INTRODUCTION
Hyperkalemia is a potentially life-threatening medical condition with an adverse prognosis, which requires early recognition and treatment. It is more common in patients with renal dysfunction, heart failure, and with recent use of certain medications that can increase the level of serum potassium (K+), such as angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), β-adrenergic receptor blockers, nonsteroidal anti-inflammatory drugs (NSAIDs), and digoxin.[1] In most cases, severe hyperkalemia requires immediate treatment due to its electrophysiological effects. Meanwhile, hyperkalemia can be challenging to manage: the types of potassium-lowering drugs, the dosage of drugs, and the time points of choice for hemodialysis treatment are all inconclusive.[2] A good approach for adjusting the drug dosage would be an early response assessment after the first round of potassium-lowering treatment. Due to a lack of prediction methods for the efficacy of potassium-lowering treatment, the drug dosage adjustment is quite difficult. Therefore, in this study, we aimed to develop a predictive model to evaluate the efficacy of potassium-lowering treatment and provide benefits for therapeutic decision-making.
METHODS
Study design and population
The study was conducted in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines for cohort studies. We included patients with hyperkalemia in the First Affiliated Hospital of Zhejiang University from January 1, 2015, to June 30, 2021. Patients who met the following criteria were excluded: (1) patients with shock; (2) patients with age ≤18 years; (3) pregnant patients; and (4) patients with important information deficits. Hyperkalemia was defined as serum K+ ≥5.5 mmol/L. Repeat serum K+ determinations were performed within 2-4 h of the initial examination. A retested serum K+ level after the first round of potassium-lowering treatment that decreased to less than 5.5 mmol/L was defined as an effective treatment. Patients were divided into the effective treatment group and the ineffective treatment group according to the retested serum K+. In the first round of potassium-lowering treatment, only drugs were used in our department. Insulin dissolved in glucose solution was the most commonly used treatment. Furosemide or other loop diuretics were given to accelerate the potassium excretion, and calcium gluconate was given to protect the heart. Sodium bicarbonate was usually used if the patient had acidosis.
Data collection
The patients were randomly divided into training dataset (80%) or validating dataset (20%) using a computerized random sampling program in SPSS. Patient data were collected from the electronic medical record in the First Affiliated Hospital of Zhejiang University. Demographic variables, including age and sex, clinical symptoms, comorbidities (diabetes mellitus, hypertension, chronic liver disease, rheumatism, tumor, and chronic renal disease), and history of recent use of hyperkalemia-related drugs, including ACEIs, ARBs, β-blockers, NSAIDs, and digoxin, were collected to identify the factors that could influence serum K+. Laboratory data, including routine blood count, biochemical indicators of liver and kidney function, and blood gases, were all first recorded after administration. Serum K+ levels were recorded before and after the first round of potassium-lowering pharmacotherapy. Drug dosage was standardized: 4 to 6 units insulin, 20 mg furosemide, 1 mg calcium gluconate, or 125 mg sodium bicarbonate were recorded as one treatment unit.
Statistical analysis
All statistical analyses were performed using the SPSS statistical software package (version 25.0). Descriptive characteristics were presented as medians with interquartile ranges (IQR) for continuous variables and numbers with percentages for categorical variables. Continuous data were evaluated using the Mann-Whitney U-test, and categorical variables were evaluated by the Chi-square test. To identify independent risk factors, we performed multivariate logistic regression analyses with a forward stepwise method, and multicollinearity was assessed through the variance inflation factor (VIF) before regression. The discrimination capacity of the predictive model was assessed by calculating the area under the receiver operating characteristic (ROC) curve. The calibration capacity was evaluated by the Hosmer-Lemeshow test. Data were considered statistically significant at a P-value <0.05.
