INTRODUCTION
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection.[1] With further research on the pathophysiological mechanism of sepsis, noticeable attention has been given to the role of microcirculation disorders.[2] Patients with sepsis may have some microcirculatory changes, such as decreased small vessel density, an increased number of poorly perfused vessels, and increased heterogeneity between vessels.[3] The mechanisms of microcirculation disorders include endothelial dysfunction, recruitment and adhesion of leukocytes, formation of microthrombi, hypoperfusion of microcirculation, and redistribution of blood flow in the intestinal wall.[4]
The vascular endothelial growth factor (VEGF) family proteins include VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and placental growth factor. The protective effects of VEGF-A on tissues are organ- and environment-dependent, and these biological properties need to be validated in different organs.[5] The increase in VEGF-A expression could be related to the repair of the alveolar-capillary membrane.[6] At present, studies on the expression and function of VEGF-A in the intestines of septic animals are rare. VEGF-A can activate the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway in endothelial cells (ECs),[7] which promotes the phosphorylation of PI3K/Akt. The PI3K/Akt signaling pathway is involved in a variety of cell and organ functions, including growth, cell survival, glucose metabolism, and protein synthesis. In ECs, the PI3K/Akt signaling pathway plays a key role in cell migration and angiogenesis.[8]
Xuebijing (XBJ) is a compound formulation composed of various herbs known for its blood-activating and stasis-removing properties, including Paeonia lactiflora Pall (Chinese: Chishao), Carthamus tinctorius L. (Chinese: Honghua), and Salvia miltiorrhiza Bge. (Chinese: Danshen), Angelica sinensis (Chinese: Danggui), and Ligusticum chuanxiong Hort. (Chinese: Chuanxiong).[9] XBJ injection has been proven effective in the treatment of sepsis,[10] and it may improve intestinal microcirculation dysfunction by antagonizing endotoxin.[11] This study aims to explore whether XBJ can improve intestinal microcirculation dysfunction in sepsis and its mechanism.
METHODS
Reagents and antibodies
XBJ (batch number: 2107201) was obtained from Chase Sun Pharmaceutical Co., Ltd., China, which has been approved by the China Food and Drug Administration (CFDA) for the treatment of sepsis (ratification number, GuoYaoZhunZi - Z20039833).
Axitinib (AG 013736) (S1005; Selleck, Inc., USA) is a potent inhibitor of VEGF receptor tyrosine kinases 1, 2, and 3. Axitinib was dissolved in normal saline containing 3% dimethyl sulfoxide (DMSO), and the final concentration of axitinib was 2.5 mg/mL. Isoflurane was obtained from EZVET Co., Ltd., China. In addition, 3,4-dihydroxybenzaldehyde, hydroxysafflor yellow A, paeoniflorin, ferulic acid, senkyunolide I, and salvianolic acid B were purchased from Selleck, Inc., USA (Cat. Nos. S3952, S9061, S2410, S2300, S3275, and S4735).
An animal tissue/cell total protein extraction kit (Column Method) (BC3790; Solarbio Co., Ltd., China), a bicinchoninic acid (BCA) protein assay kit (23225; Thermo Fisher Scientific, USA), PI3K p85 (19H8) rabbit mAb antibody (4257; Cell Signaling Technology, USA), anti-PI3K p85 alpha (phospho Y607) (ab182651; Abcam, USA), Akt antibody (9272; Cell Signaling Technology, USA), phosphorylated Akt (p-Akt) (S473) antibody (4060t; Cell Signaling Technology, USA), β-actin (13E5) rabbit mAb (4970; Cell Signaling Technology, USA), anti-VEGF-A [EPR20705] (ab214424; Abcam, USA), horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (ZB-2301; Zhongshan Jingqiao Biotechnology, China), rat tumor necrosis factor-α (TNF-α) enzyme-linked immunosorbent assay (ELISA) kit (SXR063; Senxiong Co., Ltd., China), rat interleukin 6 (IL-6) ELISA kit (SXR063; Senxiong Co., Ltd.), rat VEGF-A ELISA kit (E-EL-R2603c; Elabscience, China), and rat C-reactive protein (CRP) ELISA kit (CRE0025 4A; Biotech, China).
Instruments
Anesthesia machines for small animals (F700; EzyVet Co., Ltd., UK), microcirculation imagers (V100; Guangzhou Medsoft System, China), and non-invasive blood pressure measurement systems (DB128X; Beijing Zhishu Duobao Biotechnology Co., Ltd., China) were used.
Animals
Male Sprague-Dawley (SD) rats (body weight, 220±20 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., China (certificate No. SCXK 2016-0006). Feeding of the animals was carried out in the specific-pathogen-free (SPF) animal feeding room of Beijing University of Chinese Medicine (certificate No. SCXK 2016-0043), and the animals were adaptable to feed for one week.
Ligation of the cecum at designated positions is the major determinant of sepsis severity.[12] Cecal ligation and puncture (CLP) was used to establish a rat model of sepsis, as previously described.[13] The cecum was then ligated below the ileocecal valve without causing bowel obstruction, and the incision was punctured 5 times with an 18-gauge needle. An appropriate amount of feces was extruded from the cecum. Finally, 10 mL/kg warm normal saline was intraperitoneally injected.
A total of 30 male SD rats were divided into four groups: sham group (n=6), CLP group (n=8), XBJ + axitinib group (n=9), and XBJ group (n=7). In this study, 6 rats that died within 6 h after modeling were excluded from the study (2 rats in CLP group, 1 rat in XBJ group, and 3 rats in XBJ + axitinib group). Finally, 24 rats were included (6 rats in each group). In the sham group, only laparotomy was performed; CLP was conducted in the CLP group. In the XBJ group, 6 mL/kg XBJ was intraperitoneally injected 2 h before CLP.[14] In the XBJ + axitinib group, 6 mL/kg XBJ was intraperitoneally injected 2 h before CLP, and 25 mg/kg axitinib was injected 1 h before CLP.[15]
Drug quality control
For drug quality control, we detected the six main ingredients (3,4-dihydroxybenzaldehyde, hydroxysafflor yellow A, paeoniflorin, ferulic acid, senkyunolide I, and salvianolic acid B) in XBJ via high-performance liquid chromatography (HPLC).[16] The detection wavelengths used were 280 nm, 254 nm and 230 nm.
