INTRODUCTION
Hepatocellular carcinoma (HCC), one of the most common malignant tumors, is characterized by poor prognosis; it has the fifth-highest incidence of all cancers and is the second leading cause of cancer-related deaths worldwide.[1] Although medical science and technology are rapidly advancing, effective therapeutic options are still limited. Some emergency complications of HCC also seriously affect its prognosis.[2] The clinical symptoms of liver cancer are atypical, including indigestion, epigastria discomfort, and emaciation. However, as more than 80% of HCC develops in cirrhotic livers, some emergency and life-threatening complications, such as spontaneous rupture of the tumor and upper gastrointestinal (UGI) bleeding, have been observed. Radiofrequency ablation (RFA), surgical resection, transarterial chemoembolization, and liver transplantation may help improve patient survival. However, the exact mechanism of HCC pathogenesis and metastasis remains unknown.
Exosomes are extracellular vesicles containing DNA fragments, mRNAs, microRNAs, and many kinds of proteins. They are 30-150 nm in diameter and may be considered a tool for intercellular communication.[3] Recently, exosomes have been shown to play an important role in tumorigenesis and progression of carcinoma[4] as well as in other diseases. Generally, exosomes produced by cancer cells regulate cancer cells, as well as non-neoplastic cells in the vicinity, through paracrine/autocrine signaling mechanisms.[5,6] Cancer exosomes transfer malignant characteristics to recipient cells, including activation of invasion and metastasis, resistance to cell death, enhanced proliferation, and drug resistance.[7⇓-9] Exosomes have also been used in cell-free therapy and as drug delivery vehicles in COVID-19 management.[10] Specific populations of urinary exosomes and proteins can reflect kidney pathology in patients with primary hyperoxaluria type Ⅰ.[11] The immune cell markers of exosomes even reflect renal calcium/phosphorus physiology.[12]
Cancer cells grow in an environment that supports tumor growth; the tumor microenvironment (TME) contains normal fibroblasts (NFs), blood vessels, endothelial cells, immune cells, surrounding extracellular matrix (ECM), and other components.[13] Cancer cells interact with the microenvironment to promote tumor growth and metastasis.[14] Environmental reprogramming includes ECM remodeling, migration, angiogenesis, and the transformation of NFs into cancer-associated fibroblasts (CAFs).[15] Emerging evidence shows that cancer-derived exosomes function as mediators of communication and contribute to TME reprogramming.
In this study, we determined the mechanism by which exosomal microRNA-761 (miR-761) modifies the tumor microenvironment. The findings may provide new insights into the mechanism of HCC metastasis and improve the clinical management of patients with HCC.
METHODS
Cell lines and transfection and reagents
The HCC cell lines LM3, SK-HEP-1, HepG2, LM9, and Huh7 and the healthy liver cell line THLE-2 were maintained at our institute. The cells were cultured as described previously.[16] The human NF cell line WS1 was maintained at our institute. WS1 cells were cultured in Eagle’s minimum essential medium (Gibco, USA) supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich, USA). When the cells reached approximately 70% confluence, exosomes (20 μg/mL) were added to the medium. The morphology of the cells was examined and photographed under a microscope (AMEX1000, Thermo, China).
The miR-761 mimic and negative control (NC) mimic were obtained from GenePharma (China) and used at a concentration of 20 nmol/L. Mimic transfection was performed by using Lipofectamine 3000 (Invitrogen, USA) according to the manufacturer’s protocol. Plasmid-miR-761-sponge and plasmid-NC-sponge transfection were also performed by using Lipofectamine 3000.
AZD1480 (Cat. No. S2162) and C188-9 (Cat. No. S8605) were purchased from Selleck (USA). The concentration of AZD1480 was 5 µmol/L and that of C188-9 was 10 µmol/L. The incubation time for both drugs was 48 h.
Northern blotting
After total RNA extraction from cells and exosomes, approximately 20 µg of RNA was separated in 1.0% agarose gels and transferred to positively charged nylon membranes using a semi-dry transfer kit (BioRad, USA). After 15 min of prehybridization, the blot was incubated overnight with 32P end-labelled probes at 42 °C. The membranes were washed with a 6×SSPE solution, previously obtained from a 20×SSPE solution (3.6 mol NaCl, 0.2 mol NaH2PO4, 20 mmol/L EDTA, pH 7.4), for 30 min at 37 °C, followed by two additional washes at 4 °C. The membranes were placed in a phosphor screen cassette to obtain autoradiography images.