RESULTS
Baseline characteristics of participants in the training dataset
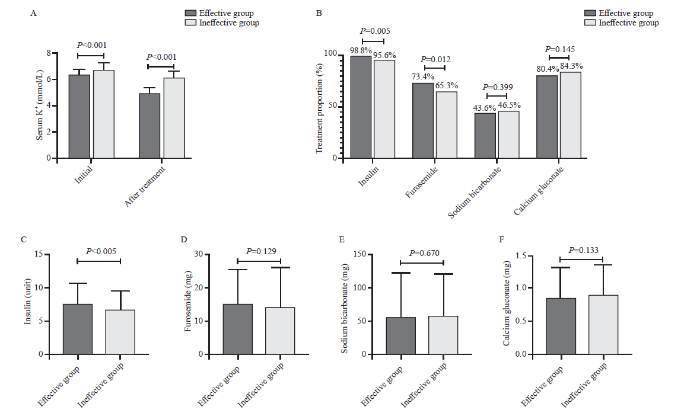
A total of 818 elderly participants were enrolled in the training dataset, including 429 patients in the effective group and 389 individuals in the ineffective group. There were no significant differences in sex between the two groups (Table 1). Patients in the effective group were younger than those in the ineffective group (63.0 [52.0-74.5] years vs. 67.0 [55.0-77.0] years, P=0.007). A higher percentage of patients in the ineffective group had peripheral edema (34.2% vs. 20.7%, P<0.001) or oliguria (24.2% vs. 14.7%, P=0.001). The initial serum K+ level was higher in the ineffective group than in the effective group (6.6 [6.3-7.0] mmol/L vs. 6.3 [6.1-6.6] mmol/L, P<0.001) (Figure 1A). Dextrose fluid mixed with insulin was the most commonly used treatment in hyperkalemia patients, while the percentage of insulin usage was higher in the effective group than in the ineffective group (98.8% vs. 95.6%, P=0.005). A higher percentage of patients in the effective group received furosemide (73.4% vs. 65.3%, P=0.012). Calcium gluconate was used in 80.4% of patients in the effective group and 84.3% in the ineffective group (Figure 1B). Almost all the laboratory results showed no significant differences between the two groups (Table 1).
Table 1. Demographic and clinical characteristics of patients enrolled in the training dataset
| Demographic and clinical characteristics | All patients (n=818) | Effective group (n=429) | Ineffective group (n=389) | P-value |
|---|---|---|---|---|
| Age, years | 65.0 (53.0-77.0) | 63.0 (52.0-74.5) | 67.0 (55.0-77.0) | 0.007 |
| Sex | 0.456 | |||
| Male | 528 (64.5) | 282 (65.7) | 246 (63.2) | |
| Female | 290 (35.5) | 147 (34.3) | 143 (36.8) | |
| Basic vital signs | ||||
| Temperature, ℃ | 36.6 (36.2-37.0) | 36.7 (36.3-37.0) | 36.5 (36.1-37.0) | 0.014 |
| Oxygen saturation, % | 99.0 (97.0-100.0) | 99.0 (98.0-100.0) | 99.0 (97.0-100.0) | 0.050 |
| Respiratory rate, breaths/min | 20.0 (19.0-20.0) | 20.0 (19.0-20.0) | 20.0 (20.0-21.0) | 0.004 |
| Heart rate, beats/min | 85.0 (73.0-100.0) | 85.0 (74.0-98.0) | 86.0 (72.5-100.0) | 0.890 |
| Mean arterial pressure, mmHg | 96.0 (83.3-109.0) | 95.0 (83.3-108.0) | 97.0 (82.7-110.3) | 0.506 |
| Comordities | ||||
| Diabetes mellitus | 245 (30.0) | 122 (28.4) | 123 (31.6) | 0.321 |
| Hypertension | 549 (67.1) | 272 (63.4) | 277 (71.2) | 0.018 |
| Chronic liver disease | 102 (12.5) | 51 (11.9) | 51 (13.1) | 0.597 |
