INTRODUCTION
Intensive care unit-acquired weakness (ICU-AW) is typically characterized by generalized and symmetrical muscle weakness, affecting both limb and respiratory muscles while sparing the facial and ocular muscles.[1⇓-3] It encompasses three distinct conditions: critical illness polyneuropathy, myopathy, and neuromyopathy. These conditions contribute to prolonged hospital stays and increased disability rates, worsening long-term outcomes. Early prevention, diagnosis, and intervention are crucial for improving these outcomes.[4] ICU-AW can be diagnosed via the Medical Research Council (MRC) score, where a total score of <48 on the 60-point scale meets the diagnostic criteria. A variety of factors contribute to the development of ICU-AW.[5,6] Some studies recommend initiating enteral nutrition (EN) within the first 24-48 h, referred to as early EN (EEN), to maintain intestinal barrier function, enhance intestinal immunity, promote nutrient absorption, reduce infection risk, and support recovery.[7,8] However, owing to common complications in critically ill patients, such as shock, contraindications to EN, and intestinal intolerance, research on the efficacy of EEN in preventing ICU-AW remains limited. The predictive value of biomarkers for ICU-AW is also not well understood.[9]
Therefore, we aimed to investigate the risk factors for ICU-AW in critically ill patients at risk of malnutrition, assess effects of EEN on ICU-AW, and evaluate the predictive value of biomarkers for early diagnosis and intervention.
METHODS
Inclusion and exclusion criteria
In this prospective, observational cohort study, we recruited critically ill patients at risk of malnutrition from the emergency intensive care unit (EICU) of the First Affiliated Hospital of Xiamen University Hospital between January 2022 and December 2023.
Nutritional Risk Screening (NRS-2002) was used to identify eligible participants at risk of malnutrition. This screening includes four components: anthropometric measurements; serum biochemical parameters; clinical evaluation; and dietary history, with a score ≥3 indicating nutritional risk.
Patients with treatment interruptions or incomplete data during the therapeutic process were excluded.
Patient group and ICU-AW diagnosis
Patients were divided into ICU-AW group and non-ICU-AW group according to whether they developed ICU-AW.
ICU-AW was diagnosed using the MRC score. In accordance with the MRC scoring system, daily assessments were conducted for each enrolled patient for diagnostic purposes.[10] For patients unable to cooperate with daily muscle strength test due to sedation, endotracheal intubation, or the need for restraints, the test was postponed until these conditions were resolved.
Nutritional support
In accordance with the 2022 American Society for Parenteral and Enteral Nutrition guidelines for nutritional support in critically ill adults, nutritional support was provided based on the recommended. Patients who received only parenteral nutrition (PN) within the first 48 h were classified into the PN group, whereas patients who started EN within 48 h were classified into the EEN group.
Data collction
For each enrolled patient, demographic information and relevant medical history were carefully recorded. The levels of biomarkers such as prealbumin (PAB), C-reactive protein (CRP), blood urea nitrogen (BUN), and creatinine (Cr) were assessed every 3 days. PAB/CRP and Cr/BUN ratios were calculated to assess nutritional status. Transferrin (TRF) levels were also monitored weekly to further assess the patient's nutritional response. The time from admission to ICU-AW diagnosis was recorded, and for patients without ICU-AW, the length of stay was recorded.
The primary outcome was the occurrence of ICU-AW. Data were reported according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (STROBE).
Statistical analysis
Statistical analyses and sample size estimation were performed via SPSS version 25.0. Data normality was assessed via the Shapiro-Wilk test. Parametric assumptions were verified through tests for homogeneity of variances and normality of distribution. For continuous variables with a normal distribution, means and standard deviations were calculated, and comparisons between groups were made via Student’s t-test for independent samples. Categorical data were compared via the Chi-square test or Fisher’s exact test, as appropriate. In cases where the data did not meet the criteria for parametric analysis, non-parametric tests, such as the Mann-Whitney U test for two independent samples or the Kruskal-Wallis test for more than two groups, were employed. Multivariate logistic regression was used to identify independent predictors of ICU-AW after adjusting for potential confounders. A two-tailed P-value <0.05 was considered statistically significant. The study was powered to detect an odds ratio (OR)/risk ratio of 85% at a significance level of 0.05.
RESULTS
Risk factors of ICU-AW
Among the 180 critically ill ICU patients, 75 developed ICU-AW, resulting in an incidence of approximately 42%. Table 1 presents the results of factors potentially associated with the development of ICU-AW. Differences were found in factors such as gender, age, body mass index (BMI), mechanical ventilation (MV), type of nutrition support, and levels of TRF, BUN, and Cr (all P<0.05).