Hemodynamic measurements
The arterial blood pressure of the rats was measured at 0, 2, 4, and 6 h using a noninvasive blood pressure measurement system. Pressure-volume signals were digitized using a biological signal convertor and recorded continuously via Medlab software. Hemodynamic parameters (heart rate [HR] and mean arterial pressure [MAP]) were obtained.
Intestinal microcirculation assessment
The intestinal microcirculation was assessed at 0 (at the beginning of CLP), 2 (after CLP), 4 (after CLP), and 6 h (after CLP). The ileum was 15-20 cm away from the ileocecal valve, and it was then moved to the incision while avoiding pulling the blood vessels. In this experiment, three different positions were selected, and the microcirculation imager probe was gently placed on the surface of the ileal serosa. The experiment was performed by a trained person who was blinded to the groups. The intestinal microcirculation was evaluated, and a video of no shorter than 3 s was recorded. After evaluation, the ileum was placed back into the abdominal cavity, and the abdominal wall was sutured. The assessment indices included the microvascular flow index (MFI), total vessel density (TVD), perfusion vessel density (PVD), and the proportion of perfused vessels (PPV).
Inflammatory cytokine detection
Rat serum was collected and tested at 6 h after CLP. The levels of IL-6, TNF-α, and CRP in the serum and of VEGF-A in the small intestine were detected by specific ELISA kits according to the manufacturer’s instructions.
Histological analysis
The rats were sacrificed by anesthetic overdose at 6 h after CLP. Fresh rat ileum tissue was removed and fixed in 4% paraformaldehyde solution for 48 h. Embedding, sectioning, dewaxing, and hematoxylin and eosin (H&E) staining were performed in sequence. Microscopic observation was conducted under a light microscope to assess the damage to the small intestine.
Microvascular endothelial cell analysis
Fresh intestinal tissue was harvested and fixed in 2.5% glutaraldehyde at 4 °C for 2-4 h. Tissue fixation, dehydration, infiltration, embedding, sectioning, and staining were carried out in sequence. Finally, changes in organelles in small intestinal microvascular endothelial cells were observed via transmission electron microscopy (TEM) at different magnifications.
Western blotting analysis
An animal tissue/cell total protein extraction kit and a BCA protein assay kit were used to extract total protein and determine protein concentrations. The proteins were separated via electrophoresis on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and subsequently transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% bovine serum albumin (BSA) for 1 h. The membranes containing the target protein were incubated overnight at 4 °C with PI3K, p-PI3K, Akt, p-Akt, VEGF-A, and β-actin antibodies. After the membranes were washed, they were incubated with a secondary antibody for 1 h at room temperature. The signal was detected by chemiluminescence using ECL luminescent solution, and the gray value was analyzed by ImageJ software.
Statistical analysis
The data were analyzed using GraphPad Prism 9.0 (GraphPad Software, Inc., USA) and SPSS 22.0 (IBM, USA) software. Continuous variables are expressed as the mean ± standard deviation (SD). The data were tested for normality and equality of variance. Student’s t test was used to compare differences between two groups if the data were normally distributed and had equal variance. Otherwise, the Mann-Whitney U test was utilized. Multiple groups were compared using analysis of variance (ANOVA) and Tukey’s test. A P-value <0.05 was considered statistical significance.
RESULTS
HPLC fingerprinting of XBJ
We detected six ingredients of XBJ, 3,4-dihydroxybenzaldehyde, hydroxysafflor yellow A, paeoniflorin, ferulic acid, senkyunolide I, and salvianolic acid B (supplementary Figure 1). The ingredients of XBJ were stable at different wavelengths.
Changes in hemodynamic parameters
There was no significant difference in the baseline HR or MAP among the four groups. Compared with the sham group, the MAP at 2 h after CLP in the other three groups significantly decreased (P<0.01). Compared with the sham group, the HR in the other three groups significantly increased at 4 h and 6 h (P<0.01) (supplementary Figure 2). In addition, the HR in the XBJ + axitinib group was lower than that in the XBJ group at 2 h (P<0.05).
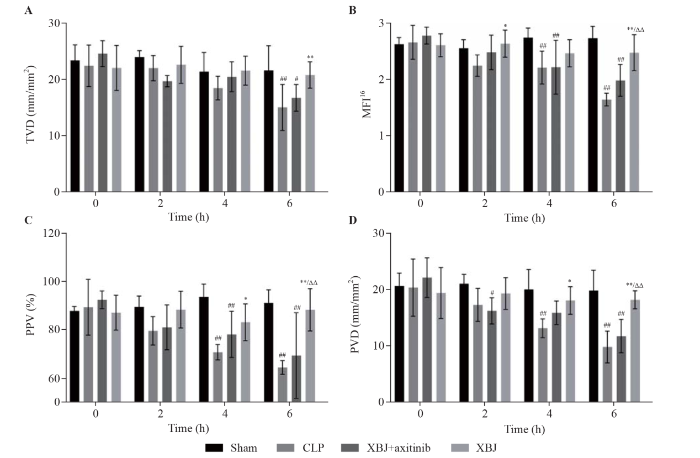
Changes in intestinal microcirculation
In the present study, time-dependent intestinal microcirculation dysfunction was found in the CLP group compared with that in the sham group. At 4 h, the MFI16, PPV, and PVD in the CLP group were significantly lower than those in the sham group (P<0.01). The TVD, MFI16, PPV, and PVD were significantly lower in the CLP group than in the sham group at 6 h (P<0.01). Compared with the CLP group, intestinal microcirculation in the XBJ group was significantly improved. The PPV and PVD improved in the XBJ group compared with those in the CLP group at 4 h (P< 0.05). At 6 h, the TVD, MFI16, PPV, and PVD in the XBJ group were significantly higher than those in the CLP group (P<0.01). Axitinib blocked the ability of XBJ to ameliorate intestinal microcirculation dysfunction In addition, the MFI16, PPV, and PVD in the XBJ + axitinib group were significantly lower than those in the XBJ group at 6 h (P<0.01) (Figure 1).