Luciferase reporter assay
A total of 100 ng luciferase reporter plasmid containing either wild-type or mutant 3’-UTR suppressor of cytokine signaling 2 (SOCS2) was co-transfected with NC or miR-761. Lipofectamine 3000 (Invitrogen, USA) was used for transfection according to the manufacturer’s protocol. Forty-eight hours after transfection, WS1 cells were collected and subjected to a dual-luciferase reporter assay (Promega, USA) according to the manufacturer’s instructions.
Statistical analysis
The SPSS version 22.0 (USA) was employed to analyze the data. The quantitative data were expressed as the mean±standard deviation. The experiments were performed in triplicate. The differences between groups were analyzed using Student’s t-test; a P-value <0.05 was considered statistically significant.
RESULTS
Identification of exosomes and exosomal miR-761
First, we isolated exosomes from five HCC cell lines and one healthy liver cell line and analyzed the expression level of exosomal miR-761 using quantitative real-time polymerase chain reaction (qRT-PCR). As shown in Figure 1A, although the expression level varied, miR-761 was detected in all six cell lines. To characterize the exosomes, the exosomal particulate fractions were examined by transmission electron microscopy. As shown in Figure 1B, the exosomes from Huh7 and LM3 cells appeared as small vesicles. Nanoparticle tracking analysis indicated that the size of exosomes ranged between 50 nm and 150 nm (Figure 1C). Next, northern blotting was performed to further confirm the presence of miR-761 in the exosomes. As shown in Figure 1D, miR-761 was detected in the exosomes of Huh7 cells. Western blotting using the markers CD81 and TSG101 was used to further confirm the presence of exosomes in Huh7 and LM3 cells (Figure 1E). We further confirmed that HCC-derived exosomal miR-761 was taken up by HCC cells, which enhanced the migration of recipient cells by targeting Mitofusin 2 (Mfn2) and promoted EMT. The data were shown in supplementary documents (supplementary Figures 1-4).
Figure 1.
Figure 1.
Characterization of exosomes from hepatocellular carcinoma (HCC) cells. A: the expression of exosomal miR-761 in five HCC cell lines and one healthy liver cell line determined by qRT-PCR; B: representative electron micrograph of exosomes isolated from Huh7 and LM3 cells (scale bar: 100 nm); C: size of exosomes in Huh7 and LM3 cells; D: detection of exosomal miR-761 secreted by Huh7 cells in northern blotting, U6 as an internal control; E: exosomes isolated from Huh7 and LM3 cells, expression of CD81 and TSG101 examined by Western blotting using specific antibodies. qRT-PCR: quantitative reverse transcription polymerase chain reaction; *P<0.01; **P<0.001. The black arrow: exosome.
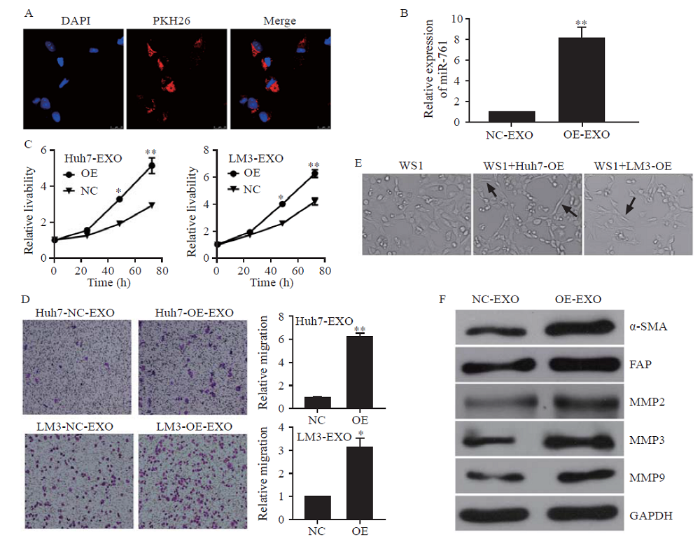
HCC exosomes transferred miR-761 into fibroblasts and increased cell migration
WS1 cells were incubated with HCC-secreted exosomes. As shown in Figure 2A, exosomes from LM3 cells labelled with PKH26 dye appeared in the cytoplasm of WS1 cells. Moreover, the exosomes increased the level of miR-761 in NFs (Figure 2B). Exosomal miR-761 secreted from both Huh7 and LM3 cells enhanced the proliferation and migration of WS1 cells (Figures 2 C and D). After co-culture with HCC exosomes, NFs presented a spindle-like shape, which is recognized as the typical morphology of CAFs (Figure 2E). As shown in Figure 2F, exosomal miR-761 increased the expression of α-smooth muscle actin (α-SMA) and fibroblast activation protein (FAP), which are regarded as CAF markers. In addition, exosomal miR-761 increased the expression of matrix metalloproteinase-2 (MMP-2), MMP-3, and MMP-9. These findings suggested that HCC-secreted exosomal miR-761 mediated TME remodeling by inducing the transformation of CAFs and increasing the expression of MMPs. Notably, the expression of Mfn2 was not detected in NFs.