Table 1. Univariate logistic regression analysis of risk factors for different outcomes
| Parameters | ICU-AW (n=75) | Non-ICU-AW (n=105) | OR | 95% CI | P-value |
|---|---|---|---|---|---|
| Male, n (%) | 45 (60.0) | 68 (64.8) | 0.336 | 0.179-0.628 | 0.001 |
| Age, years, median (IQR) | 74.0 (67.0-84.0) | 73.0 (65.5-82.0) | 1.085 | 1.049-1.123 | <0.001 |
| BMI, kg/m2, median (IQR) | 19.6 (17.8-21.6) | 21.0 (19.4-20.8) | 0.716 | 0.622-0.824 | <0.001 |
| Underlying disease, n (%) | |||||
| Hypertension | 36 (48.0) | 50 (47.6) | 0.960 | 0.544-1.782 | 0.985 |
| Diabetes | 26 (34.7) | 24 (22.9) | 0.083 | 0.246-3.185 | 0.558 |
| Malignant tumor | 17 (22.7) | 11 (10.5) | 2.505 | 1.097-5.721 | 0.029 |
| MV, n (%) | 18 (24.0) | 13 (12.4) | 2.235 | 1.018-4.905 | 0.048 |
| TRF, g/L, median (IQR) | 2.5 (2.3-3.3) | 2.6 (2.1-2.9) | 0.545 | 0.326-0.910 | 0.020 |
| PAB, mg/L, median (IQR) | 110.0 (89.0-160.0) | 124.0 (98.0-180.0) | 0.998 | 0.993-1.003 | 0.419 |
| ALB, g/L, median (IQR) | 35.2 (33.2-37.5) | 33.8 (32.5-32.7) | 1.167 | 1.047-1.300 | 0.005 |
| CRP, mg/L, median (IQR) | 77.6 (65.3-89.8) | 69.3 (63.3-80.0) | 1.007 | 1.996-1.019 | 0.205 |
| IL-6, pg/mL, median (IQR) | 63.2 (42.3-78.3) | 58.3 (36.1-66.9) | 1.016 | 1.002-1.030 | 0.022 |
| BUN, mmol/L, median (IQR) | 11.8 (10.5-12.8) | 9.1 (7.8-10.2) | 2.548 | 1.903-3.411 | <0.001 |
| Cr, μmol/L, median (IQR) | 124.0 (105.0-198.0) | 99.0 (87.0-111.0) | 1.016 | 1.009-1.023 | <0.001 |
| EEN, n (%) | 32 (42.7) | 64 (61.9) | 0.477 | 0.261-0.871 | 0.016 |
ICU-AW: intensive care unit-acquired weakness; OR: odds ratio; BMI: body mass index; MV: mechanical ventilation; TRF: transferrin; PAB: prealbumin; ALB: albumin; CRP: C-reactive protein; IL-6: interleukin-6; BUN: blood urea nitrogen; Cr: creatinine; EEN: early enteral nutrition.
A multivariate logistic regression analysis was conducted, including factors such as gender, age, and BMI, and the results are shown in Table 2. Older age was associated with a higher risk of ICU-AW (OR 1.132, 95% confidence interval [95% CI] 1.058-1.326, P<0.001). Compared with women, men had a lower risk of ICU-AW (OR 0.210, 95% CI 0.064-0.994, P=0.010). Patients receiving EEN had a lower risk of ICU-AW (OR 0.280, 95% CI 0.089-0.877, P=0.029). MV increased the risk of ICU-AW (OR 2.212, 95% CI 1.546-4.065, P=0.032). A higher BMI was associated with a lower risk of ICU-AW (OR 0.613, 95% CI 0.451-0.831, P=0.002). Higher levels of BUN and Cr were also linked to a higher risk of ICU-AW (BUN: OR 2.183, 95% CI 1.518-3.139, P<0.001; Cr: OR 1.347, 95% CI 1.197-1.986, P<0.001).
Table 2. The multivariable logistic regression analysis of risk factors for ICU-AW
| Parameters | ICU-AW (n=75) | Non-ICU-AW (n =105) | OR | 95% CI | P-value |
|---|---|---|---|---|---|
| Male, n | 45/75 | 68/105 | 0.210 | 0.064-0.994 | 0.010 |
| Age, years, median (IQR) | 74.0 (67.0-84.0) | 73.0 (65.5-82.0) | 1.132 | 1.058-1.326 | <0.001 |
| EEN, n | 32/75 | 64/105 | 0.280 | 0.889-0.887 | 0.029 |
| MV, n | 18/75 | 13/105 | 2.212 | 1.546-4.065 | 0.032 |
| BMI, kg/m2, median (IQR) | 19.6 (17.8-21.6) | 21.0 (19.4-20.8) | 0.613 | 0.451-0.831 | 0.002 |
| IL-6, pg/mL, median (IQR) | 63.2 (42.3-78.3) | 58.3 (36.1-66.9) | 1.005 | 0.981-1.560 | 0.687 |
| TRF, g/L, median (IQR) | 2.5 (2.3-3.3) | 2.6 (2.1-2.9) | 0.327 | 0.126-1.099 | 0.741 |
| Cr, μmol/L, median (IQR) | 124.0 (105.0-198.0) | 99.0 (87.0-111.0) | 1.347 | 1.197-1.986 | <0.001 |
| BUN, mmol/L, median (IQR) | 11.8 (10.5-12.8) | 9.1 (7.8-10.2) | 2.183 | 1.518-3.139 | <0.001 |
| ALB, g/L, median (IQR) | 35.2 (33.2-37.5) | 33.8 (32.5-32.7) | 1.179 | 0.930-1.495 | 0.174 |
ICU-AW: intensive care unit-acquired weakness; EEN: early enteral nutrition; MV: mechanical ventilation; BMI: body mass index; IL-6: interleukin-6; TRF: transferrin; Cr: creatinine; BUN: blood urea nitrogen; ALB: albumin; IQR: interquartile range; 95% CI: 95% confidence interval.
Comparison of the incidence of ICU-AW under different nutritional interventions
Among the 180 included patients, 96 and 84 patients were in the EEN and PN groups, respectively. Table 3 shows that baseline characteristics were similar between the two cohorts before nutritional therapy initiation (P>0.05).
