World Journal of Emergency Medicine ›› 2022, Vol. 13 ›› Issue (5): 379-385.doi: 10.5847/wjem.j.1920-8642.2022.089
• Original Articles • Previous Articles Next Articles
Xiao-hu Zhou1,2, Hao Xu1,2, Chang Xu1,2, Ying-cai Yan1,2, Lin-shi Zhang1,2, Qiang Sun1,2, Wei-lin Wang1,2( ), Yan-jun Shi1,2(
), Yan-jun Shi1,2( )
)
Received:2022-03-10
Accepted:2022-06-21
Online:2022-08-23
Published:2022-09-01
Contact:
Wei-lin Wang,Yan-jun Shi
E-mail:wam@zju.edu.cn;shiyanjun@zju.edu.cn
Xiao-hu Zhou, Hao Xu, Chang Xu, Ying-cai Yan, Lin-shi Zhang, Qiang Sun, Wei-lin Wang, Yan-jun Shi. Hepatocellular carcinoma-derived exosomal miRNA-761 regulates the tumor microenvironment by targeting the SOCS2/JAK2/STAT3 pathway[J]. World Journal of Emergency Medicine, 2022, 13(5): 379-385.
Add to citation manager EndNote|Ris|BibTeX
URL: http://wjem.com.cn/EN/10.5847/wjem.j.1920-8642.2022.089
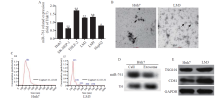
Figure 1.
Characterization of exosomes from hepatocellular carcinoma (HCC) cells. A: the expression of exosomal miR-761 in five HCC cell lines and one healthy liver cell line determined by qRT-PCR; B: representative electron micrograph of exosomes isolated from Huh7 and LM3 cells (scale bar: 100 nm); C: size of exosomes in Huh7 and LM3 cells; D: detection of exosomal miR-761 secreted by Huh7 cells in northern blotting, U6 as an internal control; E: exosomes isolated from Huh7 and LM3 cells, expression of CD81 and TSG101 examined by Western blotting using specific antibodies. qRT-PCR: quantitative reverse transcription polymerase chain reaction; *P<0.01; **P<0.001. The black arrow: exosome.
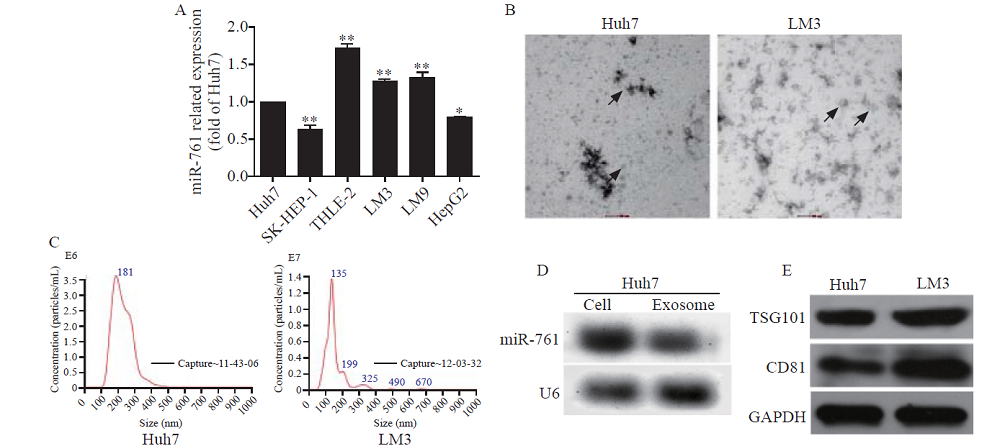
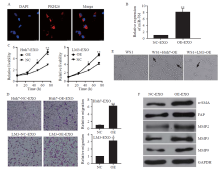
Figure 2.
HCC exosomes transferred miR-761 into fibroblasts and enhanced their migration. A: exosomes from LM3 cells taken up by fibroblasts, exosomes labelled with PKH26 dye (red), and nuclei labelled with 4’,6-diamidino-2-phenylindole (blue); Scale bar: 25 µm; B: quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis of miR-761 expression in WS1 cells after co-culture with HCC exosomes for 48 h; C: WS1 cell proliferation determined by CCK-8 assay after treatment with exosomes secreted by Huh7 or LM3 cells; D: representative images of the migration assay after WS1 cells co-cultured with HCC exosomes; E: WS1 cell morphology after treatment with HCC exosomes; F: Western blotting analysis of MMP proteins in fibroblasts after co-culture with HCC exosomes. α-SMA: α-smooth muscle actin; FAP: fibroblast activation protein; MMP-2: matrix metalloproteinase-2; MMP-3: matrix metalloproteinase-3; MMP-9: matrix metalloproteinase-9; GAPDH: glyceraldehyde-3-phosphate dehydrogenase. *P<0.01; **P<0.001. NC: negative control; EXO: exosome. The black arrow: spindle-like NFs.