Figure 1.
Figure 1.
Effects of XBJ on sepsis-induced disruption of intestinal microcirculation dysfunction (sample size for each group=6). A: total vessel density (TVD); B: microvascular flow index 16 (MFI16); C: proportion of perfused vessels (PPV); D: perfusion vessel density (PVD). XBJ: Xuebijing; CLP: cecal ligation and puncture. Compared with the sham group, #P<0.05, ##P<0.01; compared with the CLP group, *P<0.05, **P<0.01; compared with the XBJ + axitinib group, ΔΔ P<0.01.
Changes in inflammatory cytokines
Compared with those in the sham group, the serum levels of IL-6, TNF-α and CRP were significantly higher in the CLP group (P<0.01), which indicated that the rat model of sepsis was successfully established. The serum levels of IL-6 (P<0.01), TNF-α (P<0.05) and CRP (P<0.01) in the XBJ group were significantly lower than those in the CLP group, which indicated that XBJ reduced the systemic inflammatory response in septic rats. Axitinib blocked the improvement of XBJ (supplementary Figure 3).
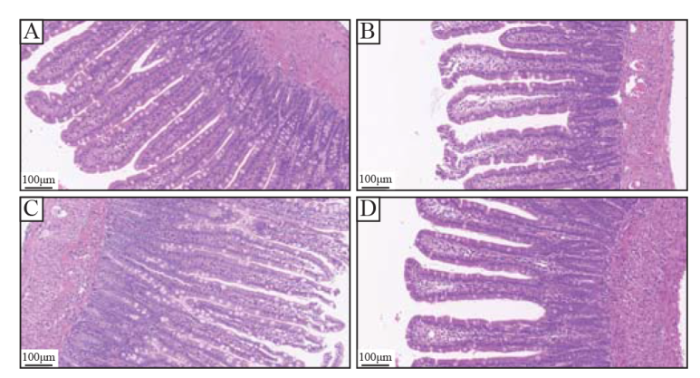
Changes in intestinal tissue
There was no obvious intestinal tissue destruction in the sham group. In the CLP group, the intestinal villi were swollen and destroyed, submucosal edema and scattered hemorrhagic necrosis foci were observed, and inflammatory cell infiltration increased in the lamina propria and submucosa. In the XBJ group, the villi of the small intestine were arranged more orderly, the degree of swelling of the villi was lower than that in the CLP group, and inflammatory cell infiltration was lower than that in the CLP group. In the XBJ + axitinib group, the intestinal villi were swollen and destroyed, submucosal edema was found, and inflammatory cell infiltration was observed in the lamina propria and submucosa (Figure 2).
Figure 2.
Figure 2.
Effect of XBJ on sepsis-induced intestinal wall destruction (H&E; 200×). A: sham group; B: CLP group; C: XBJ + axitinib group; D: XBJ group. XBJ: Xuebijing; CLP: cecal ligation and puncture.
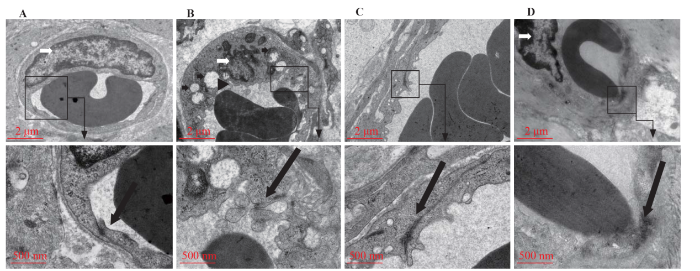
Changes in microvascular endothelial cells
There was no obvious injury to microvascular endothelial cells in the sham group. In the CLP group, there were oedematous and vacuolated mitochondria, dense and chaotic cytoplasmic processes, and nuclear deformation, and the tight junctions between endothelial cells were opened. In the XBJ group, there were few cytoplasmic processes, a regular nuclear morphology, and no special changes in the tight junctions. In the XBJ+axitinib group, the tight junctions between endothelial cells were disrupted, and many protoplasmic processes were observed. Sepsis was confirmed to result in the destruction of small intestinal microvascular endothelial cells and the opening of tight junctions between endothelial cells. XBJ ameliorated the destruction of endothelial cells and tight junctions, while axitinib blocked the ameliorating effect of XBJ (Figure 3).
Figure 3.
Figure 3.
Effect of XBJ on sepsis-induced destruction of small intestinal vascular endothelial cells. A: sham group (7,000×; 20,000×); B: CLP group (7,000×; 20,000×); C: XBJ + axitinib group (7,000×; 20,000×); D: XBJ group (7,000×; 20,000×). Triangular arrows: dense and chaotic cytoplasmic processes; long thick black arrows: tight junctions; short thick black arrows: mitochondrial edema and vacuolation; white arrows: deformed nuclei.
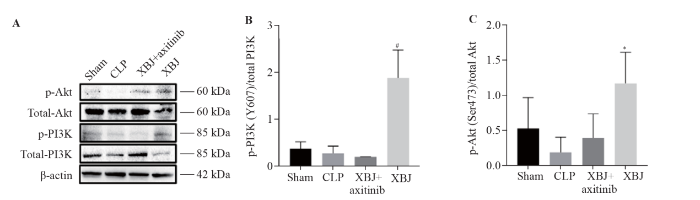
PI3K/Akt signaling pathway
The expression of p-PI3K and p-Akt in the CLP group was significantly lower than that in the sham and XBJ groups, as shown in the Western blotting images (Figure 4A). CLP inhibited the PI3K/Akt signaling pathway. XBJ upregulated the expression of p-PI3K and p-Akt (Figure 4A). According to the ratio of phosphorylated protein/total protein (Figure 4B, C), the p-PI3K/total PI3K ratio in the XBJ group was significantly higher than that in the XBJ +axitinib group (P<0.05). The p-Akt/total Akt ratio was significantly higher in the XBJ group than in the CLP group (P<0.05). Therefore, CLP downregulated the PI3K/Akt signaling pathway, XBJ upregulated the PI3K/Akt signaling pathway, and axitinib blocked the activation of the PI3K/Akt signaling pathway by XBJ.
Figure 4.
Figure 4.
