INTRODUCTION
Aortic stenosis is one of the major degenerative valvular heart diseases. The incidence is associated with the increasing age of the population.[1] Surgical aortic valve replacement (SAVR) used to be the only promising treatment option for patients with severe aortic stenosis even without solutions regarding the problems associated with high-risk surgical patients. But, this has changed since the introduction of transcatheter aortic valve replacement (TAVR) in 2002 by Cribier et al.[2] TAVR has rapidly grown to be the preferred treatment for severe aortic stenosis patients who are at increased risk for SAVR.[3,4] Despite the amelioration in technique and technology of TAVR, questions remain related to the safety and efficacy of this procedure, especially regarding to the complications that follow TAVR.[5-8] Silent ischemic cerebral infarction is one of the major concerns that have been associated with TAVR. Previous studies reported as high as 58%-91% of new silent ischemic stroke detected by diffusion-weighted magnetic resonance imaging (DW-MRI) occurred following TAVR.[6,7] Micro-embolic particles arising from the native valve during valve implantation have been thought to be one of the possible causes of ischemic lesions. As mentioned before, with most of the TAVR patients being the elderly, they are usually associated with multiple comorbidities. There was a high prevalence of pre-procedural anemia ranging from 45% to 64% among TAVR candidates.[9,10,11,12] In spite of the high prevalence of anemia, which has been reported to be associated with worse outcomes after TAVR, few studies have evaluated the impact of anemia and new ischemic lesions post TAVR. Therefore, the aim of this single-center study is to evaluate the association of pre-procedural anemia and the risk of periprocedural cerebral injury detected by DW-MRI in patients undergoing TAVR.
METHODS
Study design and patient selection
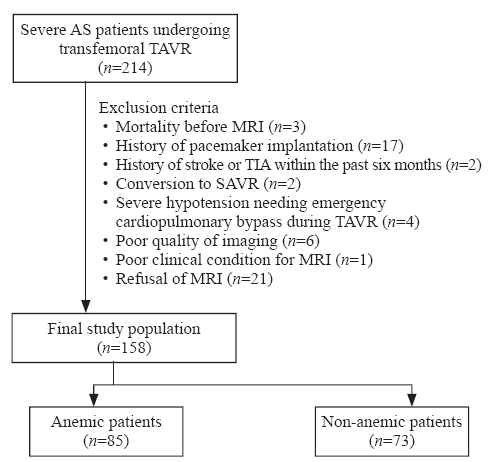
This study included 158 patients from TORCH (Transcatheter Aortic Valve Replacement Single-Center Registry in Chinese Population) registry (NCT02803294) who received TAVR from December 2016 to April 2019 based on the evaluation of a multidisciplinary heart team. The data were collected prospectively and informed consent was obtained from all patients for TAVR and the use of data for research purposes. Exclusion criteria were: (1) history of pacemaker implantation; (2) history of stroke or transient ischemic attack (TIA) within the prior six months; (3) absence of DWI-MRI imaging due to other reasons (e.g., in-hospital death, conversion to SAVR, emergency cardiopulmonary bypass during TAVR, and poor clinical condition); (4) refusal of DW-MRI; (5) poor quality imaging. TAVR procedures were performed according to the standards of care.[13] Blood samples were collected and hemoglobin levels were determined before TAVR, after the procedure, and prior to hospital discharge. Diagnosis of anemia was based on the World Health Organization criteria as hemoglobin <12 g/dL in women and <13 g/dL in men.[14] All patients completed one-year follow-up, and complications and endpoints were defined according to Valve Academic Research Consortium-2 criteria.[15]
Brain magnetic resonance imaging
All patients completed magnetic resonance imaging (MRI) before and within 4-7 days after TAVR, using either a 1.5-Tesla (1.5-T) or 3-Tesla (3-T) MRI system (GE Signa, USA). The imaging protocol consisted of transversal T2-weighted turbo spin echo (TSE); 1.5-T: repetition time (TR)/echo time (TE) 4,800/100 ms; 3-T: TR/TE 3,300/80 ms and transversal fluid-attenuated inversion recovery (FLAIR); 1.5-T: TR/TE 6,000/120 ms; 3-T: TR/TE 1,200/140 ms. DW-MRI was performed with a spin-echo echo-planar pulse sequence (1.5-T: TE 78 ms, TR 2,921 ms, matrix 128×256, total acquisition time 21.4 s; 3-T: TE 47 ms, TR 3,866 ms, matrix 128×256, total acquisition time 46.3 s) with diffusion sensitization b-values of 0 and 1,000 s/mm2. Additionally, images were obtained in 5-mm slices with a 1-mm intersection gap in both 1.5-T and 3-T systems. TAVR-associated cerebral infarction was defined as high-intensity areas (HIAs) in DW-MRI and hypointense areas on apparent diffusion coefficient (ADC) maps caused by the restricted diffusion of water.[5,7] Exclusion of other lesions or artifacts that could mimic stroke was done using T2-weighted and FLAIR sequences. The brain MRI was assessed by two independent authors using MRIcron software and reconfirmed by the neurologists. Classification of vascular territories was done according to a previous study[16]: anterior cerebral artery (ACA), middle cerebral artery (MCA), and posterior cerebral artery (PCA) for each side, respectively. Furthermore, vascular border zones (watershed zones) were defined as the area between ACA and MCA (ACA/MCA), MCA and PCA (MCA/PCA), vertebral artery and basilar artery (VA/BA). Acute periprocedural cerebrovascular events (CVEs) detected by means of DW-MRI were assessed for: (1) the total number of lesions per patient (lesion count); (2) the number of lesions located in the right and the left hemisphere; (3) the location of lesions regarding arterial distribution territories; (4) the total lesion volume per patient, accounted from the sum of all single lesion volumes; (5) the total volume/lesions in described vascular territories.
Statistical analysis
Depending on the distribution of variables, independent-samples t-test or Mann-Whitney U-test was used to compare continuous variables, and the data were presented as mean±standard deviation (SD) or median (25th to 75th percentiles). Categorical data were compared using Pearson Chi-squared test or Fisher’s exact test and were presented as frequencies (percentages). To identify the relationship between patient factors and the total volume/lesions in ACA/MCA and MCA zones, linear regression analysis was used. Individual factors were identified using univariate analysis and variables with P-value <0.2 were entered into a multivariate linear regression analysis to determine their independence. A two-sided P-value <0.05 was considered statistically significant. All statistical analyses were performed with SPSS software (version 25.0, SPSS Inc., USA).
RESULTS
Baseline characteristics
Figure 1.
Figure 1.
Patient selection flow. AS: aortic stenosis; TAVR: transcatheter aortic valve replacement; MRI: magnetic resonance imaging; TIA: transient ischemic attack; SAVR: surgical aortic valve replacement.