Figure 2.
Figure 2.
HCC exosomes transferred miR-761 into fibroblasts and enhanced their migration. A: exosomes from LM3 cells taken up by fibroblasts, exosomes labelled with PKH26 dye (red), and nuclei labelled with 4’,6-diamidino-2-phenylindole (blue); Scale bar: 25 µm; B: quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis of miR-761 expression in WS1 cells after co-culture with HCC exosomes for 48 h; C: WS1 cell proliferation determined by CCK-8 assay after treatment with exosomes secreted by Huh7 or LM3 cells; D: representative images of the migration assay after WS1 cells co-cultured with HCC exosomes; E: WS1 cell morphology after treatment with HCC exosomes; F: Western blotting analysis of MMP proteins in fibroblasts after co-culture with HCC exosomes. α-SMA: α-smooth muscle actin; FAP: fibroblast activation protein; MMP-2: matrix metalloproteinase-2; MMP-3: matrix metalloproteinase-3; MMP-9: matrix metalloproteinase-9; GAPDH: glyceraldehyde-3-phosphate dehydrogenase. *P<0.01; **P<0.001. NC: negative control; EXO: exosome. The black arrow: spindle-like NFs.
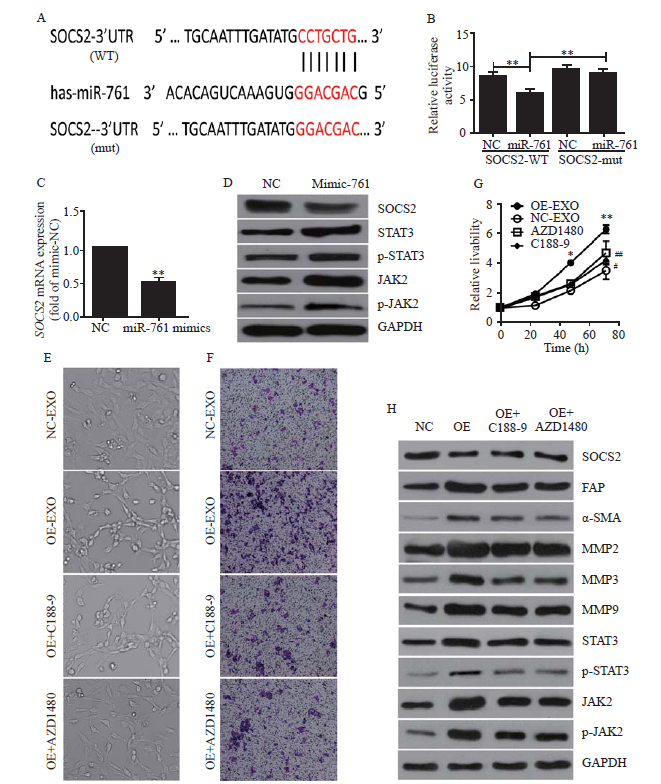
Exosomal miR-761 inhibited SOCS2 expression in WS1 cells
Since Mfn2 was not detected in WS1 cells, we sought to identify the target of miR-761 in these cells. First, we employed the TargetScan program and found a suitable candidate mRNA, SOCS2. Then, we performed a luciferase assay to verify whether SOCS2 was a downstream target of miR-761 (Figure 3A). As shown in Figure 3B, when co-transfected with miR-761, wild-type SOCS2 3’-UTR exhibited a lower translation level than mutated 3’-UTR. When WS1 cells were cultured with LM3 cell-secreted exosomes, SOCS2 expression was downregulated (Figure 3C). Therefore, SOCS2 was the downstream target of miR-761, and HCC exosomes inhibited SOCS2 expression in WS1 cells.
Figure 3.
Figure 3.