Protein expression of PI3K/Akt in the rat small intestine (sample size for each group=3). A: Western blotting images of p-Akt, total Akt, p-PI3K, and total PI3K; B: p-PI3K/total PI3K; C: p-Akt/total Akt. XBJ: Xuebijing; CLP: cecal ligation and puncture; PI3K: phosphatidylinositol 3-kinase; Akt: protein kinase B; p-Akt: phosphorylated Akt; p-PI3K: phosphorylated PI3K. Compared with the CLP group, *P<0.05; compared with the XBJ + axitinib group, #P<0.05.
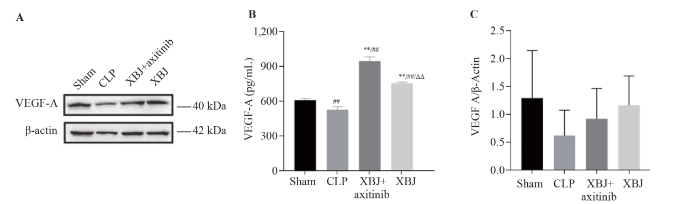
VEGF-A expression
According to the Western blotting and ELISA results, the expression level of VEGF-A was significantly lower in the CLP group than in the sham group. The expression level of VEGF-A in the XBJ group was higher than that in the CLP group, which indicated that XBJ improved the CLP-induced decrease in VEGF-A expression. The XBJ-induced increase in VEGF-A expression was not blocked by axitinib (Figure 5).
Figure 5.
Figure 5.
Protein expression of VEGF-A in the rat small intestine. A: Western blotting images of VEGF-A (sample size for each group=3); B: ELISA results for VEGF-A (sample size for each group= 6); C: VEGF-A/β-actin (sample size for each group=3). XBJ: Xuebijing; CLP: cecal ligation and puncture; VEGF-A: vascular endothelial growth factor A; ELISA: enzyme-linked immunosorbent assay.
DISCUSSION
In our study, XBJ improved intestinal microcirculation dysfunction in septic rats, alleviated the injury of small intestinal microvascular endothelial cells and small intestinal mucosa, and reduced systemic inflammation. This effect could be mediated by the VEGF-A/PI3K/Akt signaling pathway.
Intestinal microcirculation dysfunction in sepsis patients has been confirmed by several studies.[17,18] Notably, a study found microcirculation dysfunction only in moderate sepsis rats starting at 12 h.[12] The cecal ligation at the designated location, the amount of feces, and the number of perforations are the influencing factors of the severity of sepsis.[12] In the present study, the cecum of the rats was more violently injured, and more feces were extracted to induce microcirculation dysfunction as early as possible. The results of this study showed that the microcirculation dysfunction of rats with severe sepsis began at 4 h after the operation and became more serious at 6 h. The relationship between microcirculation and hemodynamics is still noteworthy, and we monitored MAP and HR. Consistent with the results of a previous study,[13] the blood pressure of the septic rats decreased continuously, which we believe is related to the development of septic shock. The early hyperdynamic circulation state was not observed in this study, and the deterioration of microcirculation and decreasing trend of blood pressure were consistent. The HR of septic rats transiently decreased at 2 h. A study reported that the efferent vagus nerve caused by sepsis regulated inflammation to the disordered state and decreased the HR.[19] The HR of septic rats continuously increased, which may be a compensatory response to shock. Our model was successful because the serum levels of inflammatory mediators (TNF-α and IL-6) were significantly elevated in septic rats. Moreover, microcirculation dysfunction in septic rats mainly manifested as a decrease in the number of perfused vessels and an increase in the number of poorly perfused vessels (slow blood flow). The microvascular endothelial cells of the small intestine of septic rats were destroyed, and the tight junctions between the vascular endothelial cells were opened, as shown by TEM.
Modern pharmacological studies have shown that XBJ can protect endothelial cells, improve microcirculation, and reduce inflammation.[20] One study investigated the pharmacokinetics of the main ingredients of XBJ and revealed that 12 herbal ingredients exhibited various biological activities related to their therapeutic effects and had good pharmacokinetic compatibility with antibiotics.[21] We used HPLC to analyze the active ingredients of XBJ, and we detected 6 active ingredients, 3,4-dihydroxybenzaldehyde, hydroxysafflor yellow A, paeoniflorin, ferulic acid, senkyunolide I, and salvianolic acid B. The results of HPLC showed that the ingredients of XBJ were stable at different wavelengths. Most of these ingredients have been confirmed to have anti-inflammatory effects, and hydroxysafflor yellow A, paeoniflorin, and ferulic acid have been shown to have endothelial protective, anticoagulant, and antithrombotic effects in inflammatory models.[22] Inflammatory reactions, endothelial damage, and microthrombosis are important factors that cause intestinal microcirculation dysfunction in patients with sepsis.[4] Therefore, it can be concluded that the hydroxyl safflower yellow A, paeoniflorin, and ferulic acid in XBJ may play important roles in improving intestinal microcirculation dysfunction in patients with sepsis. In our study, XBJ was found to ameliorate intestinal microcirculation dysfunction in septic rats. After assessing the microcirculation, it was found that XBJ significantly increased the TVD, MFI16, PPV, and PVD. Moreover, XBJ reduced injury of the small intestinal mucosa, destroyed endothelial cells and tight junctions in small intestinal microvessels, and attenuated systemic inflammation in septic rats. XBJ may be an alternative in the absence of effective treatments for microcirculation disorders.
