellular oxygen metabolism, was examined in activated peritoneal elicited macrophages (PEM) and in several inflammatory processes in vivo. LPS and TNF-alpha induced SOD-1 in PEM. SOD-1 induction by LPS was mainly via extracellular signal-regulated kinase-1 activation. Transgenic mice overexpressing SOD-1 demonstrated a significant increase in the release of TNF-alpha and of the metalloproteinases MMP-2 and MMP-9 from PEM. Disulfiram (DSF), an inhibitor of SOD-1, strongly inhibited the release of TNF-alpha, vascular endothelial growth factor, and MMP-2 and MMP-9 from cultured activated PEM. These effects were prevented by addition of antioxidants, further indicating involvement of reactive oxygen species. In vivo, transgenic mice overexpressing SOD-1 demonstrated a 4-fold increase in serum TNF-alpha levels and 2-fold stronger delayed-type hypersensitivity reaction as compared with control nontransgenic mice. Conversely, oral administration of DSF lowered TNF-alpha serum level by 4-fold, lowered the delayed-type hypersensitivity response in a dose-dependent manner, and significantly inhibited adjuvant arthritis in Lewis rats. The data suggest an important role for SOD-1 in inflammation, establish DSF as a potential inhibitor of inflammation, and raise the possibility that regulation of SOD-1 activity may be important in the treatment of immune-dependent pathologies.
Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice
DOI:10.1161/01.RES.0000092140.81594.A8 URL [Cited within: 1]
Oxidative and pre-inflammatory stress in wedge resection of pulmonary parenchyma using the radiofrequency ablation technique in a swine model
DOI:10.1186/1749-8090-7-7 URL [Cited within: 1]
The protective effect of dexmedetomidine on LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB and PI3K/Akt/mTOR pathways
DOI:S0161-5890(17)30601-6
PMID:29241031
[Cited within: 2]

The aim of present study was to evaluate the protective effects of dexmedetomidine (DEX) on lipopolysaccharide (LPS)-induced acute lung injury (ALI) and investigate its possible mechanisms mediated by HMGB1. In vivo, pulmonary pathology observation and myeloperoxidase (MPO) activity were also examined to evaluate the protective effect of DEX in the lungs. Tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) in bronchoalveolar lavage fluid (BALF), serum and lung tissues LPS-induced rats were detected. The oxidative indices including superoxide dismutase (SOD), Malondialdehyde (MDA), and glutathione peroxidase (GSH-Px) in serum were also determined. Additionally, nitric oxide (NO), TNF-α, IL-6 and IL-1β, MDA, SOD and GSH-Px in the supernatants of LPS-induced BEAS-2B cells were measured. Furthermore, we detected the protein expression of high mobility group box-1 protein (HMGB1), Toll-like receptor 4 (TLR4), myeloid differentiating factor 88 (MyD88), inhibitor of NF-κB (IκBα), p-IκBα, nuclear factor kappa-B (NF-κB), p-NF-κB, phosphatidylinositol 3'-kinase (PI3K), p-PI3K, protein kinase B (Akt), p-Akt, mammalian target of rapamycin (mTOR) and p-mTOR in LPS-induced ALI rats and LPS-induced BEAS-2B cells. Immunohistochemical and immunofluorescence analyses of HMGB1 in lung tissues or BEAS-2B cells were also conducted to evaluate the mechanisms of DEX. DEX effectively attenuated pulmonary pathology, and ameliorated the levels of MPO, SOD, MDA, GSH-Px, TNF-α, IL-6, IL-1β and NO in LPS-stimulated rats and BEAS-2B cells. Additionally, treatment with DEX inhibited the expression of HMGB1, TLR4, MyD88, p-IκB, p-NF-κB, p-PI3K, p-Akt and p-mTOR in vivo and in vitro. Immunohistochemical and immunofluorescence analyses also showed that DEX suppressed HMGB1 levels in lung sections and BEAS-2B cells. Treatment with glycyrrhizin, an inhibitor of HMGB1, confirmed that HMGB1 was involved in the mechanism of DEX on LPS-induced ALI. The transfection of HGMB1 siRNA also confirmed these findings in vitro. In conclusion, the present study showed that DEX exerted a protective effect on LPS-induced ALI rats likely through the HMGB1-mediated TLR4/NF-κB and PI3K/Akt/mTOR pathways.Copyright © 2017 Elsevier Ltd. All rights reserved.
Melatonin ameliorates chronic renal failure-induced oxidative organ damage in rats
DOI:10.1111/jpi.2004.36.issue-4 URL [Cited within: 2]
Myeloperoxidase: friend and foe
DOI:10.1189/jlb.1204697 URL [Cited within: 1]
15-epi-lipoxin A4Inhibits myeloperoxidase signaling and enhances resolution of acute lung injury
DOI:10.1164/rccm.200810-1601OC URL [Cited within: 1]
Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis
DOI:10.1016/j.redox.2017.03.001 URL [Cited within: 3]