Table 3. Baseline characteristics of patients receiving different nutritional treatment approaches
| Parameters | EEN (n=96) | PN (n=84) | P-value |
|---|---|---|---|
| Male, n (%) | 63 (65.63) | 50 (59.52) | 0.398 |
| Age, years, median (IQR) | 74.0 (67.0-82.8) | 72.5 (65.3-81.8) | 0.497 |
| BMI, kg/m2, median (IQR) | 21.0 (19.4-20.8) | 20.6 (19.2-22.1) | 0.167 |
| Underlying disease, n (%) | |||
| Hypertension | 42 (43.8) | 44 (52.4) | 0.247 |
| Diabetes | 28 (29.2) | 22 (26.2) | 0.657 |
| Malignant tumor | 17 (17.7) | 11 (13.1) | 0.394 |
| MV, n (%) | 16 (16.7) | 15 (17.9) | 0.833 |
| TRF, g/L, median (IQR) | 2.6 (2.2-3.1) | 2.6 (2.1-3.2) | 0.447 |
| PAB, mg/L, median (IQR) | 137.9 (97.3-169.5) | 144.4 (96.5-180.0) | 0.673 |
| ALB, g/L, median (IQR) | 34.5 (32.5-36.5) | 34.7 (32.5-36.5) | 0.937 |
| CRP, mg/L, median (IQR) | 73.6 (65.1-86.5) | 75.9 (62.9-89.7) | 0.654 |
| IL-6, pg/mL, median (IQR) | 56.0 (42.1-68.3) | 58.6 (35.7-76.9) | 0.500 |
| BUN, mmol/L, median (IQR) | 10.0 (8.6-10.9) | 10.5 (8.9-12.3) | 0.210 |
| Cr, μmol/L, median (IQR) | 129.8 (91.3-143.3) | 120.6 (95.3-124.0) | 0.946 |
EEN: early enteral nutrition; PN: parenteral nutrition; BMI: body mass index; MV: mechanical ventilation; TRF: transferrin; PAB: prealbumin; ALB: albumin; CRP: C-reactive protein; IL-6: interleukin-6; BUN: blood urea nitrogen; Cr: creatinine.
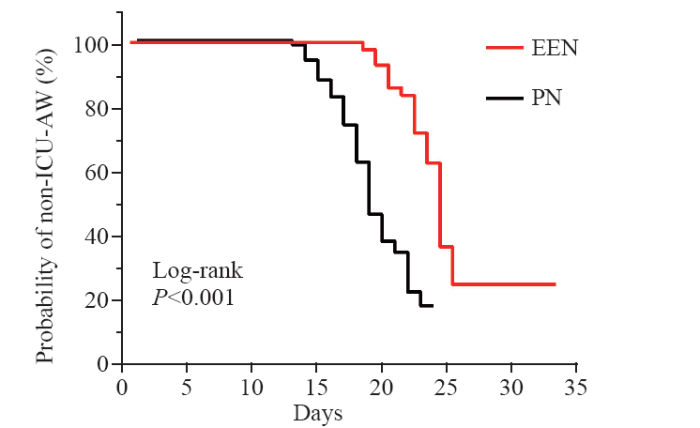
As shown in Figure 1, the median time of ICU-AW diagnosis for patients receiving EEN was 24 d, whereas it was 18 d for those receiving PN, with a significant difference observed (HR 0.324, log-rank P<0.001).
Figure 1.
Figure 1.
Kaplan-Meier curve for ICU-AW occurrence under different nutritional treatment approaches. EEN: early enteral nutrition; PN: parenteral nutrition; HR: hazard ratio; ICU-AW: intensive care unit-acquired weakness.
Diagnostic efficacy of biomarkers in ICU-AW patients
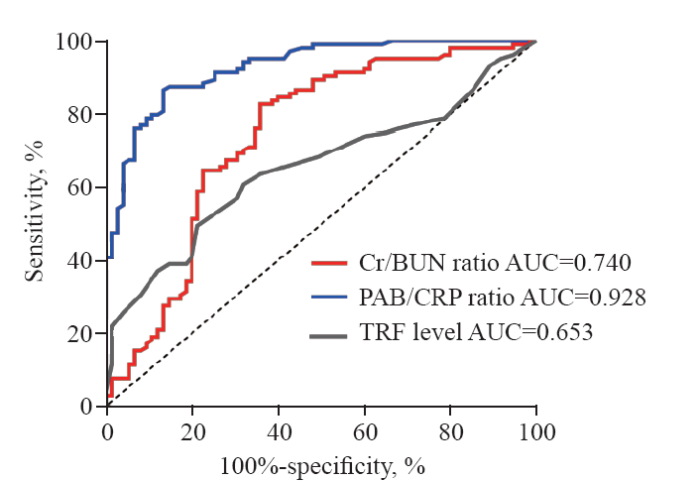
For ICU-AW patients, we calculated the PAB/CRP, Cr/BUN, and TRF levels throughout the course of their illness. For non-ICU-AW patients, we recorded these biomarkers over the duration of their hospital stay. Receiver operating characteristic (ROC) curves (Figure 2) were used to assess the diagnostic utility of these markers for ICU-AW. Among the markers, the PAB/CRP ratio demonstrated the highest accuracy (0.928, 95% CI 0.892-0.946, P<0.001), outperforming both the Cr/BUN ratio (0.740, 95% CI 0.663-0.819, P<0.001) and TRF levels (0.653, 95% CI 0.574-0.733, P<0.001). The PAB/CRP ratio also exhibited optimal sensitivity (86.7%) and specificity (86.7%), with a cutoff value of 1.508.
Figure 2.
Figure 2.
Assessment of the predictive value of various biochemical indicators for ICU-AW. ICU-AW: intensive care unit-acquired weakness; PAB/CRP: prealbumin/C-reactive protein ratio; Cr/BUN: creatinine/blood urea nitrogen ratio; TRF: transferrin.