Our study revealed that the VEGF-A/PI3K/Akt signaling pathway could mediate intestinal microcirculation dysfunction in sepsis rats. Previous studies have confirmed the role of the VEGF and PI3K/Akt signaling pathways in angiogenesis and repair of vascular injury.[5,8] A study confirmed the cascade activation of the VEGF-A/PI3K/Akt signaling pathway.[23] VEGF-A/VEGFR2 promotes the phosphorylation of PI3K, and VEGF-A also activates the Akt pathway in a PI3K-dependent manner.[24-25] VEGF-A plays an important role in the early formation of blood vessels and can be expressed in all vascularized tissues. The expression and role of VEGF-A in sepsis are organ specific.[26] The VEGF downregulation was detected in a rat model of sepsis-induced lung injury.[27] Moreover, in another study, VEGF expression was downregulated in the lungs and kidneys of septic rats.[5] Our study revealed that the expression of VEGF-A decreased in septic intestinal tissue. A network pharmacology study revealed that XBJ is an effective target of VEGF and the PI3K/Akt signaling pathway and has anti-inflammatory, anticoagulation and other biological effects.[28,29] XBJ increased the expression level of VEGF-A, which could ameliorate intestinal microcirculation dysfunction in sepsis, indicating that VEGF-A is a protective factor in the small intestinal tissue of sepsis. One study revealed that the PI3K/Akt signaling pathway was inhibited in sepsis.[30] Another study revealed that XBJ could alleviate septic injury through early activation of the PI3K/Akt signaling pathway.[31] In our study, we found that sepsis induced a decrease in the expression of p-PI3K and p-Akt in small intestinal tissues. XBJ upregulated the expression of p-PI3K and p-Akt and increased the p-PI3K/total PI3K and p-Akt/total Akt ratios. After the combination of axitinib and XBJ, we found that the improvement in the microcirculation mediated by XBJ was attenuated. In general, sepsis induces the downregulation of VEGF-A/PI3K/Akt signaling, and XBJ enhances intestinal microcirculation dysfunction by activating this signaling pathway.
Nonetheless, our study has several limitations. Our experimental data are insufficient for use in multidose studies. We still do not know whether different XBJ has dose-dependent effects on the VEGF-A/PI3K/Akt signaling pathway, which must be investigated in future experiments. In the present study, XBJ was injected before CLP, but it may be more suitable for the clinical scenario to inject XBJ after sepsis.
Funding: This study was supported by a grant from National Natural Science Foundation of China (82272196).
Ethical approval: This study was approved by the Experimental Animal Ethics Committee of Beijing University of Chinese Medicine (BUCM-4-2022010502-1052).
Conflicts of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Contributors: ALT (Aling Tang) and YL (Yan Li) are co-first authors. ALT: concept, design, manuscript preparation, experimental studies; YL: definition of intellectual content, experimental studies; LCS: data acquisition, data analysis; XYL: statistical analysis, manuscript preparation; NG: experimental studies; STY and GQZ: manuscript editing and manuscript review.
All the supplementary files in this paper are available at http://wjem.com.cn.
Reference
Analysis and validation of diagnostic biomarkers and immune cell infiltration characteristics in pediatric sepsis by integrating bioinformatics and machine learning
Endothelial cell metabolism in sepsis
Mechanisms and treatment of organ failure in sepsis
DOI:10.1038/s41581-018-0005-7
PMID:29691495
[Cited within: 1]

Sepsis is a dysregulated immune response to an infection that leads to organ dysfunction. Knowledge of the pathophysiology of organ failure in sepsis is crucial for optimizing the management and treatment of patients and for the development of potential new therapies. In clinical practice, six major organ systems - the cardiovascular (including the microcirculation), respiratory, renal, neurological, haematological and hepatic systems - can be assessed and monitored, whereas others, such as the gut, are less accessible. Over the past 2 decades, considerable amounts of new data have helped improve our understanding of sepsis pathophysiology, including the regulation of inflammatory pathways and the role played by immune suppression during sepsis. The effects of impaired cellular function, including mitochondrial dysfunction and altered cell death mechanisms, on the development of organ dysfunction are also being unravelled. Insights have been gained into interactions between key organs (such as the kidneys and the gut) and organ-organ crosstalk during sepsis. The important role of the microcirculation in sepsis is increasingly apparent, and new techniques have been developed that make it possible to visualize the microcirculation at the bedside, although these techniques are only research tools at present.
Intestinal microcirculation dysfunction in sepsis: pathophysiology, clinical monitoring, and therapeutic interventions
DOI:10.5847/wjem.j.1920-8642.2022.031
PMID:36119779
[Cited within: 2]

Intestinal microcirculation dysfunction is an important factor that causes poor prognosis in sepsis patients and is an important pathophysiological basis for the occurrence and development of sepsis.PubMed, Web of Science, and China National Knowledge Infrastructure (CNKI) were searched from inception to August 1, 2021. The search was limited to the English language only. Two reviewers independently identified studies related to intestinal microcirculation dysfunction in sepsis. Exclusion criteria were duplicate articles according to multiple search criteria.Fifty articles were included, and most of them were animal studies. These studies reported pathogenesis, including endothelial dysfunction, leukocyte recruitment and adhesion, microthrombus formation, microcirculation hypoperfusion, and redistribution of intestinal wall blood flow. The monitoring methods of intestinal microcirculation were also diverse, including handheld microscopes, intravital microscopy (IVM), laser Doppler blood flow instruments, laser speckle contrast imaging, tissue reflectance spectrophotometry, biochemical markers of intestinal ischemia, and histopathological examination. In view of the related pathogenesis of intestinal microcirculation disorder in sepsis, existing studies also have different opinions on its treatment.Limited by monitoring, there are few clinical studies on intestinal microcirculation dysfunction in sepsis. Related research mainly focuses on basic research, but some progress has also been made. Therefore, this review may provide a reference for future research on intestinal microcirculation dysfunction in sepsis.Copyright: © World Journal of Emergency Medicine.
Erythropoietin attenuates renal and pulmonary injury in polymicrobial induced-sepsis through EPO-R, VEGF and VEGF-R2 modulation
A role for vascular endothelial growth factor in acute and resolving lung injury
Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells
DOI:10.1161/01.ATV.0000110502.10593.06
PMID:14656735
[Cited within: 1]

Vascular endothelial growth factor (VEGF) is a potent angiogenic growth factor that promotes endothelial cell (EC) survival, migration, and permeability. The forkhead transcription factors FKHR, FKHRL1, and AFX are mammalian orthologues of DAF-16, a forkhead protein that controls longevity in Caenorhabditis elegans. In this study, we examined whether VEGF is coupled to phosphatidyl inositol 3-kinase (PI3K)/Akt/forkhead in ECs.We demonstrate that human ECs express members of the forkhead family (FKHR, FKHRL1, and AFX) and that VEGF modulates the phosphorylation, subcellular localization, and transcriptional activity of one or more of these isoforms by a PI3K/Akt signaling pathway. VEGF inhibited EC apoptosis, promoted DNA synthesis and the G(1)-to-S transition, and reduced expression of the cyclin-dependent kinase inhibitor p27(kip1). Each of these effects was blocked by the PI3K inhibitor LY294002 or by a phosphorylation-resistant mutant of FKHRL1, but not by wild-type FKHRL1.These results suggest that VEGF signaling in ECs is coupled to forkhead transcription factors through a PI3K/Akt-dependent pathway.