Exosomal miR-761 induced the transformation of cancer-associated fibroblasts via the SOCS2/JAK2/STAT3 signaling pathway. A: the predicted miR-761 binding site within the SOCS2 3’-UTR and its mutated version; B: luciferase assay performed in WS1 cells after cotransfection with miR-761 or NC mimics and reporter vectors carrying wild-type or mutant SOCS2 3’-UTR; C: SOCS2 expression after transfection with NC or miR-761 mimics detected by quantitative reverse transcriptase-polymerase chain reaction; D: Western blotting to determine SOCS2 expression and JAK2/STAT3 signaling pathway; E: morphology of WS1 cells; F: representative images of migration assay; G: WS1 cell proliferation detection using CCK-8 assay; H: Western blotting detection of SOCS2, JAK2, STAT3, and MMPs expression. WS1 cells were treated with LM3-secreted exosomes and JAK2 inhibitor (AZD1480), or STAT3 inhibitor (C188-9). SOCS2: suppressor of cytokine signaling 2; FAP: fibroblast activation protein; α-SMA: α-smooth muscle actin; MMP-2: matrix metalloproteinase-2; MMP-3: matrix metalloproteinase-3; MMP-9: matrix metalloproteinase-9; STAT3: signal transducer and activator of transcription 3; JAK2: Janus kinase 2; GAPDH: glyceraldehyde-3-phosphate dehydrogenase. Compared with negative control grouip, *P<0.01, **P<0.001; compared with negative control grouip, #P<0.05, ##P<0.01.
Exosomal miR-761 induced the transformation of CAFs via the SOCS2/JAK2/signal transducer and activator of transcription 3 (STAT3) signaling pathway
Exosomal miR-761 treatment decreased the protein expression of SOCS2 and enhanced the phosphorylation levels of JAK2 and STAT3 (Figure 3D). To investigate whether the transformation of CAFs and the upregulation of MMPs were caused by the activation of the JAK2/STAT3 pathway, we employed a JAK2 inhibitor, AZD1480, and a STAT3 inhibitor, C188-9. As shown in Figure 3E, both AZD1480 and C188-9 partially reversed CAF transformation. The cells treated with both inhibitors appeared rounded. AZD1480 and C188-9 also inhibited exosomal miR-761-induced increases in cell migration and proliferation (Figures 3 F and G). While these inhibitors did not affect SOCS2 expression, both AZD1480 and C188-9 inhibited JAK2 and STAT3 phosphorylation to various extents and inhibited the expression of MMPs, SMA, and FAP (Figure 3H). Collectively, these results indicated that exosomal miR-761 increased the migration of WS1 cells and the transformation of CAFs via the SOCS2/JAK2/STAT3 signaling pathway (supplementary Figure 5).
DISCUSSION
Spontaneous tumor rupture is a life-threatening complication of HCC; its incidence ranges from 12% to 15%[17] in Asia. Approximately 10% of patients with HCC die of rupture per year.[18] Tumor rupture necessitates acute intervention, including transcatheter arterial embolization (TAE), hepatic resection, and RFA. Although various methods have been proposed to manage HCC rupture, the outcomes of HCC rupture are still poor. The incidence of tumor ruptures in HCC has decreased in recent years owing to the early detection and new treatments of HCC. Another life-threatening HCC complication is UGI bleeding. As most patients with HCC have liver cirrhosis and portal hypertension, UGI bleeding is the most common acute complication. The overall incidence of UGI bleeding in patients with HCC is between 15% and 30%;[19] in some cases, UGI bleeding is the main cause of death. Early upper endoscopy is recommended in most patients with UGI bleeding. Exploring the mechanism will contribute to effectively preventing and treating HCC and decreasing the incidence of acute life-threatening complications.
Our former study has shown that miR-761 targets the 3’-UTR of the Mfn2 gene and inhibits its expression.[15]Mfn2 mediates the fusion of the mitochondrial outer membrane.[20] Our current results indicated that HCC-derived exosomes increased the expression of miR-761 and inhibited the expression of Mfn2 in HCC cells, increasing mitochondrial fission. HCC-derived exosomes enhanced the migration of recipient HCC cells, which could partially be due to increased mitochondrial fission. A similar result has shown that the downregulation of Mfn2 impairs mitochondrial fusion, contributes to mitochondrial network fragmentation, and promotes metastasis.[21] Mitochondrial fragmentation may contribute to the cancer phenotype, for example, by increasing mitotic fission, decreasing intramitochondrial calcium waves,[22] or promoting glycolysis.[23]
The EMT process is considered a key step by which tumor cells gain the capacity to invade and metastasize.[24] The main features of EMT are the loss of epithelial markers (such as E-cadherin) and the gain of mesenchymal markers (such as N-cadherin).[25] In this study, we demonstrated that HCC-derived exosomes increased the expression of N-cadherin. However, the expression of E-cadherin was not significantly affected. The increase in N-cadherin plays an important role in cancer metastasis[26,27] and may enhance cell migration even without a decrease in E-cadherin. [12] EMT promotion may also contribute to the mechanism by which HCC-derived exosomes induce the metastasis of recipient HCC cells.