Table 1 Baseline characteristics of anemic and non-anemic TAVR patients
| Variables | All patients (n=158) | Anemic patients (n=85) | Non-anemic patients (n=73) | P-value |
|---|---|---|---|---|
| Age, years | 78±7 | 80±6 | 76±6 | <0.001 |
| Male | 94 (59.5) | 53 (62.4) | 41 (56.2) | 0.430 |
| Body Mass Index, kg/m2 | 22.38±3.39 | 21.55±3.47 | 23.35±3.04 | 0.001 |
| NYHA class III/IV | 143 (90.5) | 77 (90.6) | 66 (90.4) | 0.321 |
| STS score | 7.17±4.35 | 8.77±4.73 | 5.32±2.94 | <0.001 |
| Past history | ||||
| Smoker | 27 (17.1) | 10 (11.8) | 17 (23.3) | 0.055 |
| Dyslipidemia | 18 (11.4) | 11 (12.9) | 7 (9.6) | 0.508 |
| Diabetes mellitus | 40 (25.3) | 27 (31.8) | 13 (17.8) | 0.113 |
| Hypertension | 85 (53.8) | 46 (54.1) | 39 (53.4) | 0.931 |
| Peripheral vascular disease | 13 (8.2) | 10 (11.8) | 3 (4.1) | 0.081 |
| Atrial fibrillation | 26 (16.5) | 12 (14.1) | 14 (19.2) | 0.392 |
| COPD | 40 (25.3) | 22 (25.9) | 18 (24.7) | 0.860 |
| Prior PCI | 14 (8.9) | 11 (12.9) | 3 (4.1) | 0.051 |
| Prior CABG | 0 (0) | 0 (0) | 0 (0) | - |
| Prior myocardial infarction | 2 (1.3) | 1 (1.2) | 1 (1.4) | 0.914 |
| Prior PPI | 0 (0) | 0 (0) | 0 (0) | - |
| Prior stroke | 11 (7.0) | 6 (7.1) | 5 (6.8) | 0.959 |
| Syncope | 16 (10.1) | 5 (5.9) | 11 (15.1) | 0.056 |
| Medication on admission | 0.370 | |||
| Antiplatelet | 84 (53.2) | 48 (56.5) | 36 (49.3) | |
| Anticoagulation | 18 (11.4) | 7 (8.2) | 11 (15.1) | |
| No antithrombosis | 56 (35.4) | 30 (35.3) | 26 (35.6) | |
| Laboratory data on admission | ||||
| Hemoglobin, g/dL | 12.2±1.6 | 11.2±1.3 | 13.4±0.9 | <0.001 |
| INR | 1.12±0.36 | 1.12±0.34 | 1.12±0.38 | 0.994 |
| APTT, s | 38.2±5.7 | 38.6±6.6 | 37.9±4.6 | 0.436 |
| NT-proBNP, pg/mL | 2,119 (669-5,623) | 3,101 (1,162-8,124) | 1,075 (428-2,562) | 0.015 |
| Creatinine, μmol/L | 84.66±31.63 | 90.81±37.70 | 77.49±20.66 | 0.006 |
| eGFR, mL/(min·1.73m2) | 54.49±20.58 | 48.56±18.58 | 61.39±20.77 | <0.001 |
| Echocardiography | ||||
| LVEF, % | 54.91±14.88 | 52.78±15.42 | 57.40±13.91 | 0.052 |
| Mean gradient, mmHg | 58.70±34.94 | 57.92±19.52 | 59.60±47.07 | 0.764 |
| Maximum velocity, m/s | 4.83±0.82 | 4.91±0.79 | 4.73±0.85 | 0.187 |
| Aortic valve area, cm2 | 0.56±0.23 | 0.55±0.20 | 0.57±0.26 | 0.551 |
| Carotid stenosis (II+) | 6 (3.8) | 5 (5.9) | 1 (1.4) | 0.218 |
Data were presented as mean±standard deviation, n (%), or median (interquartile range). TAVR: transcatheter aortic valve replacement; NYHA: New York Heart Association; STS: Society of Thoracic Surgeons; COPD: chronic obstructive pulmonary disease; PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting; PPI: permanent pacemaker implantation; INR: international normalized ratio; APTT: activated partial thromboplastin time; NT-proBNP: N-terminal pro-B-type natriuretic peptide; eGFR: estimated glomerular filtration rate; LVEF: left ventricular ejection fraction.
Periprocedural outcome
The results of the clinical outcome were listed in Table 2. Anemic patients were more likely to have longer hospitalization compared to the non-anemic patients (P<0.001). There was no significant difference in the incidence of stroke between the two groups (P=0.374).
Table 2 Procedural data and periprocedural clinical outcome following TAVR
| Variables | All patients (n=158) | Anemic patients (n=85) | Non-anemic patients (n=73) | P-value |
|---|---|---|---|---|
| Procedural data | ||||
| Valve type | 0.768 | |||
| Self-expanding | 126 (79.7) | 68 (80.0) | 58 (79.5) | |
| Balloon-expandable | 29 (18.4) | 16 (18.8) | 13 (17.8) | |
| Mechanically-expandable | 3 (1.9) | 1 (1.2) | 2 (2.7) | |
| Pre-dilatation | 152 (96.2) | 83 (97.6) | 69 (94.5) | 0.416 |
| Post-dilatation | 79 (50.0) | 40 (47.1) | 39 (53.4) | 0.425 |
| Duration of procedure, minutes | 64.41±32.67 | 64.60±30.49 | 64.19±35.26 | 0.938 |
| Mortality | ||||
| In-hospital | 0 (0) | 0 (0) | 0 (0) | - |
| 30-day | 0 (0) | 0 (0) | 0 (0) | - |
| 1-year | 5 (3.2) | 4 (4.7) | 1 (1.4) | 0.374 |
| Length of stay, days | 7 (7-9) | 8 (4-12) | 7 (5-9) | <0.001 |
| Myocardial infarction | 0 (0) | 0 (0) | 0 (0) | - |
| Stroke | 5 (3.2) | 4 (4.7) | 1 (1.4) | 0.374 |
| Disabling stroke | 0 (0) | 0 (0) | 0 (0) | - |
| Non-disabling stroke | 5 (3.2) | 4 (4.7) | 1 (1.4) | 0.374 |
| Bleeding | 39 (24.7) | 21 (24.7) | 18 (24.7) | 0.994 |
| Blood transfusion | 12 (7.6) | 10 (11.8) | 2 (2.7) | 0.033 |
| Acute kidney injury | 3 (1.9) | 2 (2.4) | 1 (1.4) | 1.000 |
| Vascular complication | 17 (10.8) | 10 (11.8) | 7 (9.6) | 0.660 |
| New atrial fibrillation | 6 (3.8) | 3 (3.5) | 3 (4.1) | 1.000 |
| New LBBB | 21 (13.3) | 11 (12.9) | 10 (13.7) | 0.889 |
| New atrioventricular block | 20 (12.7) | 11 (12.9) | 9 (12.3) | 0.908 |
| New pacemaker implantation | 3 (1.9) | 1 (1.2) | 2 (2.7) | 0.596 |
| Pre-discharge hemoglobin, g/dL | 10.5±1.9 | 9.6±1.7 | 11.6±1.5 | <0.001 |
Data were presented as mean±standard deviation, n (%), or median (interquartile range). LBBB: left bundle branch block.
Postprocedural DW-MRI
All patients had a follow-up of DW-MRI 4-7 days post TAVR procedures (Table 3). There was no difference between the two groups for the days of DW-MRI follow-up (P=0.195). Of the 158 patients who underwent TAVR, 126 (79.7%) patients had 718 new DW-positive lesions with a mean of 4.54±5.26 lesions per patient. These HIAs were mostly multiple and spread out in both hemispheres. Details of the lesion distribution were: ACA (n=97), ACA/MCA (n=98), MCA (n=218), MCA/PCA (n=20), PCA (n=143), and VA/BA (n=143).