Akt signaling in regulating angiogenesis
Chemical profiling and quantification of Xuebijing injection, a systematic quality control strategy using UHPLC-Q Exactive hybrid quadrupole-orbitrap high-resolution mass spectrometry
DOI:10.1038/s41598-017-17170-y
PMID:29208914
[Cited within: 1]

To clarify and quantify the chemical profiling of XueBiJing injection (XBJ) rapidly, a feasible and accurate strategy was developed by applying ultra high performance liquid chromatography-Q Exactive hybrid quadrupole-orbitrap high resolution accurate mass spectrometry (UHPLC-Q-Orbitrap HRMS). A total of 162 components were characterized, including 19 phenanthrenequinones, 33 lactones, 28 flavonoids and 12 phenolic acids and 51 other compounds. Among them, 38 major compounds were unambiguously quantified by comparing with reference standards. Meanwhile, 38 representative compounds were simultaneously detected in XBJ samples by Q-Orbitrap HRMS. Satisfactory linearity and correlation coefficient were achieved with wide linear range. The precisions, repeatability, stability and recovery were meeting requirements. The validated method was successfully applied for simultaneous determination of 38 bioactive compounds in 10 batches XBJ samples. In addition, the similarity evaluation of fingerprintings was applied to assess the quality of XBJ. And the results were evaluated by multiple statistical strategies and five compounds might be the most important chemical markers for chemical quality control of XBJ. Finally, a rapid and simple UPLC-MS/MS method was developed for determination of five markers in XBJ sample. This research established a high sensitive and efficient strategy for integrating quality control, including identification and quantification of XBJ.
Xuebijing injection in septic rats mitigates kidney injury, reduces cortical microcirculatory disorders, and suppresses activation of local inflammation
Effect of Xuebijing on intestinal microcirculation in septic rats
Immunodesign of experimental sepsis by cecal ligation and puncture
DOI:10.1038/nprot.2008.214
PMID:19131954
[Cited within: 3]

Sepsis remains a prevalent clinical challenge and the underlying pathophysiology is still poorly understood. To investigate the complex molecular mechanisms of sepsis, various animal models have been developed, the most frequently used being the cecal ligation and puncture (CLP) model in rodents. In this model, sepsis originates from a polymicrobial infectious focus within the abdominal cavity, followed by bacterial translocation into the blood compartment, which then triggers a systemic inflammatory response. A requirement of this model is that it is performed with high consistency to obtain reproducible results. Evidence is now emerging that the accompanying inflammatory response varies with the severity grade of sepsis, which is highly dependent on the extent of cecal ligation. In this protocol, we define standardized procedures for inducing sepsis in mice and rats by applying defined severity grades of sepsis through modulation of the position of cecal ligation. The CLP procedure can be performed in as little as 10 min for each animal by an experienced user, with additional time required for subsequent postoperative care and data collection.
Autonomic dysfunction in experimental sepsis induced by cecal ligation and puncture
Impacts of high-voltage electrical burn on serum platelet-related factors and platelet aggregation number in rats and the interventive effect of Xuebijing
Acceleration of proteinuria without significant impact on renal function and its protection by angiotensin II receptor blocker in rats treated with axitinib
DOI:10.1007/s11523-015-0393-6
PMID:26423685
[Cited within: 1]

Proteinuria is a dose-associated adverse event induced by anti-angiogenic agents; however, the mechanism mediating the induction of proteinuria by this type of agent remains largely unknown. The objective of this study was to assess the effects of treatment with axitinib and/or angiotensin II receptor blocker (ARB) on urinary protein excretion and renal function.Thirty-five rats were randomly selected for treatment with following agents for 4 weeks: vehicle (group A), candesartan (group B), axitinib (group C), axitinib plus candesartan (group D), or axitinib and no treatment for subsequent 2 weeks (group E).After completion of treatment schedule, urine protein-to-creatinine ratio (UPC) in group C was significantly higher than those in groups A and B, while the additional administration of candesartan resulted in the significant reduction of UPC in group D compared with group C. Following the no treatment interval for 2 weeks, UPC in group E significantly decreased compared with that in group C. There were no significant differences in serum creatinine or blood urea nitrogen level among the five groups. Furthermore, semiquantitative evaluation of immunofluorescence findings showed that the expression levels of both nephrin and podocin in rat kidneys were inversely associated with the UPC value throughout these five groups.Despite the acceleration of proteinuria involving the downregulation of slit diaphragm-associated proteins, axitinib may not have an adverse impact on renal function, and axitinib-induced proteinuria can be partially prevented by additional treatment with ARB and reversibly recovered by its transient dose-interruption.
Beneficial effect of Xuebijing against Pseudomonas aeruginosa infection in Caenorhabditis elegans
Anti-inflammatory effects of a novel iron chelator, DIBI, in experimental sepsis
DOI:10.3233/CH-179205
PMID:28869457
[Cited within: 1]

Iron catalyzes the generation of reactive oxygen species (ROS) as part of the innate antimicrobial defense. During sepsis, the dysregulated systemic inflammatory response to infection, iron homeostasis becomes disrupted, generating an excess of ROS causing damage to tissues. This can be potentially suppressed using iron chelators that selectively bind iron to prevent its participation in ROS-related inflammatory reactions.We hypothesize that administration of DIBI, a novel iron-chelator, attenuates the dysregulated systemic immune response and reduces tissue damage in experimental endotoxemia.Five groups of animals (n = 5-10) were included in this study: control, untreated endotoxemia, and endotoxemia animals treated with either DIBI-A, MAHMP, or DIBI-B. Intravital microscopy was performed on the intestine of anesthesized mice to observe leukocyte endothelial interactions and evaluate the intestinal microcirculation.Treatment of endotoxemic mice with DIBI-B reduced the number of adhering leukocytes in submucosal collecting (V1) venules by 68%. DIBI-B, MAHMP, and DIBI-A were able to restore functional capillary density (FCD) in the intestinal muscle layer by 74%, 44%, and 11%, respectively.DIBI-B reduces leukocyte recruitment and improves FCD in experimental endotoxemia, outperforming other chelators tested. These findings suggest a potential role for DIBI-B as a candidate drug for sepsis treatment.