| Rheumatism | 152 (18.6) | 84 (19.6) | 68 (17.5) | 0.441 |
| History of kidney transplantation | 72 (8.8) | 27 (6.3) | 45 (11.6) | 0.008 |
| Tumor | 149 (18.2) | 73 (17) | 76 (19.5) | 0.351 |
| Chronic renal disease | 512 (62.6) | 256 (59.7) | 256 (65.8) | 0.070 |
| ESRD a | 191 (23.3) | 84 (19.6) | 107 (27.5) | 0.007 |
| Recent use of hyperkalemia-causing drugs | 310 (37.9) | 155 (36.1) | 155 (39.8) | 0.274 |
| Symptoms | ||||
| Peripheral edema | 222 (27.1) | 89 (20.7) | 133 (34.2) | <0.001 |
| Oliguria | 157 (19.2) | 63 (14.7) | 94 (24.2) | 0.001 |
| Laboratory tests | ||||
| Red blood cell count, ×109/L | 3.4 (2.6-4.0) | 3.4 (2.7-4.0) | 3.4 (2.6-4.0) | 0.926 |
| Hemoglobin, g/L | 99.0 (79.0-116.0) | 100.0 (81.0-118.0) | 99.0 (78.0-115.0) | 0.554 |
| Platelet count, ×109/L | 192.5 (136.0-248.0) | 192.0 (133.0-240.5) | 193.0 (138.0-257.5) | 0.298 |
| Uric acid, μmol/L | 467.5 (362.8-579.3) | 461.0 (362.5-583.5) | 469.0 (363.0-574.0) | 0.885 |
| Creatinine, μmol/L | 363.5 (175.0-686.3) | 354.0 (174.0-571.5) | 372.0 (175.5-720.5) | 0.447 |
| Urea nitrogen, mmol/L | 22.4 (15.4-31.5) | 21.4 (15.3-31.6) | 23.5 (15.5-31.5) | 0.316 |
| eGFR, mL/min | 14.4 (6.5-29.8) | 15.4 (6.7-29.6) | 13.3 (6.0-30.3) | 0.353 |
| Na+, mmol/L | 138.0 (134.0-141.0) | 138.0 (134.0-141.0) | 138.0 (134.0-141.0) | 0.603 |
| Cl-, mmol/L | 102.0 (97.0-107.0) | 102.0 (97.0-107.0) | 102.8 (97.5-107.0) | 0.603 |
| pH | 7.35 (7.33-7.40) | 7.35 (7.33-7.40) | 7.35 (7.33-7.39) | 0.423 |
| PaO2, mmHg | 98.3 (78.2-111.0) | 98.7 (79.5-112.5) | 98.0 (75.3-110.0) | 0.475 |
| PaCO2, mmHg | 34.9 (30.7-37.9) | 34.9 (31.2-38.5) | 34.8 (30.0-37.7) | 0.479 |
| HCO3-, mmol/L | 19.3 (17.4-22.0) | 19.3 (17.5-22.2) | 19.2 (17.3-21.9) | 0.619 |
| Lactic acid, mmol/L | 2.0 (1.1-2.5) | 2.0 (1.2-2.6) | 1.9 (1.0-2.4) | 0.007 |
Data are presented as medians (interquartile ranges) or n (%); ESRD a: end‐stage renal disease, patients with renal disease receiving maintenance hemodialysis or peritoneal dialysis; eGFR: estimated glomerular filtration rate; PaO2: partial pressure of arterial oxygen; PaCO2: partial pressure of carbon dioxide.
Figure 1.
Figure 1.
Treatments of hyperkalemic patients. A: serum potassium levels of hyperkalemic patients between the effective and ineffective groups; B: proportion of patients receiving each therapeutic regimen; C-F: the dosages of insulin, furosemide, sodium bicarbonate, and calcium gluconate used for hyperkalemic patients in the effective and ineffective groups.