To adapt to their microenvironment and survive, cancer cells not only undergo intracellular changes but also alter the TME. Indeed, a modulatory effect of exosomes and exosomal microRNAs on the TME has been previously reported.[28,29] Cancer cell-secreted exosomes that mediate the transformation of NFs to CAFs have a key role in tumor progression and metastasis.[30] CAFs support tumor metastasis by promoting tumor angiogenesis, remodeling the ECM structure, secreting MMPs, and regulating the inflammatory microenvironment.[31] In this study, we demonstrated that HCC-derived exosomes were internalized by NFs and enhanced their migration. HCC exosomes modulated the microenvironment by inducing the transformation of NFs to CAFs and by increasing the expression of MMPs.
Interestingly, we could not detect Mfn2 expression in NFs by Western blotting or qRT-PCR. As mentioned before, miR-761 has different targets in distinct cancer cells. Here, we found that SOCS2 mRNA was a target of miR-761 in NF cells. SOCS2 is a member of the SOCS protein family. SOCS family members are classic negative regulators of the JAK/STAT signaling pathway and include SOCS1-7 and cytokine-inducible SH2 domain-containing protein.[32,33] Some studies have shown that the inactivation of SOCS proteins has a substantial impact on tumorigenesis and cancer development.[34,35] A previous study has demonstrated that exosomal miRNA-155-5p, secreted by melanoma, induces the proangiogenic transformation of cancer-related fibroblasts through the SOCS1/JAK2/STAT3 pathway.[36] In this study, we found that exosomal miR-761, secreted by HCC cells, targeted SOCS2 in WS-1 cells, leading to the continuous activation of the JAK2/STAT3 pathway and therefore of CAFs.
CONCLUSIONS
This study reveals the existence of exosomal miR-761 in HCC cells. miR-761 can be transferred to NFs via exosomes. SOCS2 was identified as the downstream target of miR-761 in NFs, and exosomal miR-761 was found to activate CAFs and modulate the TME by targeting the SOCS2 and SOCS2/JAK2/STAT3 pathways. Our study identifies a new molecular mechanism by which HCC cells and NFs communicate to promote tumor metastasis. Our findings may inspire new strategies for HCC prevention and therapy.
Funding: This work was supported by the National Natural Science Foundation of China (82072203), and Natural Science Foundation of Zhejiang Province (LQ19H160025).
Ethical approval: All specimens were obtained with informed consent.
Conflicts of interest: The authors declare that they have no competing interests.
Contributors: XHZ and HX contributed equally to the manuscript. XHZ, HX, WLW, and YJS designed the study. XHZ and CX performed the majority of the experiments and analyzed the data. YCY and LSZ performed confocal analyses. QS performed northern blotting and analyzed the data. All authors contributed to manuscript writing and approved the submitted version.
Reference
Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012
DOI:10.1002/ijc.29210 URL [Cited within: 1]
Research status and prospect of immunotherapy for liver cancer
Shedding light on the cell biology of extracellular vesicles
DOI:10.1038/nrm.2017.125 URL [Cited within: 1]
The functional role of exosome in hepatocellular carcinoma
DOI:10.1007/s00432-018-2712-7 URL [Cited within: 1]
miR-200c-3p spreads invasive capacity in human oral squamous cell carcinoma microenvironment
DOI:10.1002/mc.22744 URL [Cited within: 1]
Melanoma cell-derived exosomes promote epithelial-mesenchymal transition in primary melanocytes through paracrine/autocrine signaling in the tumor microenvironment
DOI:10.1016/j.canlet.2016.03.050 URL [Cited within: 1]
Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p
DOI:10.1186/s12943-017-0694-8 URL [Cited within: 1]
Exosome-mediated transfer of miR-222 is sufficient to increase tumor malignancy in melanoma
DOI:10.1186/s12967-016-0811-2
PMID:26912358
[Cited within: 1]

Background: Growing evidence is showing that metastatic cell populations are able to transfer their characteristics to less malignant cells. Exosomes (EXOs) are membrane vesicles of endocytic origin able to convey their cargo of mRNAs, microRNAs (miRs), proteins and lipids from donors to proximal as well as distant acceptor cells. Our previous results indicated that miR-221&222 are key factors for melanoma development and dissemination. The aim of this study was to verify whether the tumorigenic properties associated with miR-222 overexpression can be also propagated by miR-222-containing EXOs. Methods: EXOs were isolated by UltraCentrifugation or Exoquick-TC (R) methods. Preparations of melanoma-derived vesicles were characterized by using the Nanosight (TM) technology and the expression of exosome markers analyzed by western blot. The expression levels of endogenous and exosomal miRNAs were examined by real time PCR. Confocal microscopy was used to evaluate transfer and uptake of microvesicles from donor to recipient cells. The functional significance of exosomal miR-222 was estimated by analyzing the vessel-like process formation, as well as cell cycle rates, invasive and chemotactic capabilities. Results: Besides microvesicle marker characterization, we evidenced that miR-222 exosomal expression mostly reflected its abundance in the cells of origin, correctly paralleled by repression of its target genes, such as p27Kip1, and induction of the PI3K/AKT pathway, thus confirming its functional implication in cancer. The possible differential significance of PI3K/AKT blockade was assessed by using the BKM120 inhibitor in miR-222-transduced cell lines. In addition, in vitro cultures showed that vesicles released by miR-222-overexpressing cells were able to transfer miR-222-dependent malignancy when taken-up by recipient primary melanomas. Results were confirmed by antago-miR-221&222 treatments and by functional observations after internalization of EXOs devoid of these miRs. Conclusion: All together these data, besides generally confirming the role of miR-222 in melanoma tumorigenesis, supported its responsibility in the exosome-associated melanoma properties, thus further indicating this miR as potential diagnostic and prognostic biomarker and its abrogation as a future therapeutic option.