Table 3 Post-procedural DW-MRI findings
| Variables | All patients (n=158) | Anemic patients (n=85) | Non-anemic patients (n=73) | P-value |
|---|---|---|---|---|
| Time of post-procedural MRI, days | 5 (4-7) | 5 (4-7) | 5 (4-6) | 0.195 |
| Patients with new lesions | 126 (79.7) | 69 (81.2) | 57 (78.1) | 0.629 |
| Lesion location (patients) | 0.107 | |||
| Left hemisphere lesions | 20 (15.9) | 11 (15.9) | 9 (15.8) | |
| Right hemisphere lesions | 22 (17.5) | 7 (10.1) | 15 (26.3) | |
| Bihemispheric lesions | 84 (66.7) | 51 (73.9) | 33 (57.9) | |
| Total number of lesions | 718 | 379 | 339 | |
| Number of lesions per patient | 4.54±5.26 | 4.46±4.73 | 4.64±5.84 | 0.826 |
| ACA lesions (n=97) | 0.61±1.13 | 0.58±1.02 | 0.66±1.25 | 0.654 |
| Total volume ACA lesions, mm3 | 45.13±102.68 | 38.59±77.02 | 52.74±126.33 | 0.389 |
| Total volume/lesions in ACA, mm3 | 24.75±51.58 | 24.71±52.41 | 24.79±50.97 | 0.991 |
| ACA/MCA lesions* (n=98) | 0.62±1.25 | 0.73±1.25 | 0.49±1.26 | 0.239 |
| Total volume ACA/MCA lesions, mm3 | 50.06±112.45 | 63.06±126.90 | 34.93±91.40 | 0.109 |
| Total volume/lesions in ACA/MCA, mm3 | 25.05±48.59 | 31.89±55.78 | 17.08±37.39 | 0.049 |
| MCA lesions (n=218) | 1.38±2.12 | 1.51±2.07 | 1.23±2.18 | 0.422 |
| Total volume MCA lesions, mm3 | 124.43±246.70 | 147.41±262.18 | 97.67±226.20 | 0.207 |
| Total volume/lesions in MCA, mm3 | 44.93±63.78 | 54.54±74.72 | 33.75±46.03 | 0.034 |
| MCA/PCA lesions* (n=20) | 0.13±0.49 | 0.12±0.52 | 0.14±0.45 | 0.805 |
| Total volume MCA/PCA lesions, mm3 | 8.54±34.06 | 8.35±35.52 | 8.77±32.53 | 0.940 |
| Total volume/lesions in MCA/PCA, mm3 | 6.00±23.97 | 5.91±26.75 | 6.10±20.45 | 0.962 |
| PCA lesions (n=143) | 0.90±1.72 | 0.69±1.53 | 1.14±1.90 | 0.112 |
| Total volume PCA lesions, mm3 | 175.00±1,232.21 | 70.71±262.21 | 296.44±1,789.64 | 0.252 |
| Total volume/lesions in PCA, mm3 | 43.42±160.58 | 28.43±53.78 | 60.87±228.65 | 0.207 |
| VA/BA lesions* (n=143) | 0.91±1.45 | 0.84±1.49 | 0.99±1.42 | 0.517 |
| Total volume VA/BA lesions, mm3 | 180.70±638.99 | 164.00±600.66 | 200.14±684.61 | 0.724 |
| Total volume/lesions in VA/BA, mm3 | 104.48±468.14 | 110.46±558.37 | 97.51±337.72 | 0.863 |
Data were presented as mean±standard deviation, n (%), or median (interquartile range). DW-MRI: diffusion weighted magnetic resonance imaging; ACA: anterior cerebral artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; VA: vertebral artery; BA: basilar artery. *: vascular border zones (watershed zones).
In our univariable linear regression analysis, we identified that NYHA functional class, smoker, dyslipidemia, atrial fibrillation, anemia, INR, activated partial thromboplastin time (APTT), aortic mean gradient, maximum velocity, and procedural time were the predictors of volume/lesions in the ACA/MCA region. Meanwhile, the predictors for the volume/lesions in the MCA region were NYHA functional class, prior history of percutaneous coronary intervention (PCI), anemia, APTT, valve types, and procedural time. However, based on multivariable linear regression analysis, only NYHA functional class (β= -14.361, 95% confidence interval [95% CI] -24.807 to -3.915, P=0.007), anemia (β=16.796, 95% CI 2.001 to 31.591, P=0.026) and aortic mean gradient (β=0.218, 95% CI 0.007 to 0.430, P=0.043) were independently associated with the volume/lesions in the ACA/MCA region; while, only anemia (β=0.020, 95% CI 0.001 to 0.040, P=0.041) and valve types (β= -0.025, 95% CI -0.046 to -0.004, P=0.022) remained as the independent predictors of the volume/lesions in the MCA zone (Table 4).
Table 4 Linear regression analysis for the prediction of the volume/lesion in ACA/MCA and MCA region (on post-TAVR DW-MRI)
| Variables | ACA/MCA region | MCA region | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||||||
| R | P-value | β | t | P-value | 95% CI | R | P-value | β | t | P-value | 95% CI | ||
| Age | 0.007 | 0.930 | 0.025 | 0.757 | |||||||||
| Male | 0.019 | 0.813 | 0.045 | 0.575 | |||||||||
| NYHA class | 0.195 | 0.014 | -14.361 | -2.716 | 0.007 | -24.807 to -3.915 | 0.106 | 0.186 | |||||
| STS score | 0.027 | 0.736 | 0.034 | 0.674 | |||||||||
| Medication on admission | 0.041 | 0.610 | 0.068 | 0.394 | |||||||||
| Past history | |||||||||||||
| Smoker | 0.111 | 0.166 | 0.092 | 0.252 | |||||||||
| Diabetes mellitus | 0.016 | 0.844 | 0.069 | 0.388 | |||||||||
| Hypertension | 0.004 | 0.960 | 0.037 | 0.642 | |||||||||
| Dyslipidemia | 0.121 | 0.130 | 0.061 | 0.443 | |||||||||
| Prior myocardial infarction | 0.059 | 0.465 | 0.027 | 0.740 | |||||||||
| Prior PCI | 0.036 | 0.649 | 0.102 | 0.203 | |||||||||
| Prior stroke | 0.046 | 0.566 | 0.027 | 0.735 | |||||||||
| Peripheral vascular disease | 0.008 | 0.923 | 0.066 | 0.408 | |||||||||
| Atrial fibrillation | 0.119 | 0.135 | 0.059 | 0.458 | |||||||||
| Anemia | 0.152 | 0.056 | 16.796 | 2.243 | 0.026 | 2.001 to 31.591 | 0.163 | 0.041 | 0.020 | 2.039 | 0.041 | 0.001 to 0.040 | |
| Laboratory data | |||||||||||||
| Hemoglobin | 0.095 | 0.237 | 0.059 | 0.460 | |||||||||
| INR | 0.103 | 0.196 | 0.069 | 0.390 | |||||||||
| APTT | 0.108 | 0.175 | 0.109 | 0.174 | |||||||||
| Pre-TAVR echocardiography | |||||||||||||
| Mean gradient | 0.152 | 0.056 | 0.218 | 2.043 | 0.043 | 0.007 to 0.430 | 0.009 | 0.910 | |||||
| Aortic valve area | 0.038 | 0.640 | 0.059 | 0.458 | |||||||||
| Maximum velocity | 0.179 | 0.025 | 0.052 | 0.519 | |||||||||
| Carotid stenosis (II+) | 0.093 | 0.243 | 0.025 | 0.751 | |||||||||
| Procedural data | |||||||||||||
| Valve types | 0.097 | 0.224 | 0.197 | 0.013 | -0.025 | -2.316 | 0.022 | -0.046 to -0.004 | |||||
| Pre-dilatation | 0.011 | 0.887 | 0.036 | 0.651 | |||||||||
| Post-dilatation | 0.075 | 0.348 | 0.074 | 0.355 | |||||||||
| Procedural time | 0.137 | 0.086 | 0.139 | 0.082 | |||||||||
ACA: anterior cerebral artery; MCA: middle cerebral artery; TAVR: transcatheter aortic valve replacement; DW-MRI: diffusion-weighted magnetic resonance imaging; NYHA: New York Heart Association; STS: Society of Thoracic Surgeons; PCI: percutaneous coronary intervention; INR: international normalized ratio; APTT: activated partial thromboplastin time; CI: confidence interval.