Development of the predictive model
Based on the primary analyses, we selected parameters with a P-value <0.05 in multicollinearity assessment with VIF before multivariate logistic regression analysis. There was no multicollinearity among the variables (all VIF values <10, supplementary Table 1). After regression analysis by using the forward stepwise method, age (P=0.001), peripheral edema (P<0.001), oliguria (P=0.050), history of kidney transplantation (P=0.001), ESRD (P=0.016), insulin (P<0.001), and initial potassium (P<0.001) were identified as independent predictors for the therapeutic effect of potassium-lowering treatment (supplementary Table 2). The following equation was derived:
logit(P)=10.626 - 0.015×age - 0.704×peripheral edema - 0.399×oliguria - 0.955×history of kidney transplantation - 0.450×ESRD + 0.515×insulin - 1.504×initial potassium; P means the probability of effective potassium-lowering treatments.
Validation of the predictive model
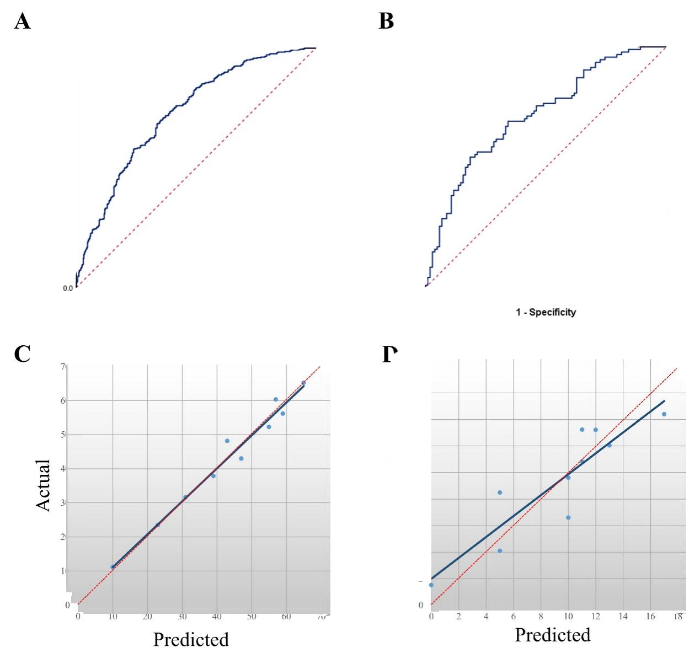
The developed predictive model was evaluated in the training dataset (n=818) and validating dataset (n=196). The discrimination of the model was valued by using ROC analysis, and the area under the curve (AUC) in the training dataset was 0.729 (95% confidence interval [95% CI] 0.694-0.763) and 0.717 (95% CI 0.646-0.788) in the validating dataset (Figures 2 A-B), which suggested that the predictive model had good performance. The calibration curve of the model demonstrated good agreement between prediction and observation in the training dataset and validating dataset (Figures 2 C-D). The Hosmer-Lemeshow test suggested that there was no departure from perfect fit (P=0.870 in the training dataset, P=0.220 in the validation dataset).
DISCUSSION
The main finding of this study is that age, peripheral edema, oliguria, history of kidney transplantation, ESRD, insulin, and initial potassium were all independently associated with favorable treatment effects, which could be used as factors for predicting treatment effects in patients with hyperkalemia. Patients with hyperkalemia who were older, had renal dysfunction or higher initial serum K+, and received lower doses of insulin were prone to have an ineffective therapeutic effect after the first round of potassium-lowering treatment.