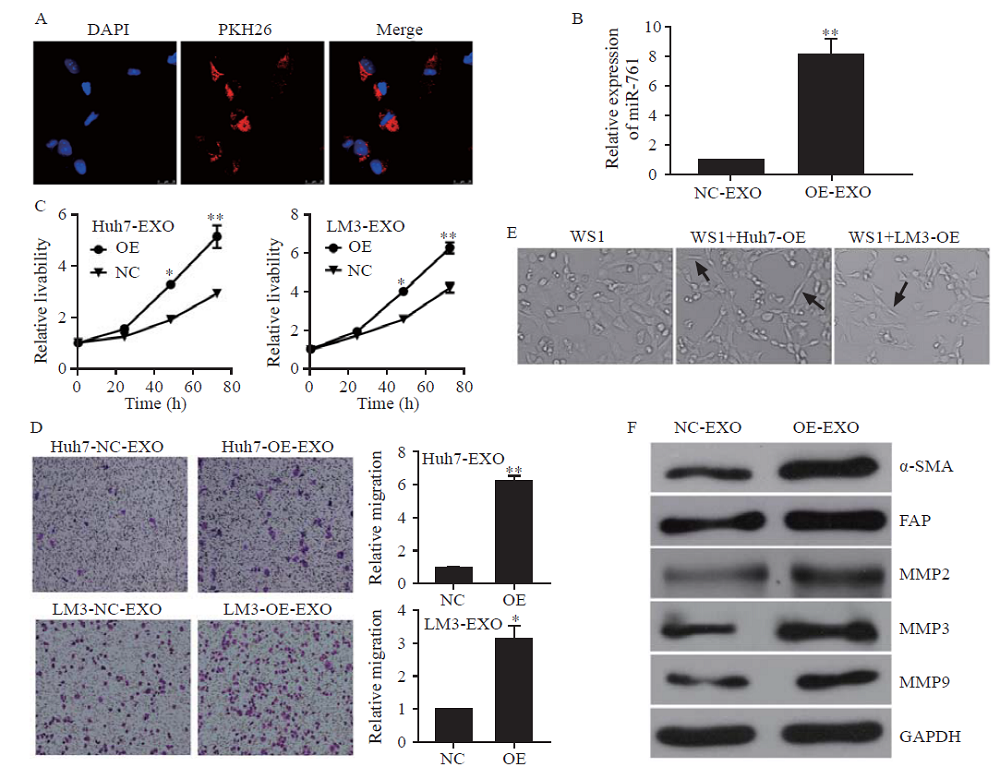
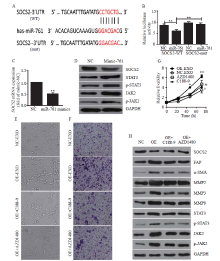
Figure 3.
Exosomal miR-761 induced the transformation of cancer-associated fibroblasts via the SOCS2/JAK2/STAT3 signaling pathway. A: the predicted miR-761 binding site within the SOCS2 3’-UTR and its mutated version; B: luciferase assay performed in WS1 cells after cotransfection with miR-761 or NC mimics and reporter vectors carrying wild-type or mutant SOCS2 3’-UTR; C: SOCS2 expression after transfection with NC or miR-761 mimics detected by quantitative reverse transcriptase-polymerase chain reaction; D: Western blotting to determine SOCS2 expression and JAK2/STAT3 signaling pathway; E: morphology of WS1 cells; F: representative images of migration assay; G: WS1 cell proliferation detection using CCK-8 assay; H: Western blotting detection of SOCS2, JAK2, STAT3, and MMPs expression. WS1 cells were treated with LM3-secreted exosomes and JAK2 inhibitor (AZD1480), or STAT3 inhibitor (C188-9). SOCS2: suppressor of cytokine signaling 2; FAP: fibroblast activation protein; α-SMA: α-smooth muscle actin; MMP-2: matrix metalloproteinase-2; MMP-3: matrix metalloproteinase-3; MMP-9: matrix metalloproteinase-9; STAT3: signal transducer and activator of transcription 3; JAK2: Janus kinase 2; GAPDH: glyceraldehyde-3-phosphate dehydrogenase. Compared with negative control grouip, *P<0.01, **P<0.001; compared with negative control grouip, #P<0.05, ##P<0.01.