DISCUSSION
Despite a known high prevalence of anemia and periprocedural stroke risk among TAVR patients, few studies have evaluated the relationship between anemia and new ischemic lesions detected by DW-MRI. In this study, we revealed that anemia was not directly related to the incidence of new ischemic lesions and did not affect the number of lesions in post TAVR patients. However, patients with anemia were shown to have bigger total volume/lesions in the ACA/MCA and MCA regions compared with the non-anemic group.
Anemia in TAVR
The prevalence of pre-procedural anemia among the TAVR candidates has been reported to be higher than that in cardiac surgery candidates, ranging from 45% to 64%[9,10,11,12] in the former and from 16% to 36% in the latter.[17] Although little is known about the causes of anemia in patients with aortic stenosis, the occurrence of recurrent hemorrhage or shear stress-dependent intravascular hemolysis has been reported in up to 25% of patients with severe aortic stenosis.[18] Furthermore, a potentially reversible cause of anemia was detected in almost 90% of patients in one study. Interestingly, more than half of these patients were identified to have iron deficiency. In addition, anemia incidence is also known to be associated with the increasing age.[10] As most TAVR procedures are offered to elderly patients, they contribute to the higher proportions of baseline anemia.
Pre-procedural anemia interrelates with worse outcomes after TAVR. Some of the risks that have been reported include a higher risk for acute kidney injury (AKI), blood transfusion, prolonged hospitalizations, and long-term mortality.[9,10,11,12] Furthermore, a study found that a lower pre-discharge hemoglobin level could predict early readmission. In fact, a high percentage of TAVR patients are anemic to a certain extent at hospital discharge and are at greater risk for readmission. This could be explained by the connection between low hemoglobin levels and heart failure decompensation that happen in anemic patients post-TAVR.[19]
*Similar to the previous study,[12] the anemic patients in our current study also demonstrated longer hospitalization time, which might be related to the higher demand for blood transfusion. Correspondingly, Seiffert et al[11] reported an independent association between anemia and in-hospital blood transfusion. Red blood cell (RBC) transfusions have been reported to be associated with a higher risk of one-year mortality, major cerebrovascular events, and AKI after TAVR.[12] In the current study, the anemic patients were transfused about four times more frequent compared to the non-anemic patients. A reasonable explanation behind this could be the lower hemoglobin level in anemic patients that reaches the threshold for transfusion faster even in the absence of overt bleeding. Despite being transfused, our patients did not show any signs of AKI, which was discordant with a previous study.[10]
Similar to the previous study,[12] the anemic patients in our current study also demonstrated longer hospitalization time, which might be related to the higher demand for blood transfusion. Correspondingly, Seiffert et al[11] reported an independent association between anemia and in-hospital blood transfusion. Red blood cell (RBC) transfusions have been reported to be associated with a higher risk of one-year mortality, major cerebrovascular events, and AKI after TAVR.[12] In the current study, the anemic patients were transfused about four times more frequent compared to the non-anemic patients. A reasonable explanation behind this could be the lower hemoglobin level in anemic patients that reaches the threshold for transfusion faster even in the absence of overt bleeding. Despite being transfused, our patients did not show any signs of AKI, which was discordant with a previous study.[10]
Anemia and stroke
Anemia is believed to have a clear relationship with CVEs, as anemia may impair tissue oxygen delivery.[20] As arterial oxygen content is dependent on hemoglobin level, anemia may affect the oxygen delivery to the brain.[21] Even though hypertension and atherosclerosis have been reported to be the main cause of stroke, there are also other less common causes of stroke that includes cardioembolism, hematologic disorders, substance abuse, trauma, dissections, oral contraceptive use, connective tissue disorder, pregnancy, postpartum stage, and migraine.[20] In particular, according to the INTERSTROKE study,[22] ten risk factors were associated with 90% of the risk of stroke. Even though anemia was not listed in the INTERSTROKE study, it is consistently present in patients with acute stroke and TIA, ranging from 15% to 29%.[20,23] That is why the importance of anemia, particularly the value of hemoglobin and hematocrit is still debatable among the numerous well-known risk factors for ischemic stroke.
Iron deficiency anemia (IDA) has been suggested as an etiological factor for ischemic stroke and there is significant evidence for this in pediatric TIA and ischemic stroke.[24] Even though the evidence that IDA confers risk for ischemic stroke in adults is less well-established, a previous study[25] have reported its possible associations with ischemic stroke. Being the top cause of anemia, IDA is a global health problem, as it affects more than 2 billion people worldwide.[26] Its prevalence has been confirmed by the analysis of numerous reports on the burden of disease in 187 countries between 1990 and 2010. The systemic analysis also found out that there was a high rate of iron deficiency in the aging population.[27]
Few mechanisms have been proposed by which anemia, precisely IDA, could contribute to the development of stroke, including thrombocytosis, altered erythrocyte deformability, blood flow, anemic hypoxia, and inflammation related endothelial dysfunction.[25] In the settings of IDA, erythropoietin (EPO) levels are commonly increased and can lead to secondary thrombocytosis. It is primarily due to the resemblance of EPO and thrombopoietin (TPO) molecules, hence causing the interaction of EPO and TPO receptors on the surface of megakaryocytes. Additionally, the changes in erythrocyte deformability may impact tissue perfusion, either through decreased oxygen capacity or diminution of blood supply. Apart from that, in the case of hypoxic anemia, inflammatory endothelial dysfunction can also lead to ischemic injury of the brain.[20,25] Despite all previous studies that have evaluated the association of anemia and stroke, in our current study, we did not find any significant difference in the incidence of stroke between the anemic and non-anemic groups due to the small sample size.
TAVR and DW-MRI lesions
TAVR has been known to be associated with frequent occurrence of cerebral complications. Few studies have reported the incidence of clinically apparent stroke about 3.3%-9.1% in the early postoperative period.[6,7] Additionally, the perioperative incidence of MRI-defined cerebral infarction ranges from 58% to 91%.[6,7,28,29] Consistently, our present study found that 79.7% of patients presented with new ischemic lesions on post-procedural DW-MRI.