DISCUSSION
Since the initial report of ICU-AW, awareness has increased alongside its recognition as irreversible and associated with poor prognosis.[11⇓-13] Research indicates that ICU-AW has a multifactorial etiology involving risk factors. Modifiable risks include hyperglycemia, PN, and certain medications (e.g., blood pressure medications, steroids, muscle relaxants, and sedatives), whereas non-modifiable risks include disease severity, sepsis, inflammation, organ failure, elevated lactate levels, female, and muscle atrophy.[13⇓-15] The findings of this study support previous research identifying female sex, advanced age, PN, MV, low BMI, and elevated Cr and BUN levels as independent risk factors for ICU-AW.[16,17] Studies have shown that inflammation plays a significant role in the development of ICU-AW.[18,19] Our analysis identified IL-6 as a risk factor for ICU-AW, although multivariable logistic regression analysis did not identify it as an independent risk factor.[20,21] This highlights the complex etiology of ICU-AW, indicating the need to consider multiple factors when assessing risk. These findings also underscore the importance of further understanding inflammatory markers such as IL-6 in the management of ICU-AW.
Although the influence of nutritional therapy on ICU-AW development was previously unclear,[3] increasing evidence suggests that nutritional interventions might reduce its incidence.[22⇓-24] One RCT has indicated that PN may precipitate muscle atrophy more than EEN does, potentially accelerating ICU-AW onset.[25] Despite these insights, the lack of comprehensive nutritional assessments and interventions early in ICU admission has limited our understanding of the influence of EEN on ICU-AW.[12]
Research has suggested that between 40% and 75% of patients with severe infections may develop ICU-AW. In our study, approximately 45% of patients developed ICU-AW. Notably, patients receiving EEN had a lower incidence of ICU-AW (33.3%), whereas those receiving PN had a higher incidence (51.2%), which aligns with findings from earlier studies and confirms the high occurrence of ICU-AW in this patient population.[15,18,26] These results highlight the potential protective effect of EEN in curbing ICU-AW onset while validating the increased risk associated with PN approaches.
As a protein produced by liver cells, TRF serves dual roles as an indicator of nutritional status and degree of inflammation within the body. Research has established the involvement of iron metabolism in skeletal muscle synthesis.[27⇓-29] However, the findings from the present study revealed that TRF is not an independent ICU-AW risk factor. This suggests a complex interplay of various factors in the pathogenesis of ICU-AW, corroborating findings from prior studies.[30] Moreover, the ROC curve analysis demonstrated that TRF has limited predictive power for ICU-AW.
Nutritional status and inflammation significantly contribute to the development of ICU-AW, with the nutritional state influenced by the body’s inflammatory response. BUN, a byproduct of protein metabolism, is affected by both nutritional status and the severity of inflammation. Similarly, Cr, a product of muscle metabolism, is influenced by renal function and the extent of muscle catabolism. Studies have identified the Cr/BUN ratio as an indicator of skeletal muscle status, with lower ratios observed in patients with ICU-AW than in those without ICU-AW.[31,32] The Cr/BUN ratio was found to be a moderate predictor of ICU-AW. However, the Cr/BUN ratio has limitations, as Cr levels can be influenced by kidney function, which is often impaired in patients with severe infections. PAB is an albumin precursor with a half-life of 2-4 d, whereas albumin has a half-life of 20-22 d. Measuring PAB levels provides information about short-term impairments in energy intake and the effectiveness of nutritional support. The PAB/CRP ratio has been widely studied as a prognostic indicator, with a low ratio associated with poor outcomes in critically ill patients.[33⇓-35] However, there is limited research on the use of the PAB/CRP ratio to predict ICU-AW specifically. Our study revealed that the PAB/CRP ratio may be a predictor of ICU-AW, with a threshold of 1.508. These findings suggest that the PAB/CRP ratio could serve as an early marker for ICU-AW in critically ill patients, warranting further research to explore its potential in identifying patients at risk for ICU-AW.
Limitations
This study had several limitations. Although this study provides valuable insights, it is not without limitations. This study did not investigate the potential impact of APACHE II scores, medications, or rehabilitation therapies on ICU-AW, nor did it fully explore the effects of nutritional therapy in severely ill patients. Future research should incorporate a broader range of treatments and patient populations to better understand the risk factors influencing ICU-AW.
CONCLUSION
Female sex, advanced age, PN, MV, low BMI, and elevated Cr and BUN levels have been identified as independent risk factors for ICU-AW onset. EEN may delay ICU-AW development in critically ill patients. Additionally, the PAB/CRP ratio may have predictive value in the diagnosis of ICU-AW.
Funding: The authors received no funding for this study.