Tetrahydrobiopterin improves microcirculation in experimental sepsis
DOI:10.3233/CH-160207
PMID:28598830
[Cited within: 1]

Tetrahydrobiopterin (BH4), an endogenous nucleic acid derivative, acts as an important cofactor for several enzymes found within the vascular endothelium, which is deranged in sepsis.We hypothesized that BH4 would improve capillary density and decrease inflammation within the intestinal microcirculation of septic rats.We conducted a randomized, controlled trial using two previously validated models of sepsis in rats: 1) A fecal peritonitis model using a stent perforating the ascending colon, and 2) An endotoxemia model using lipopolysaccharide (LPS) toxin from E. coli. Experimental groups receiving BH4 (60 mg/kg) were compared to otherwise healthy controls and to untreated groups with sepsis-like physiology.BH4 decreased leukocyte-endothelial adhesion by 55% and 58% (P < 0.05) in the peritonitis model and endotoxemia models, respectively. In the endotoxemia model but not the peritonitis model, BH4 improved functional capillary density in capillary beds within the intestine (141.3 vs. 106.7 mm/cm2, p < 0.05). Macrohemodynamic parameters were no different between placebo treatment and BH4-treated groups.This study demonstrates that BH4 improves capillary density and inflammation in two separate models of sepsis. BH4 may represent a novel adjunct in the treatment of sepsis and septic shock in clinical practice. Further dose-finding studies and clinical trials are warranted.
Gut microcirculatory and mitochondrial effects of hyperdynamic endotoxaemic shock and norepinephrine treatment
DOI:10.1093/bja/aer379
PMID:22157851
[Cited within: 1]

Microcirculatory and mitochondrial dysfunction are important factors in the development of septic shock. In this study, we investigated the effects of fluid resuscitated endotoxaemic shock and norepinephrine treatment on intestinal microcirculation and mitochondrial function in sheep.Eight anaesthetized sheep received an i.v. infusion of endotoxin. After 24 h, mean arterial pressure (MAP) was restored to baseline levels with a norepinephrine infusion. Five sheep served as sham experiments. Central and regional haemodynamics were monitored, and ileal microcirculation was evaluated with laser Doppler and sidestream dark-field videomicroscopy techniques. Gut mucosal acidosis was assessed by air tonometry, and ileal wall biopsies were analysed for mitochondrial activity.After 24 h of endotoxaemia, the animals had developed hyperdynamic shock with systemic and mucosal acidosis. Although superior mesenteric artery (SMA) flow was higher than the baseline values, ileal microcirculatory perfusion and mitochondrial complex I activity decreased. After norepinephrine was started, SMA flow, ileal microcirculation, and mucosal acidosis remained unchanged. Although no statistically significant difference could be demonstrated, norepinephrine increased mitochondrial complex I activity in five of the six animals from which ileal biopsies were taken.Although fluid resuscitated endotoxaemic shock increased regional blood flow, microcirculatory and mitochondrial alterations were still present. Restoring MAP with norepinephrine did not affect ileal microcirculation or mucosal acidosis, indicating that perfusion pressure manipulation is of limited importance to the intestinal microcirculation in established endotoxaemic shock.
Tropisetron inhibits sepsis by repressing hyper-inflammation and regulating the cardiac action potential in rat models
DOI:S0753-3322(18)35470-2
PMID:30529771
[Cited within: 1]

The objective of the present investigation was to explore the possible effect of the 5-HT3 receptor antagonist tropisetron on the expression levels of the inflammatory factors interleukin 6 (IL-6), creatine kinase isoenzyme (CK-MB), soluble growth stimulating gene 2 protein (sST2) and immunoglobulin E (IgE), as well as the cardiac action potential in septic rats.The cecal ligation and perforation (CLP) method was utilized to construct abdominal infarction in rats. A total of 68 male adult Sprague Dawley rats were used, including 40 for assessing survival and 28 for detecting the expression levels of IL-6 and IgE, myocardial injury, cardiac dysfunction and the cardiac action potential. These 28 rats were divided into the sham (6 rats), sham + Tropisetron (6 rats), CLP (8 rats) and CLP + Tropisetron (8 rats) groups. Twenty-four hours after establishment of the sepsis rat model, immunohistochemistry was used to analyze 5-HT3 receptor protein expression, and enzyme-linked immunosorbent assay (ELISA) was employed to monitor the serum levels of IL-6, CKMB, sST2 and IgE. Furthermore, the structure of the myocardium in various groups was examined by H&E staining.The levels of IL-6, CK-MB, sST2 and IgE in the sepsis group were significantly higher than those of the sham group (P < 0.01). Furthermore, the heart rate in the sepsis group was lower than that of the sham group (P < 0.01), and the time of atrial ventricular action potential in the sepsis group was longer than that of the sham group (P < 0.05). In addition, immunohistochemical analyses showed that the area, intensity and index of 5-HT3 receptor in the sepsis group were significantly lower than those of the sham group (P < 0.01). Importantly, the 5-HT3 receptor antagonist Tropisetron exhibited significant inhibitory effects IL-6, CK-MB, sST2 and IgE expression levels, and inductive effects on atrial ventricular action potential in the sepsis group.Sepsis leads to systemic inflammatory reaction, resulting in myocardial injury, structural changes and immune imbalance. The inhibitory effect of tropisetron on inflammation, and the regulatory inflammatory disorder by the efferent vagus nerve may be one of the important mechanisms leading to cardiac electrophysiological changes in sepsis.Copyright © 2018. Published by Elsevier Masson SAS.