In the ADVANCE trial, using the CoreValves exclusively, about half of the strokes were reported to occur between day 2 and day 30, while the other half occurred on the day of the procedure. This suggests that the risk of stroke is not limited to the procedure itself.[30] Furthermore, in the Claret Embolic Protection and TAVI (CLEAN-TAVI) randomized clinical trial, numerous materials were captured and removed by the filters that included old and fresh thrombus, endothelium, atheromatous plaque, valve tissue, and calcium.[31] It becomes evident that the causes of cerebral injury are multifactorial and that the embolic risk persists even after a TAVR procedure. With regard to the potential risk factors for the development of new MRI-defined cerebral infarction, several studies have found an association between age and the number of lesions after these procedures.[28,29]
In our study, patients with anemia were significantly older than the non-anemic patients. Despite statistical insignificance, the anemic group had more patients with new lesions. Furthermore, the total volume/lesions in ACA/MCA and MCA regions were larger in this group. These findings are conceivable as in healthy awake patients, anterior and middle cerebral arteries, which are the branches of internal carotid arteries, contribute about 70% of the brain’s blood supply. The remaining 30% of cerebral blood flow arises from two vertebral arteries, which supply the posterior cerebral circulation. Therefore, based on the blood flow distribution, two-third of all cardiogenic emboli would be expected in the anterior and middle circulation.[32] This problem can be exacerbated with the presence of anemia, in which there is a reduced blood flow to the brain. In other words, anemia could magnify the effects of microemboli by impairing their washout.
Moreover, the multivariable linear regression analysis revealed that NYHA class, anemia, and mean gradient were the independent predictors for the total volume/lesions in ACA/MCA zone. Meanwhile, anemia and valve types were the only independent predictors for the total volume/lesions in the MCA region. In a previous study,[33] a linear relationship was found between hemoglobin decrease and a larger final infarct volume and greater and faster infarct growth. The causal relationship is conceivable as anemia entails a decrease of oxygen-carrying capacity of blood, which might exert a negative effect on penumbra evolution.[34] In addition, with the presence of worse NYHA class, it means that the heart will have less ability to compensate for procedural stress during TAVR. Meanwhile, a higher mean transaortic gradient usually reflects a more severe aortic valve stenosis, which is often accompanied by a higher degree of aortic valve calcification. Similarly, a study by Samim et al[6] revealed that only peak transaortic gradient was independently associated with post-procedural total infarct volume. Furthermore, with the majority of the patients having self-expandable valves, there are few possible mechanisms that can explain the association of valve types and volume/lesions in the MCA region. Firstly, the slow and stepwise implantation of self-expandable valves might have caused a higher number of HIA in DW-MRI. Secondly, the continuously expanding properties of self-expandable valves for approximately 10 days to best fit the surrounding anatomical structures might have caused the debris from the native valve or surrounding tissue to slough off. Lastly, the larger attachment area of self-expandable valves might impact not only the native valve and surrounding tissue, but also on the aortic root area, which can result in greater debris dissemination to the brain.[5]
Although the consequences of silent ischemic brain injuries are still uncertain, they have been associated with cognitive decline and increased risk of dementia and depression.[35,36] Therefore, any risk factors that can pose a higher risk to cerebral injury post TAVR should be clearly assessed prior to a TAVR procedure.
Study limitations
This single-center, non-randomized study had several limitations that warranted mention. First and foremost, this study involved only a small number of patients, and therefore needs to be confirmed with a larger randomized clinical trial. Inter-ethnic differences between Asian and other population might also played as a confounding factor.[37] Secondly, the risk strata of our cohort were heterogeneous, with a mixture of low-risk, intermediate-risk, and high-risk patients. In addition, analysis of the causes of anemia was not systematically performed, so a potentially correctable cause of anemia was not identified. Last but not least, this present study still requires longer follow-up to see the result of the negative impact of anemia on mortality, stroke, neurological, and cognitive function assessment.
CONCLUSIONS
Patients with anemia may have bigger total volume/lesions in the ACA/MCA and MCA regions compared to the non-anemic patients. Nonetheless, the incidence of new ischemic lesions as well as the number of new ischemic lesions is not statistically different between the two groups. An independent association is found between NYHA functional class, anemia and aortic mean gradient and the volume/lesions in the ACA/MCA region. However, only anemia and valve types are independently associated with the volume/lesions in the MCA zone. Whether the consequences of bigger total volume/lesions impact neurological and cognitive outcomes remains to be investigated.
Funding: This study was funded by Zhejiang Province Science and Technology Department Key R&D Program (2018C03084, 2021C03097).
Ethics approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine.
Conflicts of interests: The authors have no conflicts of interest to declare that are relevant to the content of this article.
Contributors: SN and QFZ contributed equally to the manuscript. XBL, JAW, and JBJ established the registry, conceived, and designed the study. SN and QFZ drafted the manuscript. CHL and JQF performed the MRI image analysis. SN, YMX, and QFZ analyzed data and interpreted the results. All authors edited and revised the manuscript. All authors read and approved the final manuscript. XBL and JAW are the study guarantors, responsible for overall content of the manuscript.
Reference
Burden of valvular heart diseases: a population-based study
DOI:10.1016/S0140-6736(06)69208-8 URL [Cited within: 1]
Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description
PMID:12473543
[Cited within: 1]

The design of a percutaneous implantable prosthetic heart valve has become an important area for investigation. A percutaneously implanted heart valve (PHV) composed of 3 bovine pericardial leaflets mounted within a balloon-expandable stent was developed. After ex vivo testing and animal implantation studies, the first human implantation was performed in a 57-year-old man with calcific aortic stenosis, cardiogenic shock, subacute leg ischemia, and other associated noncardiac diseases. Valve replacement had been declined for this patient, and balloon valvuloplasty had been performed with nonsustained results.With the use of an antegrade transseptal approach, the PHV was successfully implanted within the diseased native aortic valve, with accurate and stable PHV positioning, no impairment of the coronary artery blood flow or of the mitral valve function, and a mild paravalvular aortic regurgitation. Immediately and at 48 hours after implantation, valve function was excellent, resulting in marked hemodynamic improvement. Over a follow-up period of 4 months, the valvular function remained satisfactory as assessed by sequential transesophageal echocardiography, and there was no recurrence of heart failure. However, severe noncardiac complications occurred, including a progressive worsening of the leg ischemia, leading to leg amputation with lack of healing, infection, and death 17 weeks after PHV implantation.Nonsurgical implantation of a prosthetic heart valve can be successfully achieved with immediate and midterm hemodynamic and clinical improvement. After further device modifications, additional durability tests, and confirmatory clinical implantations, PHV might become an important therapeutic alternative for the treatment of selected patients with nonsurgical aortic stenosis.