Application of exosomes in the diagnosis and treatment of gastrointestinal tumors
Extracellular vesicle-based therapy for COVID-19: promises, challenges and future prospects
DOI:10.3390/biomedicines9101373 URL [Cited within: 1]
Specific populations of urinary extracellular vesicles and proteins differentiate type 1 primary hyperoxaluria patients without and with nephrocalcinosis or kidney stones
DOI:10.1186/s13023-019-1279-y URL [Cited within: 1]
Excretion of urine extracellular vesicles bearing markers of activated immune cells and calcium/phosphorus physiology differ between calcium kidney stone formers and non-stone formers
DOI:10.1186/s12882-021-02417-8 URL [Cited within: 2]
The tumor microenvironment and its role in promoting tumor growth
DOI:10.1038/onc.2008.271
PMID:18836471
[Cited within: 1]

The tumor microenvironment is created by the tumor and dominated by tumor-induced interactions. Although various immune effector cells are recruited to the tumor site, their anti-tumor functions are downregulated, largely in response to tumor-derived signals. Infiltrates of inflammatory cells present in human tumors are chronic in nature and are enriched in regulatory T cells (T(reg)) as well as myeloid suppressor cells (MSC). Immune cells in the tumor microenvironment not only fail to exercise antitumor effector functions, but they are co-opted to promote tumor growth. Sustained activation of the NF-kappaB pathway in the tumor milieu represents one mechanism that appears to favor tumor survival and drive abortive activation of immune cells. The result is tumor escape from the host immune system. Tumor escape is accomplished through the activation of one or several molecular mechanisms that lead to inhibition of immune cell functions or to apoptosis of anti-tumor effector cells. The ability to block tumor escape depends on a better understanding of cellular and molecular pathways operating in the tumor microenvironment. Novel therapeutic strategies that emerge are designed to change the pro-tumor microenvironment to one favoring acute responses and potent anti-tumor activity.
Role of tumor microenvironment in tumorigenesis
DOI:10.7150/jca.17648 URL [Cited within: 1]
MicroRNAs transported by exosomes in body fluids as mediators of intercellular communication in cancer
Micro RNA-761 is upregulated in hepatocellular carcinoma and regulates tumorigenesis by targeting Mitofusin-2
DOI:10.1111/cas.12904 URL [Cited within: 1]
Management of spontaneous rupture of hepatocellular carcinoma: single-center experience
PMID:11533094
[Cited within: 1]

To report the management of patients with spontaneous rupture of hepatocellular carcinoma (HCC) in a single center over a 10-year period and to evaluate a two-stage therapeutic approach.A retrospective study was performed on all 1,716 patients with HCC who presented from 1989 to 1998. The two-stage therapeutic approach to manage ruptured HCC consisted of initial management by conservative method, hemostasis by transarterial embolization (TAE) or surgical means, followed by second-stage hepatic resection or transarterial oily chemoembolization (TOCE). Results of definitive treatment were compared with patients with no history of rupture during the same study period.During the study period, 154 patients (9%) had spontaneous HCC rupture. Initial intervention to control bleeding included TAE in 42 patients, surgical hemostasis in 35 patients, and conservative management only in 53 patients. The 30-day mortality rate was 38%. Independent factors on presentation affecting 30-day mortality were shock on admission, hemoglobin, serum total bilirubin, and known diagnosis of inoperable tumor. After initial stabilization and clinical evaluation, 33 patients underwent hepatic resection and 30 patients received TOCE. Median survival of the hepatectomy patients was 25.7 months; that of the TOCE patients was 9.7 months. Compared with patients with no rupture, survival after hepatectomy (25.7 months v 49.2 months, P =.003) was inferior but still substantially long, whereas survival after TOCE was comparable (9.7 months v 8.7 months, P =.904).Early mortality of spontaneous rupture of HCC was dependent on prerupture disease state, liver function, and severity of bleeding. Although it was a catastrophic presentation, prolonged survival could be achieved in selected patients with second-stage hepatic resection or TOCE.