Hyperkalemia is one of the most serious electrolyte abnormalities in the clinic since it can cause severe electrophysiologic disturbances.[1] A retrospective study of 932 hospitalized patients suggested that the rates of arrhythmia (35.2%) and cardiac arrest (43.3%) in patients with serum K+ levels >6.5 mmol/L were quite high.[3] Researchers found that patients with serum K+ >5.1 mmol/L had an increased duration of hyperkalemia and mortality.[4] Another study showed that critically ill patients with hyperkalemia had higher mortality, and it was considered an independent predictor of death.[5]
Due to renal dysfunction in potassium homeostasis, hyperkalemia is common in patients with chronic kidney disease (CKD).[6] Some drugs that are beneficial to patients with CKD, such as renin-angiotensin-aldosterone-system antagonists, are also the common causative or contributing factors of hyperkalemia.[7] Hyperkalemia has been shown to be associated with increased mortality in patients with CKD, especially in ESRD patients on maintenance hemodialysis or in kidney transplant recipients, emphasizing the importance of maintaining serum K+ levels in the physiologically normal range.[8,9] In our study, we found that renal disease, including histories of kidney transplantation, chronic renal disease, and ESRD, were all risk factors for adverse outcomes after potassium-lowering treatment. In addition, patients with peripheral edema or oliguria were also prone to have ineffective outcomes, and these symptoms might also suggest renal dysfunction.[10]
The main therapeutic methods for hyperkalemia include: (1) shifting serum K+ from the extracellular to the intracellular cell; (2) accelerating the excretion of K+ from the body; and (3) stabilizing the potential in the myocardium. Among these treatments, dialysis may be the most effective method of lowering serum K+, by removing potassium from the blood with the dialysate and shifting potassium from the extracellular to the intracellular compartment as the acidosis is reversed.[11,12] However, a long preparation period is required before patients can receive hemodialysis. Thus, drug treatments are required before hemodialysis. Insulin can lower plasma potassium levels by promoting K+ entry into cells.[13,14] Among these types of potassium-lowering treatments, we found that insulin was the only independent predictor of the therapeutic effect, suggesting that insulin may be the most effective potassium-lowering drug in the emergency treatment of hyperkalemia patients before hemodialysis.
Using the predictive model we developed, clinicians may adjust the drug dosage based on the predicted effect of potassium-lowering treatment. If the predictive model suggests that the probability of potassium dropping to the normal range after the first round of pharmacotherapy is very low (<50%), clinicians should start a second round of potassium-lowering treatment and prepare the patient for dialysis. Rapid detection and intervention of hyperkalemia are crucial for preventing serious complications.[15]
There are also limitations in our study. First, this was a retrospective cohort study, and there might be some residual confounding. Therefore, we increased the sample size to reduce the error. Second, because the exact time interval between the two serum K+ tests was difficult to obtain, the time-effect relationship could not be determined. Each repeated serum K+ determination was performed within 2-4 h of the initial examination. Finally, this was a single-center study; to make the sample more representative and to optimize the model, we plan to conduct research in multiple centers.
CONCLUSIONS
Patients with hyperkalemia who were older, had renal dysfunction or higher initial serum K+, and received lower doses of insulin, were prone to have an ineffective therapeutic effect after the first round of potassium-lowering treatment. The developed predictive model could provide early prediction of therapeutic outcomes for hyperkalemic patients receiving potassium-lowering treatment, which could also help clinicians identify high-risk hyperkalemic patients and adjust the dosage of potassium-lowering medication.
Figure 2.
Figure 2.
Validation of the predictive model for assessing the effect of potassium-lowering treatment. A, B: ROC curve of the predictive model; the AUC showed that this predictive model had a quite good discriminative capacity; C, D: calibration curve of the predictive model. The Y-axis represents the actual number of patients with effective potassium-lowering treatment; the X-axis represents the predicted number of patients with effective potassium-lowering treatment. The orange line represents a perfect prediction by an ideal model. The blue line represents the performance of the novel model, of which a closer fit to the orange line represents a better prediction. ROC: receiver operating characteristic; AUC: area under the curve; 95% CI: 95% confidence interval.
ACKNOWLEDGEMENTS
We would like to thank Wen Fang (the First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China) for providing assistance with language editing.
Funding: This research was supported by the Key Research and Development Program of Zhejiang Province (2019C03076).
Ethical approval: This study was approved by the Ethical Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (2022971). Written informed consent was obtained from each patient or his/her authorized representatives following a full explanation of the study. All methods and procedures in this study were carried out in accordance with relevant guidelines and regulations.
Conflicts of interest: The authors declare that they have no competing interests.