2017 ESC/EACTS guidelines for the management of valvular heart disease
2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines
Self-expandable transcatheter aortic valve replacement is associated with frequent periprocedural stroke detected by diffusion-weighted magnetic resonance imaging
DOI:10.1016/j.jjcc.2019.01.013 URL [Cited within: 3]
Silent ischemic brain lesions after transcatheter aortic valve replacement: lesion distribution and predictors
DOI:10.1007/s00392-014-0798-8 URL [Cited within: 4]
Topographical distribution of perioperative cerebral infarction associated with transcatheter aortic valve implantation
DOI:10.1016/j.ahj.2017.12.008 URL [Cited within: 4]
Exploring the difference in post-procedural stroke rates between patients with aortic stenosis who undergo transcatheter aortic valve replacement versus surgical aortic valve replacement
Blood disorders in patients undergoing transcatheter aortic valve replacement: a review
DOI:10.1016/j.jcin.2018.09.041 URL [Cited within: 3]
Effect on outcomes and exercise performance of anemia in patients with aortic stenosis who underwent transcatheter aortic valve replacement
DOI:10.1016/j.amjcard.2014.11.033
PMID:25549880
[Cited within: 5]

The objectives of this study were to determine the causes and impact of anemia and hemoglobin level on functional status, physical performance, and quality of life in the preprocedural evaluation and follow-up of transcatheter aortic valve replacement (TAVR) candidates. A total of 438 patients who underwent TAVR were included. Anemia was defined as a hemoglobin level <12 g/dl in women and <13 g/dl in men. Before TAVR, anemia was encountered in 282 patients (64.4%). A potential treatable cause of anemia was detected in 90.4% of patients and was attributed to iron deficiency in 53% of them. The occurrence of anemia was an independent predictor of poorer performance in the 6-minute walk test (6MWT), a lower Duke Activity Status Index score, and Kansas City Cardiomyopathy Questionnaires overall, clinical, and social limitation scores (p <0.05 for all). A lower hemoglobin level was associated with a higher prevalence of New York Heart Association class III to IV (p <0.001) and correlated negatively with the results of all functional tests (p <0.02 for all). At follow-up, anemia was found in 62% of patients and was associated with poorer performance in the 6MWT (p = 0.023). A lower hemoglobin level after TAVR was a predictor of poorer New York Heart Association class (p = 0.020) and correlated negatively with the distance walked in the 6MWT (r = -0.191, p = 0.004) and Duke Activity Status Index score (r = -0.158, p = 0.011) at 6-month follow-up. In conclusion, anemia was very common in TAVR candidates and was attributed to iron deficiency in more than half of them. The presence of anemia and lower hemoglobin levels determined poorer functional status before and after the TAVR procedure. These results highlight the importance of implementing appropriate measures for the diagnosis and treatment of this frequent co-morbidity to improve both the accuracy of preprocedural evaluation and outcomes of TAVR candidates. Copyright © 2015 Elsevier Inc. All rights reserved.
Baseline anemia and its impact on midterm outcome after transcatheter aortic valve implantation
DOI:10.1002/ccd.26563 URL [Cited within: 4]
Prognostic impact of anemia and iron-deficiency anemia in a contemporary cohort of patients undergoing transcatheter aortic valve implantation
DOI:10.1016/j.ijcard.2017.06.024 URL [Cited within: 6]
Transcatheter aortic valve implantation: current and future approaches
DOI:10.1038/nrcardio.2011.164
PMID:22083020
[Cited within: 1]

The first human transcatheter aortic valve implantation (TAVI) in 2002, and several subsequent single-center series, showed the feasibility of this new approach for the treatment of patients with severe aortic stenosis who were considered to be at very high or prohibitive surgical risk. More-recent multicenter registries have confirmed the safety and efficacy of this procedure, despite a very-high-risk patient profile. Moreover, the randomized, controlled PARTNER trial has confirmed both the superiority of TAVI over medical treatment in patients not considered to be candidates for standard surgical aortic valve replacement and the noninferiority of TAVI compared with surgical aortic valve replacement in high-risk patients. The hemodynamics of transcatheter valves are usually excellent, although residual paravalvular aortic regurgitation (usually trivial or mild) is frequent. Stroke, major vascular complications, and conduction disturbances leading to permanent pacemaker implantation remain among the most-concerning periprocedural complications of TAVI. Nevertheless, promising preliminary data exist for long-term outcomes following TAVI, 'valve-in-valve' TAVI for surgical prosthesis dysfunction, and for the treatment of lower-risk patients. Improvements in transcatheter valve technology, optimization of procedural and midterm results, and confirmation of long-term durability of transcatheter valve prostheses will determine the expansion of TAVI towards the treatment of a broader spectrum of patients.
Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document
DOI:10.1016/j.jacc.2012.09.001
PMID:23036636
[Cited within: 1]

The aim of the current Valve Academic Research Consortium (VARC)-2 initiative was to revisit the selection and definitions of transcatheter aortic valve implantation (TAVI) clinical endpoints to make them more suitable to the present and future needs of clinical trials. In addition, this document is intended to expand the understanding of patient risk stratification and case selection.A recent study confirmed that VARC definitions have already been incorporated into clinical and research practice and represent a new standard for consistency in reporting clinical outcomes of patients with symptomatic severe aortic stenosis (AS) undergoing TAVI. However, as the clinical experience with this technology has matured and expanded, certain definitions have become unsuitable or ambiguous.Two in-person meetings (held in September 2011 in Washington, DC, USA, and in February 2012 in Rotterdam, the Netherlands) involving VARC study group members, independent experts (including surgeons, interventional and non-interventional cardiologists, imaging specialists, neurologists, geriatric specialists, and clinical trialists), the US Food and Drug Administration (FDA), and industry representatives, provided much of the substantive discussion from which this VARC-2 consensus manuscript was derived. This document provides an overview of risk assessment and patient stratification that need to be considered for accurate patient inclusion in studies. Working groups were assigned to define the following clinical endpoints: mortality, stroke, myocardial infarction, bleeding complications, acute kidney injury, vascular complications, conduction disturbances and arrhythmias, and a miscellaneous category including relevant complications not previously categorized. Furthermore, comprehensive echocardiography recommendations are provided for the evaluation of prosthetic valve (dys)function. Definitions for the quality of life assessments are also reported. These endpoints formed the basis for several recommended composite endpoints.This VARC-2 document has provided further standardization of endpoint definitions for studies evaluating the use of TAVI, which will lead to improved comparability and interpretability of the study results, supplying an increasingly growing body of evidence with respect to TAVI and/or surgical aortic valve replacement. This initiative and document can furthermore be used as a model during current endeavors of applying definitions to other transcatheter valve therapies (for example, mitral valve repair).Copyright © 2012 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Cerebral white matter lesion burden is associated with the degree of aortic valve calcification and predicts peri-procedural cerebrovascular events in patients undergoing transcatheter aortic valve implantation (TAVI)
DOI:10.1002/ccd.v91.4 URL [Cited within: 1]
Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery
DOI:10.1161/CIRCULATIONAHA.106.653501 URL [Cited within: 1]
Acquired hematological abnormalities in aortic stenosis
DOI:10.1177/1076029610375424 URL [Cited within: 1]
Incidence, causes, and predictors of early (≤30 days) and late unplanned hospital readmissions after transcatheter aortic valve replacement
DOI:10.1016/j.jcin.2015.07.022 URL [Cited within: 1]
Anemia and stroke: Where do we stand?