Spontaneous rupture of hepatocellular carcinoma: a review of 172 Japanese cases
PMID:1846058
[Cited within: 1]

The spontaneous rupture of a primary hepatocellular carcinoma (HCC) accounts for 10% mortality of HCC patients in Japan. Because this problem occurs much less frequently in western countries, it is often difficult for clinicians practicing in such countries to decide upon the best course of action during the crisis accompanying the spontaneous rupture of a HCC. In an effort to advance the general knowledge of this disease and clarify a selection for its treatment, we review 172 cases of spontaneous rupture of a HCC reported in the Japanese literature. The chief complaint of the patients was sudden epigastralgia or right hypochondriac pain. Abdominal paracentesis was positive in 86% of the patients. Liver failure was the cause of death in 42% of the patients. Therefore, it is important to evaluate liver reserve quickly. In addition, emergency hemostatic procedures must be implemented to avoid hemorrhagic shock. Although two-stage hepatectomy was performed in only 12% of the cases, these had the highest survival rates. Consequently, this is the procedure of choice for the treatment of spontaneous rupture of a HCC.
Acute variceal bleeding
DOI:10.1055/s-0032-1301734
PMID:22447260
[Cited within: 1]

Bleeding from gastroesophageal varices is a frequent complication of cirrhosis. Mortality from a variceal bleeding episode has decreased in the last 2 decades from 40% to 15 to 20% due to the implementation of effective treatments and improvement in the general medical care. Initial treatment should include adequate fluid resuscitation and transfusion to maintain hemoglobin around 7 to 8 g/dL, and prophylactic antibiotics (norfloxacin or ceftriaxone). It is currently recommended that a vasoactive drug be started as soon as variceal bleeding is suspected. Vasoactive therapy should be maintained for up to 5 days to prevent early rebleeding. Where available, terlipressin, a vasopressin derivative, is the preferred agent because of its safety profile; it represents the only drug with proven efficacy in improving survival. Somatostatin and octreotide are used and are as effective as terlipressin in control of bleeding but have not been shown to reduce mortality. Endoscopic therapy must be performed within the first 12 hours after admission when the patient is stable. Variceal band ligation is the recommended endoscopic treatment, but injection sclerotherapy is an alternative if band ligation is technically difficult. Despite this standard of care, 10 to 20% of patients may still exhibit initial failure to control bleeding or early rebleeding within the first 5 days. In failures to control bleeding the use of rescue transjugular intrahepatic portosystemic shunt (TIPS) using covered stents is the best alternative. In mild early rebleeding a second course of endoscopic therapy may be attempted. If rebleeding is severe, placement of TIPS using covered stents is the first-choice rescue treatment. In refractory variceal bleeding episodes, balloon tamponade may be used as a temporary bridge to TIPS. Identification of patients that are at high risk of treatment failure may guide new strategies to improve outcomes. Indeed, a recent trial has shown that placement of TIPS, using covered stents, within 72 hours of admission in patients at high risk of treatment failure (i.e., those Child B with active bleeding or Child C less than 14 points) markedly decreased rebleeding and improved survival.Thieme Medical Publishers 333 Seventh Avenue, New York, NY 10001, USA.
Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion
DOI:10.1091/mbc.e09-03-0252 URL [Cited within: 1]
Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer
DOI:10.1096/fj.11-196543 URL [Cited within: 1]
Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis
DOI:10.1016/j.molcel.2004.09.026
PMID:15469822
[Cited within: 1]

By transiently or stably overexpressing the mitochondrial fission factor dynamin-related protein-1 (Drp-1), we evaluated the role of mitochondrial division in organelle Ca2+ homeostasis and apoptotic signaling. Quantitative 3D digital microscopy revealed a split mitochondrial network in Drp-1-overexpressing cells without changes in cell viability. High-speed mitochondrial [Ca2+] ([Ca2+]m) imaging revealed propagating intramitochondrial Ca2+ waves in intact cells, which were blocked in the Drp-1-fragmented network, leaving a fraction of individual mitochondria without substantial [Ca2+]m elevation. Consequently, in Drp-1-expressing cells the apoptotic efficacy of ceramide, which causes a Ca2+-dependent perturbation of mitochondrial structure and function, was drastically reduced. Conversely, the sensitivity to staurosporine-induced apoptosis, previously shown to be directly triggered by Drp-1-dependent recruitment of proapoptotic proteins to mitochondria, was enhanced. These results demonstrate that the regulated process of mitochondrial fusion and fission controls the spatiotemporal properties of mitochondrial Ca2+ responses and, thus, physiological and pathological consequences of cellular Ca2+ signals.