| 1 |
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136(5): E359-86.
doi: 10.1002/ijc.29210 |
| 2 | Du YW. Research status and prospect of immunotherapy for liver cancer. J Pract Oncol. 2021; 36(5): 393-8. |
| 3 |
van Niel G, d’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018; 19(4): 213-28.
doi: 10.1038/nrm.2017.125 |
| 4 |
Liu H, Li B. The functional role of exosome in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2018; 144(11): 2085-95.
doi: 10.1007/s00432-018-2712-7 |
| 5 |
Kawakubo-yasukochi T, Morioka M, Hazekawa M, Yasukochi A, Nishinakagawa T, Ono K, et al. miR-200c-3p spreads invasive capacity in human oral squamous cell carcinoma microenvironment. Mol Carcinog. 2018; 57(2): 295-302.
doi: 10.1002/mc.22744 |
| 6 |
Xiao D, Barry S, Kmetz D, Egger M, Pan J, Rai SN, et al. Melanoma cell-derived exosomes promote epithelial-mesenchymal transition in primary melanocytes through paracrine/autocrine signaling in the tumor microenvironment. Cancer Lett. 2016; 376(2): 318-27.
doi: 10.1016/j.canlet.2016.03.050 |
| 7 |
Wei F, Ma C, Zhou T, Dong X, Luo Q, Geng L, et al. Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol Cancer. 2017; 16(1): 132.
doi: 10.1186/s12943-017-0694-8 |
| 8 |
Felicetti F, de Feo A, Coscia C, Puglisi R, Pedini F, Pasquini L, et al. Exosome-mediated transfer of miR-222 is sufficient to increase tumor malignancy in melanoma. J Transl Med. 2016; 14: 56.
doi: 10.1186/s12967-016-0811-2 pmid: 26912358 |
| 9 | Zhang DH. Application of exosomes in the diagnosis and treatment of gastrointestinal tumors. J Pract Oncol. 2021; 36(4): 300-5. |
| 10 |
Karn V, Ahmed S, Tsai LW, Dubey R, Ojha S, Singh HN, et al. Extracellular vesicle-based therapy for COVID-19: promises, challenges and future prospects. Biomedicines. 2021; 9(10): 1373.
doi: 10.3390/biomedicines9101373 |
| 11 |
Jayachandran M, Yuzhakov SV, Kumar S, Larson NB, Enders FT, Milliner DS, et al. Specific populations of urinary extracellular vesicles and proteins differentiate type 1 primary hyperoxaluria patients without and with nephrocalcinosis or kidney stones. Orphanet J Rare Dis. 2020; 15(1): 1-14.
doi: 10.1186/s13023-019-1279-y |
| 12 |
Zhang J, Kumar S, Jayachandran M, Herrera Hernandez LP, Wang S, Wilson EM, et al. Excretion of urine extracellular vesicles bearing markers of activated immune cells and calcium/phosphorus physiology differ between calcium kidney stone formers and non-stone formers. BMC Nephrol. 2021; 22(1): 204.
doi: 10.1186/s12882-021-02417-8 |
| 13 |
Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008; 27(45): 5904-12.
doi: 10.1038/onc.2008.271 pmid: 18836471 |
| 14 |
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017; 8(5): 761-73.
doi: 10.7150/jca.17648 |
| 15 | Salido-guadarrama I, Romero-cordoba S, Peralta-zaragoza O, Hidalgo-miranda A, Rodríguez-dorantes M. MicroRNAs transported by exosomes in body fluids as mediators of intercellular communication in cancer. Onco Targets Ther. 2014; 7: 1327-38. |
| 16 |
Zhou X, Zhang L, Zheng B, Yan Y, Zhang Y, Xie H, et al. Micro RNA-761 is upregulated in hepatocellular carcinoma and regulates tumorigenesis by targeting Mitofusin-2. Cancer Sci. 2016; 107(4): 424-32.
doi: 10.1111/cas.12904 |
| 17 |
Liu CL, Fan ST, Lo CM, Tso WK, Poon RTP, Lam CM, et al. Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol. 2001; 19(17): 3725-32.