Ethical approval: This study was approved by the Ethics Committee of the First Affiliated Hospital of Xiamen University Hospital.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Contributors: QLZ proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
Reference
ICU-acquired weakness
DOI:S0012-3692(16)47575-6
PMID:27063347
[Cited within: 1]

Survivorship after critical illness is an increasingly important health-care concern as ICU use continues to increase while ICU mortality is decreasing. Survivors of critical illness experience marked disability and impairments in physical and cognitive function that persist for years after their initial ICU stay. Newfound impairment is associated with increased health-care costs and use, reductions in health-related quality of life, and prolonged unemployment. Weakness, critical illness neuropathy and/or myopathy, and muscle atrophy are common in patients who are critically ill, with up to 80% of patients admitted to the ICU developing some form of neuromuscular dysfunction. ICU-acquired weakness (ICUAW) is associated with longer durations of mechanical ventilation and hospitalization, along with greater functional impairment for survivors. Although there is increasing recognition of ICUAW as a clinical entity, significant knowledge gaps exist concerning identifying patients at high risk for its development and understanding its role in long-term outcomes after critical illness. This review addresses the epidemiologic and pathophysiologic aspects of ICUAW; highlights the diagnostic challenges associated with its diagnosis in patients who are critically ill; and proposes, to our knowledge, a novel strategy for identifying ICUAW.Copyright © 2016 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
ICU-acquired weakness and recovery from critical illness
Intensive care unit-acquired weakness: unanswered questions and targets for future research
Respective contribution of intensive care unit-acquired limb muscle and severe diaphragm weakness on weaning outcome and mortality: a post hoc analysis of two cohorts
ICU-acquired weakness
DOI:10.1007/s00134-020-05944-4
PMID:32076765
[Cited within: 1]

Critically ill patients often acquire neuropathy and/or myopathy labeled ICU-acquired weakness. The current insights into incidence, pathophysiology, diagnostic tools, risk factors, short- and long-term consequences and management of ICU-acquired weakness are narratively reviewed. PubMed was searched for combinations of "neuropathy", "myopathy", "neuromyopathy", or "weakness" with "critical illness", "critically ill", "ICU", "PICU", "sepsis" or "burn". ICU-acquired weakness affects limb and respiratory muscles with a widely varying prevalence depending on the study population. Pathophysiology remains incompletely understood but comprises complex structural/functional alterations within myofibers and neurons. Clinical and electrophysiological tools are used for diagnosis, each with advantages and limitations. Risk factors include age, weight, comorbidities, illness severity, organ failure, exposure to drugs negatively affecting myofibers and neurons, immobility and other intensive care-related factors. ICU-acquired weakness increases risk of in-ICU, in-hospital and long-term mortality, duration of mechanical ventilation and of hospitalization and augments healthcare-related costs, increases likelihood of prolonged care in rehabilitation centers and reduces physical function and quality of life in the long term. RCTs have shown preventive impact of avoiding hyperglycemia, of omitting early parenteral nutrition use and of minimizing sedation. Results of studies investigating the impact of early mobilization, neuromuscular electrical stimulation and of pharmacological interventions were inconsistent, with recent systematic reviews/meta-analyses revealing no or only low-quality evidence for benefit. ICU-acquired weakness predisposes to adverse short- and long-term outcomes. Only a few preventive, but no therapeutic, strategies exist. Further mechanistic research is needed to identify new targets for interventions to be tested in adequately powered RCTs.
Rapidly progressive brain atrophy in ventilated patients: a retrospective descriptive study
Effects of early standardized enteral nutrition on preventing acute muscle loss in the acute exacerbation of chronic obstructive pulmonary disease patients with mechanical ventilation
DOI:10.5847/wjem.j.1920-8642.2023.046
PMID:37152533
[Cited within: 1]

To investigate the effects of early standardized enteral nutrition (EN) on the cross-sectional area of erector spine muscle (ESMcsa), plasma growth differentiation factor-15 (GDF-15), and 28-day mortality of acute exacerbation of chronic obstructive pulmonary disease (AECOPD) patients with invasive mechanical ventilation (MV).A total of 97 AECOPD patients with invasive MV were screened in the ICUs of the First People's Hospital of Lianyungang. The conventional EN group (stage I) and early standardized EN group (stage II) included 46 and 51 patients, respectively. ESMcsa loss and GDF-15 levels on days 1 and 7 of ICU admission and 28-day survival rates were analyzed.On day 7, the ESMcsa of the early standardized EN group was significantly higher than that of the conventional EN group, while the plasma GDF-15 levels were significantly lower than those in the conventional EN group (ESMcsa: 28.426±6.130 cm vs. 25.205±6.127 cm; GDF-15: 1661.608±558.820 pg/mL vs. 2541.000±634.845 pg/mL; all <0.001]. The 28-day survival rates of the patients in the early standardized EN group and conventional EN group were 80.40% and 73.90%, respectively (=0.406).ESMcsa loss in AECOPD patients with MV was correlated with GDF-15 levels, both of which indicated acute muscular atrophy and skeletal muscle dysfunction. Early standardized EN may prevent acute muscle loss and intensive care unit-acquired weakness (ICU-AW) in AECOPD patients.Copyright: © World Journal of Emergency Medicine.