Contributors: CYS and JYZ contributed equally to this work. YQL conceived and designed the experiments. JYZ and CYS collected and analyzed the data. CYS and WH contributed to the writing of the manuscript. JYZ and YQL revised the manuscript.
Reference
How dangerous is hyperkalemia?
DOI:10.1681/ASN.2016121344
PMID:28778861
[Cited within: 2]

Hyperkalemia is a potentially life-threatening electrolyte disorder appreciated with greater frequency in patients with renal disease, heart failure, and with use of certain medications such as renin angiotensin aldosterone inhibitors. The traditional views that hyperkalemia can be reliably diagnosed by electrocardiogram and that particular levels of hyperkalemia confer cardiotoxic risk have been challenged by several reports of patients with atypic presentations. Epidemiologic data demonstrate strong associations of morbidity and mortality in patients with hyperkalemia but these associations appear disconnected in certain patient populations and in differing clinical presentations. Physiologic adaptation, structural cardiac disease, medication use, and degree of concurrent illness might predispose certain patients presenting with hyperkalemia to a lower or higher threshold for toxicity. These factors are often overlooked; yet data suggest that the clinical context in which hyperkalemia develops is at least as important as the degree of hyperkalemia is in determining patient outcome. This review summarizes the clinical data linking hyperkalemia with poor outcomes and discusses how the efficacy of certain treatments might depend on the clinical presentation.Copyright © 2017 by the American Society of Nephrology.
Emergency management and commonly encountered outpatient scenarios in patients with hyperkalemia
Severe hyperkalemia requiring hospitalization: predictors of mortality
DOI:10.1186/cc11872 URL [Cited within: 1]
Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes
DOI:10.5114/aoms.2014.42577
PMID:24904657
[Cited within: 1]

The aim of the study was to investigate predictors of mortality in patients hospitalized with hyperkalemia.Data among hospitalized patients with hyperkalemia (serum potassium ≥ 5.1 mEq/l) were collected. Patients with end-stage renal disease on dialysis were excluded.Of 15,608 hospitalizations, 451 (2.9%) episodes of hyperkalemia occurred in 408 patients. In patients with hyperkalemia, chronic kidney disease, hypertension, diabetes, coronary artery disease and heart failure were common comorbidities. Acute kidney injury (AKI) and metabolic acidosis were common metabolic abnormalities, and 359 patients (88%) were on at least one drug associated with hyperkalemia. Mean duration to resolution of hyperkalemia was 12 ±9.9 h. Nonsteroidal anti-inflammatory drugs (HR = 1.59), highest potassium level (HR = 0.61), tissue necrosis (HR = 0.61), metabolic acidosis (HR = 0.77), and AKI (HR = 0.77) were significant independent determinants of duration prior to hyperkalemia resolution. Tissue necrosis (OR = 4.55), potassium supplementation (OR = 5.46), metabolic acidosis (OR = 4.84), use of calcium gluconate for treatment of hyperkalemia (OR = 4.62), AKI (OR = 3.89), and prolonged duration of hyperkalemia (OR = 1.06) were significant independent predictors of in-hospital mortality.Tissue necrosis, potassium supplementation, metabolic acidosis, calcium gluconate for treatment of hyperkalemia, AKI and prolonged duration of hyperkalemia are independent predictors of in-hospital mortality.