DOI:10.1111/ane.12657
PMID:27480069
[Cited within: 4]

Anemia seems to have a clear relationship with cerebrovascular events (CVEs), as there is a direct connection between central nervous system, blood supply, and tissue oxygen delivery. Anemia is considered a hyperkinetic state which disturbs endothelial adhesion molecule genes that may lead to thrombus formation. Furthermore, blood flow augmentation and turbulence may result in the migration of this thrombus, thus producing artery-to-artery embolism. It is for this reason that anemia is characterized as "the fifth cardiovascular risk factor." Anemia is consistently present in patients with acute stroke, ranging from 15% to 29%, while the mortality rate was significantly higher in patients suffering from anemia at the time of admission. Different types of anemia (sickle cell disease, beta thalassemia, iron deficiency anemia [IDA]) have been associated with increased cardiovascular and CVE risk. The relation between hemoglobin level and stroke would require further investigation. Unfortunately, treatment of anemia in cardiovascular and cerebrovascular disease still lacks clear targets and specific therapy has not developed. However, packed red blood cell transfusion is generally reserved for therapy in patients with CVEs. What is more, treatment of IDA prevents thrombosis and the occurrence of stroke; although iron levels should be checked, chronic administration favors thrombosis. Regarding erythropoietin (EPO), as there is lack of studies in anemic stroke patients, it would be desirable to utilize both neuroprotective and hematopoietic properties of EPO in anemic stroke patients. This review aims to clarify the poorly investigated and defined issues concerning the relation of anemia and CVEs.© 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Red blood cell transfusion increases cerebral oxygen delivery in anemic patients with subarachnoid hemorrhage
DOI:10.1161/STROKEAHA.109.556159 URL [Cited within: 1]
Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study
DOI:10.1016/S0140-6736(16)30506-2
PMID:27431356
[Cited within: 1]

Stroke is a leading cause of death and disability, especially in low-income and middle-income countries. We sought to quantify the importance of potentially modifiable risk factors for stroke in different regions of the world, and in key populations and primary pathological subtypes of stroke.We completed a standardised international case-control study in 32 countries in Asia, America, Europe, Australia, the Middle East, and Africa. Cases were patients with acute first stroke (within 5 days of symptom onset and 72 h of hospital admission). Controls were hospital-based or community-based individuals with no history of stroke, and were matched with cases, recruited in a 1:1 ratio, for age and sex. All participants completed a clinical assessment and were requested to provide blood and urine samples. Odds ratios (OR) and their population attributable risks (PARs) were calculated, with 99% confidence intervals.Between Jan 11, 2007, and Aug 8, 2015, 26 919 participants were recruited from 32 countries (13 447 cases [10 388 with ischaemic stroke and 3059 intracerebral haemorrhage] and 13 472 controls). Previous history of hypertension or blood pressure of 140/90 mm Hg or higher (OR 2·98, 99% CI 2·72-3·28; PAR 47·9%, 99% CI 45·1-50·6), regular physical activity (0·60, 0·52-0·70; 35·8%, 27·7-44·7), apolipoprotein (Apo)B/ApoA1 ratio (1·84, 1·65-2·06 for highest vs lowest tertile; 26·8%, 22·2-31·9 for top two tertiles vs lowest tertile), diet (0·60, 0·53-0·67 for highest vs lowest tertile of modified Alternative Healthy Eating Index [mAHEI]; 23·2%, 18·2-28·9 for lowest two tertiles vs highest tertile of mAHEI), waist-to-hip ratio (1·44, 1·27-1·64 for highest vs lowest tertile; 18·6%, 13·3-25·3 for top two tertiles vs lowest), psychosocial factors (2·20, 1·78-2·72; 17·4%, 13·1-22·6), current smoking (1·67, 1·49-1·87; 12·4%, 10·2-14·9), cardiac causes (3·17, 2·68-3·75; 9·1%, 8·0-10·2), alcohol consumption (2·09, 1·64-2·67 for high or heavy episodic intake vs never or former drinker; 5·8%, 3·4-9·7 for current alcohol drinker vs never or former drinker), and diabetes mellitus (1·16, 1·05-1·30; 3·9%, 1·9-7·6) were associated with all stroke. Collectively, these risk factors accounted for 90·7% of the PAR for all stroke worldwide (91·5% for ischaemic stroke, 87·1% for intracerebral haemorrhage), and were consistent across regions (ranging from 82·7% in Africa to 97·4% in southeast Asia), sex (90·6% in men and in women), and age groups (92·2% in patients aged ≤55 years, 90·0% in patients aged >55 years). We observed regional variations in the importance of individual risk factors, which were related to variations in the magnitude of ORs (rather than direction, which we observed for diet) and differences in prevalence of risk factors among regions. Hypertension was more associated with intracerebral haemorrhage than with ischaemic stroke, whereas current smoking, diabetes, apolipoproteins, and cardiac causes were more associated with ischaemic stroke (p<0·0001).Ten potentially modifiable risk factors are collectively associated with about 90% of the PAR of stroke in each major region of the world, among ethnic groups, in men and women, and in all ages. However, we found important regional variations in the relative importance of most individual risk factors for stroke, which could contribute to worldwide variations in frequency and case-mix of stroke. Our findings support developing both global and region-specific programmes to prevent stroke.Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Canadian Stroke Network, Health Research Board Ireland, Swedish Research Council, Swedish Heart and Lung Foundation, The Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland (Sweden), AstraZeneca, Boehringer Ingelheim (Canada), Pfizer (Canada), MSD, Chest, Heart and Stroke Scotland, and The Stroke Association, with support from The UK Stroke Research Network.Copyright © 2016 Elsevier Ltd. All rights reserved.
The influence of Anemia on clinical presentation and outcome of patients with first-ever atherosclerosis-related ischemic stroke
DOI:10.1016/j.jocn.2008.08.014
PMID:19285409
[Cited within: 1]

There is considerable debate regarding whether anemia qualifies as a prognostic factor for stroke. The purpose of this study was twofold: first, to assess the influence of anemia on vascular risk factors and clinical presentations in patients with first-ever atherosclerosis-related ischemic stroke and, second, to evaluate whether anemia may be of prognostic importance. A total of 774 consecutive patients with first-ever atherosclerosis-related ischemic stroke were prospectively investigated. Vascular risk factors, clinical presentations and outcomes were recorded and compared between those patients with and without anemia. Stroke recurrence and mortality were recorded at the 3-year follow-up. Of the study population, 168 (21.7%) were anemic. Multivariate analysis revealed that patients with anemia were more likely to be older than 70 years (p<0.001) and have chronic renal insufficiency (p<0.001). After a mean follow-up period of 958 days, 21 (12.5%) and 24 (4.0%) of the patients in the anemic and control groups, respectively, died. Within 3 years of initial onset, the mortality rate was significantly higher in patients with anemia (p=0.021). The Kaplan-Meier analysis for patients with and without anemia showed different survival curves (Log-rank test p<0.001). Within 3 years of the onset of first-ever atherosclerosis-related ischemic stroke, patients who had anemia at the time of the initial admission had an associated higher mortality rate. The stroke risk factors of being older than 70 years and having chronic renal insufficiency were more frequently observed in those patients with anemia.
Association between iron-deficiency anemia and stroke in young children
DOI:10.1542/peds.2007-0502 URL [Cited within: 1]
Iron deficiency anemia prevalence at first stroke or transient ischemic attack
DOI:10.1017/S0317167100013214 URL [Cited within: 3]
A systematic analysis of global anemia burden from 1990 to 2010
DOI:10.1182/blood-2013-06-508325
PMID:24297872
[Cited within: 1]

Previous studies of anemia epidemiology have been geographically limited with little detail about severity or etiology. Using publicly available data, we estimated mild, moderate, and severe anemia from 1990 to 2010 for 187 countries, both sexes, and 20 age groups. We then performed cause-specific attribution to 17 conditions using data from the Global Burden of Diseases, Injuries and Risk Factors (GBD) 2010 Study. Global anemia prevalence in 2010 was 32.9%, causing 68.36 (95% uncertainty interval [UI], 40.98 to 107.54) million years lived with disability (8.8% of total for all conditions [95% UI, 6.3% to 11.7%]). Prevalence dropped for both sexes from 1990 to 2010, although more for males. Prevalence in females was higher in most regions and age groups. South Asia and Central, West, and East sub-Saharan Africa had the highest burden, while East, Southeast, and South Asia saw the greatest reductions. Iron-deficiency anemia was the top cause globally, although 10 different conditions were among the top 3 in regional rankings. Malaria, schistosomiasis, and chronic kidney disease-related anemia were the only conditions to increase in prevalence. Hemoglobinopathies made significant contributions in most populations. Burden was highest in children under age 5, the only age groups with negative trends from 1990 to 2010.