Machine learning predictive model for aspiration risk in early enteral nutrition patients with severe acute pancreatitis
A cross-sectional study of the impact of ICU-acquired weakness: prevalence, associations, and severity
Coagulopathy correlates with muscle titin injury in critically ill patients
Lesions of peripheral nerves developing during coma
DOI:10.1001/jama.1956.02960360041008 PMID:13271159 [Cited within: 1]
Effect of early mobilization combined with early nutrition on acquired weakness in critically ill patients (EMAS): a dual-center, randomized controlled trial
Intensive care unit-acquired weakness: a review of recent progress with a look toward the future
Intensive care unit-acquired weakness: questions the clinician should ask
Risk factors for ICU-acquired weakness in sepsis patients: a retrospective study of 264 patients
Intensive care unit-acquired weakness and mechanical ventilation: a reciprocal relationship
DOI:10.12998/wjcc.v12.i18.3644
PMID:38983411
[Cited within: 1]

Intensive care unit-acquired weakness (ICU-AW; ICD-10 Code: G72.81) is a syndrome of generalized weakness described as clinically detectable weakness in critically ill patients with no other credible cause. The risk factors for ICU-AW include hyperglycemia, parenteral nutrition, vasoactive drugs, neuromuscular blocking agents, corticosteroids, sedatives, some antibiotics, immobilization, the disease severity, septicemia and systemic inflammatory response syndrome, multiorgan failure, prolonged mechanical ventilation (MV), high lactate levels, older age, female sex, and pre-existing systemic morbidities. There is a definite association between the duration of ICU stay and MV with ICU-AW. However, the interpretation that these are modifiable risk factors influencing ICU-AW, appears to be flawed, because the relationship between longer ICU stays and MV with ICU-AW is reciprocal and cannot yield clinically meaningful strategies for the prevention of ICU-AW. Prevention strategies must be based on other risk factors. Large multicentric randomized controlled trials as well as meta-analysis of such studies can be a more useful approach towards determining the influence of these risk factors on the occurrence of ICU-AW in different populations.©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
A systematic review and meta-analysis of risk factors for intensive care unit acquired weakness
Pathophysiology of skeletal muscle during sepsis
DOI:10.1254/fpj.23040
PMID:38432919
[Cited within: 2]

While sepsis mortality is reducing in developed countries due to advances in intensive care medicine, morbidity is increasing due to aging and obesity. ICU-acquired weakness (ICU-AW) is a respiratory and limb muscle weakness experienced by many sepsis survivors and is present in 50-75% of sepsis patients. ICU-AW can persist for several years, making reintegration of sepsis survivors difficult and leading to a secondary decrease in long-term survival. Exposure of septic patients to multiple muscle-damaging factors during ICU admission, including hyperglycemia, immobility, mechanical ventilation, administration of muscle relaxants, and administration of steroidal anti-inflammatory drugs, may compound the hyper cytokine, hyper nitric oxide, and hyper oxidative conditions, leading to the development of ICU-AW. However, the pathogenesis of ICU-AW remains unclear, and the pathophysiology of ICU-AW awaits further elucidation to develop therapeutic strategies. Recent ICU-AW studies have also revealed that skeletal muscle itself is a key organ in the inflammatory response and metabolic abnormalities in sepsis. In this article, we review the pathophysiology of skeletal muscle in sepsis and international trends in the development of therapeutic agents based on our research results.
Intensive care unit-acquired weakness: Recent insights
Prediction of sepsis within 24 hours at the triage stage in emergency departments using machine learning
DOI:10.5847/wjem.j.1920-8642.2024.074
PMID:39290601
[Cited within: 1]

Sepsis is one of the main causes of mortality in intensive care units (ICUs). Early prediction is critical for reducing injury. As approximately 36% of sepsis occur within 24 h after emergency department (ED) admission in Medical Information Mart for Intensive Care (MIMIC-IV), a prediction system for the ED triage stage would be helpful. Previous methods such as the quick Sequential Organ Failure Assessment (qSOFA) are more suitable for screening than for prediction in the ED, and we aimed to find a light-weight, convenient prediction method through machine learning.We accessed the MIMIC-IV for sepsis patient data in the EDs. Our dataset comprised demographic information, vital signs, and synthetic features. Extreme Gradient Boosting (XGBoost) was used to predict the risk of developing sepsis within 24 h after ED admission. Additionally, SHapley Additive exPlanations (SHAP) was employed to provide a comprehensive interpretation of the model's results. Ten percent of the patients were randomly selected as the testing set, while the remaining patients were used for training with 10-fold cross-validation.For 10-fold cross-validation on 14,957 samples, we reached an accuracy of 84.1%±0.3% and an area under the receiver operating characteristic (ROC) curve of 0.92±0.02. The model achieved similar performance on the testing set of 1,662 patients. SHAP values showed that the five most important features were acuity, arrival transportation, age, shock index, and respiratory rate.Machine learning models such as XGBoost may be used for sepsis prediction using only a small amount of data conveniently collected in the ED triage stage. This may help reduce workload in the ED and warn medical workers against the risk of sepsis in advance.Copyright: © World Journal of Emergency Medicine.
Exploring the impact of interleukins on sarcopenia development: a systematic review and meta-analysis
Relationship between critical care nutrition and post-intensive care syndrome in surviving ventilated patients with COVID-19: a multicenter prospective observational study
DOI:10.3164/jcbn.23-66
PMID:38292118
[Cited within: 1]

The impact of nutrition therapy in the acute phase on post-intensive care syndrome (PICS) remains unclear. We conducted a multicenter prospective study on adult patients with COVID-19 who required mechanical ventilation for more than three days. The questionnaire was mailed after discharge. Physical PICS, defined as less than 90 points on the Barthel index (BI), was assigned as the primary outcome. We examined the types of nutrition therapy in the first week that affected PICS components. 269 eligible patients were evaluated 10 months after discharge. Supplemental parenteral nutrition (SPN) >400 kcal/day correlated with a lower occurrence of physical PICS (10% vs 21.92%, = 0.042), whereas the amounts of energy and protein provided, early enteral nutrition, and a gradual increase in nutrition delivery did not, and none correlated with cognitive or mental PICS. A multivariable regression analysis revealed that SPN had an independent impact on physical PICS (odds ratio 0.33, 95% CI 0.12-0.92, = 0.034), even after adjustments for age, sex, body mass index and severity. Protein provision ≥1.2 g/kg/day was associated with a lower occurrence of physical PICS (odds ratio 0.42, 95% CI 0.16-1.08, = 0.071). In conclusion, SPN in the acute phase had a positive impact on physical PICS for ventilated patients with COVID-19.Copyright © 2024 JCBN.