Association between hyperkalemia at critical care initiation and mortality
DOI:10.1007/s00134-012-2636-7
PMID:22806439
[Cited within: 1]

To investigate the association between potassium concentration at the initiation of critical care and all-cause mortality.We performed a retrospective observational study on 39,705 patients, age ≥18 years, who received critical care between 1997 and 2007 in two tertiary care hospitals in Boston, Massachusetts. The exposure of interest was the highest potassium concentration on the day of critical care initiation and categorized a priori as 4.0-4.5, 4.5-5.0, 5.0-5.5, 5.5-6.0, 6.0-6.5, or ≥6.5 mEq/l. Logistic regression examined death by days 30, 90, and 365 post-critical care initiation, and in-hospital mortality. Adjusted odds ratios were estimated by multivariable logistic regression models.The potassium concentration was a strong predictor of all-cause mortality 30 days following critical care initiation with a significant risk gradient across potassium groups following multivariable adjustment: K = 4.5-5.0 mEq/l OR 1.25 (95 % CI, 1.16-1.35; P < 0.0001); K = 5.0-5.5 mEq/l OR 1.42 (95 % CI, 1.29-1.56; P < 0.0001); K = 5.5-6.0 mEq/l OR 1.67 (95 % CI, 1.47-1.89; P < 0.0001); K = 6.0-6.5 mEq/l OR 1.63 (95 % CI, 1.36-1.95; P < 0.0001); K > 6.5 mEq/l OR 1.72 (95 % CI, 1.49-1.99; P < 0.0001); all relative to patients with K = 4.0-4.5 mEq/l. Similar significant associations post multivariable adjustments are seen with in-hospital mortality and death by days 90 and 365 post-critical care initiation. In patients whose hyperkalemia decreases ≥1 mEq/l in 48 h post-critical care initiation, the association between high potassium levels and mortality is no longer significant.Our study demonstrates that a patient's potassium level at critical care initiation is robustly associated with the risk of death even at moderate increases above normal.
Management of hyperkalaemia in chronic kidney disease
DOI:10.1038/nrneph.2014.168
PMID:25223988
[Cited within: 1]

Hyperkalaemia is common in patients with chronic kidney disease (CKD), in part because of the effects of kidney dysfunction on potassium homeostasis and in part because of the cluster of comorbidities (and their associated treatments) that occur in patients with CKD. Owing to its electrophysiological effects, severe hyperkalaemia represents a medical emergency that usually requires prompt intervention, whereas the prevention of hazardous hyperkalaemic episodes in at-risk patients requires measures aimed at the long-term normalization of potassium homeostasis. The options for effective and safe medical interventions to restore chronic potassium balance are few, and long-term management of hyperkalaemia is primarily limited to the correction of modifiable exacerbating factors. This situation can result in a difficult trade-off in patients with CKD, because drugs that are beneficial to these patients (for example, renin-angiotensin-aldosterone-system antagonists) are often the most prominent cause of their hyperkalaemia. Maintaining the use of these beneficial medications while implementing various strategies to control potassium balance is desirable; however, discontinuation rates remain high. The emergence of new medications that specifically target hyperkalaemia could lead to a therapeutic paradigm shift, emphasizing preventive management over ad hoc treatment of incidentally discovered elevations in serum potassium levels.
The frequency of hyperkalemia and its significance in chronic kidney disease
DOI:10.1001/archinternmed.2009.132
PMID:19546417
[Cited within: 1]

Hyperkalemia is a potential threat to patient safety in chronic kidney disease (CKD). This study determined the incidence of hyperkalemia in CKD and whether it is associated with excess mortality.This retrospective analysis of a national cohort comprised 2 103 422 records from 245 808 veterans with at least 1 hospitalization and at least 1 inpatient or outpatient serum potassium record during the fiscal year 2005. Chronic kidney disease and treatment with angiotensin-converting enzyme inhibitors and/or angiotensin II receptor blockers (blockers of the renin-angiotensin-aldosterone system [RAAS]) were the key predictors of hyperkalemia. Death within 1 day of a hyperkalemic event was the principal outcome.Of the 66 259 hyperkalemic events (3.2% of records), more occurred as inpatient events (n = 34 937 [52.7%]) than as outpatient events (n = 31 322 [47.3%]). The adjusted rate of hyperkalemia was higher in patients with CKD than in those without CKD among individuals treated with RAAS blockers (7.67 vs 2.30 per 100 patient-months; P <.001) and those without RAAS blocker treatment (8.22 vs 1.77 per 100 patient-months; P <.001). The adjusted odds ratio (OR) of death with a moderate (potassium, >or=5.5 and <6.0 mEq/L [to convert to mmol/L, multiply by 1.0]) and severe (potassium, >or=6.0 mEq/L) hyperkalemic event was highest with no CKD (OR, 10.32 and 31.64, respectively) vs stage 3 (OR, 5.35 and 19.52, respectively), stage 4 (OR, 5.73 and 11.56, respectively), or stage 5 (OR, 2.31 and 8.02, respectively) CKD, with all P <.001 vs normokalemia and no CKD.The risk of hyperkalemia is increased with CKD, and its occurrence increases the odds of mortality within 1 day of the event. These findings underscore the importance of this metabolic disturbance as a threat to patient safety in CKD.