High degree of pharmacokinetic compatibility exists between the five-herb medicine Xuebijing and antibiotics comedicated in sepsis care
DOI:10.1016/j.apsb.2019.06.003
PMID:31649852
[Cited within: 1]

Managing the dysregulated host response to infection remains a major challenge in sepsis care. Chinese treatment guideline recommends adding XueBiJing, a five-herb medicine, to antibiotic-based sepsis care. Although adding XueBiJing further reduced 28-day mortality modulating the host response, pharmacokinetic herb-drug interaction is a widely recognized issue that needs to be studied. Building on our earlier systematic chemical and human pharmacokinetic investigations of XueBiJing, we evaluated the degree of pharmacokinetic compatibility for XueBiJing/antibiotic combination based on mechanistic evidence of interaction risk. Considering both XueBiJing‒antibiotic and antibiotic‒XueBiJing interaction potential, we integrated informatics-based approach with experimental approach and developed a compound pair-based method for data processing. To reflect clinical reality, we selected for study XueBiJing compounds bioavailable for drug interactions and 45 antibiotics commonly used in sepsis care in China. Based on the data of interacting with drug metabolizing enzymes and transporters, no XueBiJing compound could pair, as perpetrator, with the antibiotics. Although some antibiotics could, due to their inhibition of uridine 5'-diphosphoglucuronosyltransferase 2B15, organic anion transporters 1/2 and/or organic anion-transporting polypeptide 1B3, pair with senkyunolide I, tanshinol and salvianolic acid B, the potential interactions (resulting in increased exposure) are likely desirable due to these XueBiJing compounds' low baseline exposure levels. Inhibition of aldehyde dehydrogenase by 7 antibiotics probably results in undesirable reduction of exposure to protocatechuic acid from XueBiJing. Collectively, XueBiJing/antibiotic combination exhibited a high degree of pharmacokinetic compatibility at clinically relevant doses. The methodology developed can be applied to investigate other drug combinations.© 2019 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Treatment effects of Xuebijing injection in severe septic patients with disseminated intravascular coagulation
Matrine combined with cisplatin synergistically inhibited urothelial bladder cancer cells via down-regulating VEGF/PI3K/Akt signaling pathway
VEGF-A engages at least three tyrosine kinases to activate PI3K/Akt
Association between innate immunity gene polymorphisms and neonatal sepsis development: a systematic review and meta-analysis
DOI:10.1007/s12519-022-00569-7
PMID:35666457
[Cited within: 1]

The aim of this meta-analysis was to analyze all available data from studies investigating associations between polymorphisms in genes responsible for innate immunity and neonatal sepsis development.A comprehensive literature search, reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses-S guidelines, was performed with no language restriction. Studies derived using the PICO (population, intervention, comparison and outcomes) strategy, with data on the genotype distribution for innate immunity gene polymorphisms in newborns with and without sepsis. Data were analyzed using Review Manager. The Cochran-Mantel-Haenszel test was used to calculate odds ratios with 95% confidence intervals. Heterogeneity was tested using the I index.From a total of 9428 possibly relevant articles, 33 qualified for inclusion in this systematic review. According to the STrengthening the REporting of Genetic Association Studies, 23 studies were found to be of moderate quality, while 10 were of low quality. The results showed an association of the mannose-binding lectin (MBL) exon 1 genetic polymorphism with the risk of culture-proven sepsis. Toll-like receptor (TLR) 4 rs4986791 genotype distribution suggests its association with the increased risk of culture-proven sepsis. The certainty of evidence per GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) varied from very low to low. Publication bias was not detected.Out of the 11 investigated single-nucleotide polymorphisms, this meta-analysis found a possible association between the risk for culture-proven sepsis and MBL exon 1 and TLR4 rs4986791 polymorphisms. There is an evident need for larger well-designed, multicentric observational studies investigating inflammatory gene polymorphisms in neonatal sepsis.© 2022. Children's Hospital, Zhejiang University School of Medicine.
Anger emotional stress influences VEGF/VEGFR2 and its induced PI3K/Akt/mTOR signaling pathway
Time-dependent alterations of VEGF and its signaling molecules in acute lung injury in a rat model of sepsis
DOI:10.1007/s10753-011-9337-1
PMID:21528367
[Cited within: 1]

Molecular mechanisms of sepsis-associated acute lung injury (ALI) are poorly defined. Since vascular endothelial growth factor (VEGF) is a potent vascular permeability and mitogenic factor, it might contribute to the development of ALI in sepsis. Thus, using lipopolysaccharide (LPS)-induced (15 mg/kg, intraperitoneal) endotoxemic rat model, we studied the timeline (1, 3, 6, and 10 h) of pulmonary VEGF expression and its signaling machinery. Levels of pulmonary VEGF and its angiogenic-mediating receptor, Flk-1, were downregulated by LPS in a time-dependent manner; levels of plasma VEGF and its permeability-mediating receptor, Flt-1, in contrast, was upregulated with time. In addition, blockade of Flt-1 could improve the downregulated pulmonary VEGF level and attenuate the elevated plasma and pulmonary levels of TNF-α, followed by improvement of arterial oxygenation and wet-to-dry weight ratio of the lung. Expression of signaling, pro- and or apoptotic factors after LPS administration were as follows: phosphorylated Akt, a downstream molecule was downregulated time dependently; endothelial nitric oxide synthase levels were significantly reduced; pro-apoptotic markers caspase 3 and Bax were upregulated whereas levels of Bcl-2 were downregulated. The present findings show that VEGF may play a role through the expression of Flt-1 in LPS-induced ALI. Moreover, downregulation of VEGF signaling cascade may account for LPS-induced apoptosis and impaired physiological angiogenesis in lung tissues, which in turn may contribute to the development of ALI induced by LPS.
Network pharmacology to explore the anti-inflammatory mechanism of Xuebijing in the treatment of sepsis
Examining the effector mechanisms of Xuebijing injection on COVID-19 based on network pharmacology
Dapagliflozin ameliorates sepsis-induced heart injury by inhibiting cardiomyocyte apoptosis and electrical remodeling through the PI3K/Akt pathway
Xuebijing injection protects against sepsis-induced myocardial injury by regulating apoptosis and autophagy via mediation of PI3K/Akt/mTOR signaling pathway in rats