Achievement of adequate nutrition contributes to maintaining the skeletal muscle area in patients with sepsis undergoing early mobilization: a retrospective observational study
Effect of intermittent or continuous feed on muscle wasting in critical illness: a phase 2 clinical trial
DOI:S0012-3692(20)30584-5
PMID:32247714
[Cited within: 1]

Acute skeletal muscle wasting in critical illness is associated with excess morbidity and mortality. Continuous feeding may suppress muscle protein synthesis as a result of the muscle-full effect, unlike intermittent feeding, which may ameliorate it.Does intermittent enteral feed decrease muscle wasting compared with continuous feed in critically ill patients?In a phase 2 interventional single-blinded randomized controlled trial, 121 mechanically ventilated adult patients with multiorgan failure were recruited following prospective informed consultee assent. They were randomized to the intervention group (intermittent enteral feeding from six 4-hourly feeds per 24 h, n = 62) or control group (standard continuous enteral feeding, n = 59). The primary outcome was 10-day loss of rectus femoris muscle cross-sectional area determined by ultrasound. Secondary outcomes included nutritional target achievements, plasma amino acid concentrations, glycemic control, and physical function milestones.Muscle loss was similar between arms (-1.1% [95% CI, -6.1% to -4.0%]; P =.676). More intermittently fed patients received 80% or more of target protein (OR, 1.52 [1.16-1.99]; P <.001) and energy (OR, 1.59 [1.21-2.08]; P =.001). Plasma branched-chain amino acid concentrations before and after feeds were similar between arms on trial day 1 (71 μM [44-98 μM]; P =.547) and trial day 10 (239 μM [33-444 μM]; P =.178). During the 10-day intervention period the coefficient of variation for glucose concentrations was higher with intermittent feed (17.84 [18.6-20.4]) vs continuous feed (12.98 [14.0-15.7]; P <.001). However, days with reported hypoglycemia and insulin usage were similar in both groups. Safety profiles, gastric intolerance, physical function milestones, and discharge destinations did not differ between groups.Intermittent feeding in early critical illness is not shown to preserve muscle mass in this trial despite resulting in a greater achievement of nutritional targets than continuous feeding. However, it is feasible and safe.ClinicalTrials.gov; No.: NCT02358512; URL: www.clinicaltrials.gov.Copyright © 2020 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Effects of early enteral nutrition on immune function and prognosis of patients with sepsis on mechanical ventilation
DOI:10.1177/0885066618809893
PMID:30384813
[Cited within: 1]

To explore the therapeutic effects of early enteral nutrition (EEN) on patients with sepsis on mechanical ventilation.Patients with sepsis on mechanical ventilation in the medical intensive care unit (ICU) from January 2013 to March 2016 were treated with enteral nutrition. Patients treated within 48 hours of initiation of mechanical ventilation were assigned to the EEN group, and the rest were assigned to the delayed enteral nutrition (DEN) group. Peripheral blood Th17 cells and Treg cells, endotoxin (ET) level, 28-day mortality, duration of mechanical ventilation, lengths of ICU stay and hospital stay, and incidence of ICU-acquired weakness (ICU-AW) were analyzed between the 2 groups.The proportion of Th17 cells and ET levels in the EEN group were significantly lower than those in the DEN group, whereas the proportion of Treg cells in the EEN group was remarkably higher than that in the DEN group (<.05). The duration of mechanical ventilation, lengths of ICU stay and hospital stay, and incidence of ICU-AW were higher in the DEN group than in the EEN group (<.05), but there was no significant difference in the 28-day mortality between the 2 groups.Patients with sepsis mainly present with an increased proportion of Th17 cells in the early stage, manifesting as enhanced immune response. Early enteral nutrition can inhibit the excessive immune response, shorten the duration of mechanical ventilation, lengths of ICU stay and hospital stay, and reduce the incidence of ICU-AW, but it has no obvious effect on 28-day mortality.
An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit-acquired weakness in adults
Transferrin receptor 1 plays an important role in muscle development and denervation-induced muscular atrophy
DOI:10.4103/1673-5374.301024
PMID:33318410
[Cited within: 1]

Previous studies demonstrate an accumulation of transferrin and transferrin receptor 1 (TfR1) in regenerating peripheral nerves. However, the expression and function of transferrin and TfR1 in the denervated skeletal muscle remain poorly understood. In this study, a mouse model of denervation was produced by complete tear of the left brachial plexus nerve. RNA-sequencing revealed that transferrin expression in the denervated skeletal muscle was upregulated, while TfR1 expression was downregulated. We also investigated the function of TfR1 during development and in adult skeletal muscles in mice with inducible deletion or loss of TfR1. The ablation of TfR1 in skeletal muscle in early development caused severe muscular atrophy and early death. In comparison, deletion of TfR1 in adult skeletal muscles did not affect survival or glucose metabolism, but caused skeletal muscle atrophy and motor functional impairment, similar to the muscular atrophy phenotype observed after denervation. These findings suggest that TfR1 plays an important role in muscle development and denervation-induced muscular atrophy. This study was approved by the Institutional Animal Care and Use Committee of Beijing Institute of Basic Medical Sciences, China (approval No. SYXK 2017-C023) on June 1, 2018.