Prevalence and factors associated with hyperkalemia in predialysis patients followed in a low-clearance clinic
DOI:10.2215/CJN.01150112 URL [Cited within: 1]
Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system
DOI:10.1056/NEJMra035279 URL [Cited within: 1]
Immunosenescence: a critical factor associated with organ injury after sepsis
DOI:10.3389/fimmu.2022.917293
URL
[Cited within: 1]

Progressive immune dysfunction associated with aging is known as immunosenescence. The age-related deterioration of immune function is accompanied by chronic inflammation and microenvironment changes. Immunosenescence can affect both innate and acquired immunity. Sepsis is a systemic inflammatory response that affects parenchymal organs, such as the respiratory system, cardiovascular system, liver, urinary system, and central nervous system, according to the sequential organ failure assessment (SOFA). The initial immune response is characterized by an excess release of inflammatory factors, followed by persistent immune paralysis. Moreover, immunosenescence was found to complement the severity of the immune disorder following sepsis. Furthermore, the immune characteristics associated with sepsis include lymphocytopenia, thymus degeneration, and immunosuppressive cell proliferation, which are very similar to the characteristics of immunosenescence. Therefore, an in-depth understanding of immunosenescence after sepsis and its subsequent effects on the organs may contribute to the development of promising therapeutic strategies. This paper focuses on the characteristics of immunosenescence after sepsis and rigorously analyzes the possible underlying mechanism of action. Based on several recent studies, we summarized the relationship between immunosenescence and sepsis-related organs. We believe that the association between immunosenescence and parenchymal organs might be able to explain the delayed consequences associated with sepsis.
Improving outcomes by changing hemodialysis practice patterns
DOI:10.1097/MNH.0b013e328365b34c URL [Cited within: 1]
Application of regional citrate anticoagulation in patients at high risk of bleeding during intermittent hemodialysis: a prospective multicenter randomized controlled trial
DOI:10.1631/jzus.B2200082 [Cited within: 1]
Diagnosis and treatment of hyperkalemia
DOI:10.3949/ccjm.84a.17056 URL [Cited within: 1]
The successful use of extracorporeal membrane oxygenation combined with continuous renal replacement therapy for a cardiac arrest patient with refractory hypokalemia and diabetic ketoacidosis
DOI:10.5847/wjem.j.1920-8642.2022.066 PMID:35837568 [Cited within: 1]
Management of severe hyperkalemia
DOI:10.1097/CCM.0b013e31818f222b
PMID:18936701
[Cited within: 1]

Hyperkalemia is one of the few potentially lethal electrolyte disturbances. Prompt recognition and expeditious treatment of severe hyperkalemia are expected to save lives. This review is intended to provide intensivists and other interested clinicians with an understanding of the pathophysiology that underlies hyperkalemia, and a rational approach to its management.This article reviews and analyzes literature relevant to the pathophysiology and management of severe hyperkalemia. Methods include search of MEDLINE, and bibliographic search of current textbooks and journal articles.A more complete understanding of potassium homeostasis in recent years has led to new approaches to the management of severe hyperkalemia. The physiologically based sequential approach still applies. The efficacy, pitfalls, and risks of the agents available for use at each step in the sequence are critically reviewed. Rational use of the available tools will allow clinicians to successfully treat severe hyperkalemia.