pmid: 11533094 |
| 18 |
Miyamoto M, Sudo T, Kuyama T. Spontaneous rupture of hepatocellular carcinoma: a review of 172 Japanese cases. Am J Gastroenterol. 1991; 86(1): 67-71.
pmid: 1846058 |
| 19 |
García-pagán JC, Reverter E, Abraldes JG, Bosch J. Acute variceal bleeding. Semin Respir Crit Care Med. 2012; 33(1): 46-54.
doi: 10.1055/s-0032-1301734 pmid: 22447260 |
| 20 |
Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009; 20(15): 3525-32.
doi: 10.1091/mbc.e09-03-0252 |
| 21 |
Rehman J, Zhang HJ, Toth PT, Zhang YM, Marsboom G, Hong ZG, et al. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J. 2012; 26(5): 2175-86.
doi: 10.1096/fj.11-196543 |
| 22 |
Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004; 16(1): 59-68.
doi: 10.1016/j.molcel.2004.09.026 pmid: 15469822 |
| 23 |
Liang J, Yang YP, Bai L, Li F, Li EX. DRP1 upregulation promotes pancreatic cancer growth and metastasis through increased aerobic glycolysis. J Gastroenterol Hepatol. 2020; 35(5): 885-95.
doi: 10.1111/jgh.14912 |
| 24 |
Aiello NM, Kang YB. Context-dependent EMT programs in cancer metastasis. J Exp Med. 2019; 216(5): 1016-26.
doi: 10.1084/jem.20181827 |
| 25 |
Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018; 18(2): 128-34.
doi: 10.1038/nrc.2017.118 pmid: 29326430 |
| 26 | Nguyen PT, Kudo Y, Yoshida M, Kamata N, Ogawa I, Takata T. N-cadherin expression is involved in malignant behavior of head and neck cancer in relation to epithelial-mesenchymal transition. Histol Histopathol. 2011; 26(2): 147-56. |
| 27 |
Lascombe I, Clairotte A, Fauconnet S, Bernardini S, Wallerand H, Kantelip B, et al. N-cadherin as a novel prognostic marker of progression in superficial urothelial tumors. Clin Cancer Res. 2006; 12(9): 2780-7.
doi: 10.1158/1078-0432.CCR-05-2387 |
| 28 |
Bell E, Taylor MA. Functional roles for exosomal microRNAs in the tumour microenvironment. Comput Struct Biotechnol J. 2017; 15: 8-13.
doi: 10.1016/j.csbj.2016.10.005 |
| 29 | Gong W, Wu X, Ma J, Li T. The role of exosomes in tumor stem cell maintenance, tumorigenesis and tumor development. J Pract Oncol. 2020; 36(1): 79-82. |
| 30 |
Yang FM, Ning ZQ, Ma L, Liu WT, Shao CC, Shu YQ, et al. Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts. Mol Cancer. 2017; 16(1): 148.
doi: 10.1186/s12943-017-0718-4 |
| 31 |
Erdogan B, Webb DJ. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans. 2017; 45(1): 229-36.
doi: 10.1042/BST20160387 |
| 32 |
Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. 2000; 18: 143-64.
pmid: 10837055 |
| 33 |
Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007; 7(6): 454-65.
doi: 10.1038/nri2093 pmid: 17525754 |
| 34 |
Hussain S, Singh N, Salam I, Bandil K, Yuvaraj M, Akbar Bhat M, et al. Methylation-mediated gene silencing of suppressor of cytokine signaling-1 (SOCS-1)gene in esophageal squamous cell carcinoma patients of Kashmir valley. J Recept Signal Transduct. 2011; 31(2): 147-56.
doi: 10.3109/10799893.2011.553836 |
| 35 | Chu PY, Yeh CM, Hsu NC, Chang YS, Chang JG, Yeh KT. Epigenetic alteration of the SOCS1 gene in hepatocellular carcinoma. Swiss Med Week. 2010; 140: w13065. |
| 36 |
Zhou X, Yan T, Huang C, Xu Z, Wang L, Jiang E, et al. Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway. J Exp Clin Cancer Res. 2018; 37(1): 242.
doi: 10.1186/s13046-018-0911-3 |
| [1] | Xiao-fang Guo, Shuang-shuang Gu, Jun Wang, Hao Sun, Yu-juan Zhang, Peng-fei Yu, Jin-song Zhang, Lei Jiang. Protective effect of mesenchymal stem cell-derived exosomal treatment of hippocampal neurons against oxygen-glucose deprivation/reperfusion-induced injury [J]. World Journal of Emergency Medicine, 2022, 13(1): 46-53. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