The combination of lactoferrin and creatine ameliorates muscle decay in a sarcopenia murine model
Iron limitation promotes the atrophy of skeletal myocytes, whereas iron supplementation prevents this process in the hypoxic conditions
DOI:10.3892/ijmm.2018.3481
PMID:29436580
[Cited within: 1]

There is clinical evidence that patients with heart failure and concomitant iron deficiency have increased skeletal muscle fatigability and impaired exercise tolerance. It was expected that a skeletal muscle cell line subjected to different degrees of iron availability and/or concomitant hypoxia would demonstrate changes in cell morphology and in the expression of atrophy markers. L6G8C5 rat skeletal myocytes were cultured in normoxia or hypoxia at optimal, reduced or increased iron concentrations. Experiments were performed to evaluate the iron content in cells, cell morphology, and the expression of muscle specific atrophy markers [Atrogin1 and muscle‑specific RING‑finger 1 (MuRF1)], a gene associated with the atrophy/hypertrophy balance [mothers against decapentaplegic homolog 4 (SMAD4)] and a muscle class‑III intermediate filament protein (Desmin) at the mRNA and protein level. Hypoxic treatment caused, as compared to normoxic conditions, an increase in the expression of Atrogin‑1 (P<0.001). Iron‑deficient cells exhibited morphological abnormalities and demonstrated a significant increase in the expression of Atrogin‑1 (P<0.05) and MuRF1 (P<0.05) both in normoxia and hypoxia, which indicated activation of the ubiquitin proteasome pathway associated with protein degradation during muscle atrophy. Depleted iron in cell culture combined with hypoxia also induced a decrease in SMAD4 expression (P<0.001) suggesting modifications leading to atrophy. In contrast, cells cultured in a medium enriched with iron during hypoxia exhibited inverse changes in the expression of atrophy markers (both P<0.05). Desmin was upregulated in cells subjected to both iron depletion and iron excess in normoxia and hypoxia (all P<0.05), but the greatest augmentation of mRNA expression occurred when iron depletion was combined with hypoxia. Notably, in hypoxia, an increased expression of Atrogin‑1 and MuRF1 was associated with an increased expression of transferrin receptor 1, reflecting intracellular iron demand (R=0.76, P<0.01; R=0.86, P<0.01). Hypoxia and iron deficiency when combined exhibited the most detrimental impact on skeletal myocytes, especially in the context of muscle atrophy markers. Conversely, iron supplementation in in vitro conditions acted in a protective manner on these cells.
Serum iron level is independently associated with sarcopenia: a retrospective study
DOI:10.1038/s41598-024-61429-0
PMID:38719903
[Cited within: 1]

Sarcopenia greatly reduces the quality of life of the elderly, and iron metabolism plays an important role in muscle loss. This study aimed to investigate the association between iron status and sarcopenia. A total of 286 adult patients hospitalized between 2019 and 2021 were included in this study, of which 117 were diagnosed with sarcopenia. Serum iron, total iron binding capacity (TIBC), transferrin, and transferrin saturation levels were compared between groups with and without sarcopenia and were included in the logistic analyses, with significant variables further included in the logistic regression model for the prediction of sarcopenia. Serum iron, TIBC, and transferrin levels decreased significantly in the sarcopenia group (p < 0.05), and were negatively associated with handgrip strength, relative skeletal muscle index, and multiple test performances (p < 0.05). Multivariate logistic analysis showed that sex, age, body mass index (BMI), and serum iron level were independent risk factors for sarcopenia. In the final logistic regression model, male sex (odds ratio [OR] 3.65, 95% confidence interval [CI] 1.67-7.98), age > 65 years (OR 5.40, 95% CI 2.25-12.95), BMI < 24 kg/m (OR 0.17, 95% CI 0.08-0.36), and serum iron < 10.95 μmol/L (OR 0.39, 95% CI 0.16-0.93) were included. Our study supported the impact of iron metabolism on muscle strength and performance.© 2024. The Author(s).
Dynamic changes of diaphragm and limb skeletal muscle in patients with sepsis assessed by bedside ultrasound and their correlation with blood urea/creatinine ratio
Association of calf circumference with clinical and biochemical markers in older adults with COVID-19 admitted at intensive care unit: a retrospective cross-sectional study
Diabetes-related cardiovascular and all-cause mortality in asian american subgroups
Clinical characteristics and prognosis of Klebsiella pneumoniae meningitis in adults
The clinical characteristics of non-cystic fibrosis bronchiectasis patients with positive serum tumor markers: a retrospective study
DOI:10.1186/s12890-023-02816-7
PMID:38191360
[Cited within: 1]

Serum tumor markers (STM), extensively used for the diagnosis, monitoring and prognostic assessment of tumors, can be increased in some non-malignant lung diseases. To date, there is a paucity of studies regarding the clinical characteristics of non-cystic fibrosis bronchiectasis patients with positive STMs.To investigate the clinical characteristics and indicators of bronchiectasis with positive STMs.The clinical data of 377 bronchiectasis patients was retrospectively collected from January 2017 to December 2019 from Beijing Chaoyang Hospital. Patients were divided into the STM negative group, the single STM positive group and the ≥2 STMs positive group according to the number of the positive STMs. The clinical characteristics are described and compared separately. The multivariate logistic regression analysis model was used to investigate the indicators regarding positive STMs.Patients in the ≥2 STMs positive group were older (P = 0.015), had higher mMRC scores (P < 0.001) and developed higher fever (P = 0.027). Additionally, these patients also had lower Albumin/Globulin Ratio (A/G), albumin (ALB), prealbumin (PAB) (P < 0.001, P < 0.001, P < 0.001, respectively) and higher CRP, ESR and Fbg (P < 0.001, P < 0.001 and P < 0.001, respectively). Age (OR 1.022, 95%CI 1.003-1.042; P = 0.026) and the number of affected lobes (OR 1.443, 95%CI 1.233-1.690; P < 0.001) were independently associated with one and ≥ 2 positive STMs in bronchiectasis patients.The ≥2 positive STMs are associated with a higher inflammation status and severer radiologic manifestations in bronchiectasis patients.© 2024. The Author(s).