DRP1 upregulation promotes pancreatic cancer growth and metastasis through increased aerobic glycolysis
DOI:10.1111/jgh.14912 URL [Cited within: 1]
Context-dependent EMT programs in cancer metastasis
DOI:10.1084/jem.20181827 URL [Cited within: 1]
EMT in cancer
DOI:10.1038/nrc.2017.118
PMID:29326430
[Cited within: 1]

Similar to embryonic development, changes in cell phenotypes defined as an epithelial to mesenchymal transition (EMT) have been shown to play a role in the tumorigenic process. Although the first description of EMT in cancer was in cell cultures, evidence for its role in vivo is now widely reported but also actively debated. Moreover, current research has exemplified just how complex this phenomenon is in cancer, leaving many exciting, open questions for researchers to answer in the future. With these points in mind, we asked four scientists for their opinions on the role of EMT in cancer and the challenges faced by scientists working in this fast-moving field.
N-cadherin expression is involved in malignant behavior of head and neck cancer in relation to epithelial-mesenchymal transition
N-cadherin as a novel prognostic marker of progression in superficial urothelial tumors
DOI:10.1158/1078-0432.CCR-05-2387 URL [Cited within: 1]
Functional roles for exosomal microRNAs in the tumour microenvironment
DOI:10.1016/j.csbj.2016.10.005 URL [Cited within: 1]
The role of exosomes in tumor stem cell maintenance, tumorigenesis and tumor development
Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts
DOI:10.1186/s12943-017-0718-4 URL [Cited within: 1]
Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis
DOI:10.1042/BST20160387 URL [Cited within: 1]
Negative regulation of cytokine signaling pathways
PMID:10837055
[Cited within: 1]

The Janus family of protein tyrosine kinases (JAKs) and STAT transcription factors regulate cellular processes involved in cell growth, differentiation, and transformation through their association with cytokine receptors. The CIS family of proteins (also referred to as the SOCS or SSI family) has been implicated in the regulation of signal transduction by a variety of cytokines. Most of them appear to be induced after stimulation with several different cytokines, and at least three of them (CIS1, CIS3/SOCS3, and JAB/SOCS1) negatively regulate cytokine signal transduction by various means: CIS1 inhibits STAT5 activation by binding to cytokine receptors that recruit STAT5, whereas JAB/SOCS-1 and CIS3/SOCS-3 directly bind to the kinase domain of JAKs, thereby inhibiting tyrosine-kinase activity. Therefore, these CIS family members seem to function in a classical negative feedback loop of cytokine signaling. Biochemical characterization as well as gene disruption studies indicate that JAB/SOCS1/SSI-1 is an important negative regulator of interferon gamma signaling. The mechanisms by which these inhibitors of cytokine signal transduction exert their effects have been extensively studied and will provide useful information for regulating tyrosine-kinase activity.
SOCS proteins, cytokine signalling and immune regulation
DOI:10.1038/nri2093
PMID:17525754
[Cited within: 1]

Suppressor of cytokine signalling (SOCS) proteins are inhibitors of cytokine signalling pathways. Studies have shown that SOCS proteins are key physiological regulators of both innate and adaptive immunity. These molecules positively and negatively regulate macrophage and dendritic-cell activation and are essential for T-cell development and differentiation. Evidence is also emerging of the involvement of SOCS proteins in diseases of the immune system. In this Review we bring together data from recent studies on SOCS proteins and their role in immunity, and propose a cohesive model of how cytokine signalling regulates immune-cell function.
Methylation-mediated gene silencing of suppressor of cytokine signaling-1 (SOCS-1)gene in esophageal squamous cell carcinoma patients of Kashmir valley
DOI:10.3109/10799893.2011.553836 URL [Cited within: 1]
Epigenetic alteration of the SOCS1 gene in hepatocellular carcinoma
Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway
DOI:10.1186/s13046-018-0911-3 URL [Cited within: 1]