Diffusion-weighted MRI determined cerebral embolic infarction following transcatheter aortic valve implantation: assessment of predictive risk factors and the relationship to subsequent health status
DOI:10.1136/heartjnl-2011-300065
PMID:21737581
[Cited within: 2]

'Silent' cerebral infarction and stroke are complications of transcatheter aortic valve implantation (TAVI).To assess the occurrence of cerebral infarction, identify predictive risk factors and examine the impact on patient health-related quality of life (HRQoL).Cerebral diffusion weighted MRI of 31 patients with aortic stenosis undergoing CoreValve TAVI was carried out. HRQoL was assessed at baseline and at 30 days by SF-12v2 and EQ5D questionnaires.New cerebral infarcts occurred in 24/31 patients (77%) and stroke in 2 (6%). Stroke was associated with a greater number and volume of cerebral infarcts. Age (r=0.37, p=0.042), severity of atheroma (arch and descending aorta; r=0.91, p<0.001, r=0.69, p=0.001, respectively) and catheterisation time (r=0.45, p=0.02) were predictors of the number of new cerebral infarcts. HRQoL improved overall: SF-12v2 physical component summary increased significantly (32.4±6.2 vs 36.5±7.2; p=0.03) with no significant change in mental component summary (43.5±11.7 vs. 43.1±14.3; p=0.85). The EQ5D score and Visual Analogue Scale showed no significant change (0.56±0.26 vs. 0.59±0.31; p=0.70, and 54.2±19 vs. 58.2±24; p=0.43).Multiple small cerebral infarcts occurred in 77% of patients with TAVI. The majority of infarcts were 'silent' with clinical stroke being associated with a both higher infarct number and volume. Increased age and the severity of aortic arch atheroma were independent risk factors for the development of new cerebral infarcts. Overall HRQoL improved and there was no association between the number of new cerebral infarcts and altered health status.
Magnetic resonance imaging evaluation of cerebral embolization during percutaneous aortic valve implantation: comparison of transfemoral and trans-apical approaches using Edwards Sapiens valve
DOI:10.1016/j.ejcts.2010.11.070
PMID:21256045
[Cited within: 2]

Cerebral embolization during trans-catheter aortic valve implantation (TAVI) has not been assessed clearly in the literature. Therefore, we compared the rate of cerebral embolisms with diffusion-weighted magnetic resonance imaging (DWI) in transfemoral (TF) and trans-apical (TA) approaches.Eighty patients benefited from TAVI between January 2008 and June 2010. Out of these, 35 were included in the study. Twenty-one were TF (group 1) and 14 TA (group 2). During the same period, 285 patients benefited from a conventional aortic valve surgery (aortic valve replacement (AVR)). Thirteen of these were also analyzed and considered as the control group (group 3). We systematically performed a DWI the day before the procedure and 48 h after. DWI studies were blindly analyzed by a neuroradiologist, and all patients had a clinical neurological assessment before and after the procedure, according the National Institutes of Health Stroke Scale (NIHSS).Thirty-two patients in the TAVI group had new cerebral lesions: 19 in the TF group and 13 in the trans-apical group (p=NS). Mean number of embolic lesions per patient was 6.6 in group I and 6.0 in group II (p=NS). Mean volume of embolic lesions was 475.0 mm³ in group I and 2170.5 mm³ in group II (p=NS). In group III, one patient had one new cerebral lesion (p<0.05 vs TAVI) of 36.5 mm³ (p=NS vs TAVI). All patients were neurologically asymptomatic.The incidence of silent cerebral embolic lesions after TAVI is significantly higher compared with the standard surgical AVR. The number of emboli is similar in the TF and TA groups but the volume tended to be higher in the TA group. However, there is no clinical impact of those lesions.Copyright © 2010 European Association for Cardio-Thoracic Surgery. Published by Elsevier B.V. All rights reserved.
The incidence and predictors of early- and mid-term clinically relevant neurological events after transcatheter aortic valve replacement in real-world patients
DOI:S0735-1097(15)02445-6
PMID:26184612
[Cited within: 1]

Transcatheter aortic valve replacement (TAVR) enables treatment of high-risk patients with symptomatic aortic stenosis without open-heart surgery; however, the benefits are mitigated by the potential for neurological events.This study sought to determine the timing and causes of clinically relevant neurological events after self-expandable TAVR.We enrolled 1,015 patients, of whom 996 underwent TAVR with a self-expandable system at 44 TAVR-experienced centers in Europe, Colombia, and Israel. Neurological events were evaluated for 3 distinct time periods: periprocedural (0 to 1 days post TAVR); early (2 to 30 days); and late (31 to 730 days). In this real-world study, neurological events were first referred to the site neurologist and then reviewed by an independent neurologist.The overall stroke rate was 1.4% through the first day post-procedure, 3.0% at 30 days, and 5.6% at 2 years. There were no significant predictors of periprocedural stroke or stroke/transient ischemic attack (TIA) combined. Significant predictors of early stroke were acute kidney injury (p = 0.03), major vascular complication (p = 0.04), and female sex (p = 0.04). For stroke/TIA combined, prior atrial fibrillation (p = 0.03) and major vascular complication (p = 0.009) were predictive. Coronary artery bypass graft surgery was the only significant predictor of late stroke (p = 0.007) or late stroke/TIA (p = 0.06).Treatment of high-risk patients with aortic stenosis using a self-expandable system was associated with a low stroke rate at short- and long-term follow-up. Multivariable predictors of clinically relevant neurological events differed on the basis of the timing after TAVR. (CoreValve Advance International Post Market Study; NCT01074658).Copyright © 2015 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI Randomized Clinical Trial
DOI:10.1001/jama.2016.10302 URL [Cited within: 1]
Blood flow distribution in cerebral arteries
DOI:10.1038/jcbfm.2014.241 URL [Cited within: 1]
Association of anemia and hemoglobin decrease during acute stroke treatment with infarct growth and clinical outcome
Loss of penumbra by impaired oxygen supply? Decreasing hemoglobin levels predict infarct growth after acute ischemic stroke: Stroke: Relevant Impact of Hemoglobin, Hematocrit and Transfusion (STRAIGHT) - an observational study
DOI:10.1159/000343731
PMID:23599701
[Cited within: 1]

The association of mortality and poor outcome with reduced levels of hemoglobin (Hb) and hematocrit (Hct) in patients admitted for ischemic stroke was recently demonstrated. The mechanisms behind this have remained unclear.Here, we aimed to investigate a putative association between low Hb and Hct levels and infarct growth.All consecutive patients who received intravenous thrombolysis based on multimodal magnetic resonance imaging during the years 1998-2009 were screened. Laboratory data as well as admission magnetic resonance images and follow-up computed tomography scans of 257 patients were assessed. Overall, data of 100 patients were of sufficient quality and further analyzed.Decrease in Hb and Hct as well as perfusion-weighted imaging volume, mismatch volume, and final infarct size on follow-up computed tomography were associated with infarct growth. A linear regression model revealed Hb decrease (β = 0.23, p = 0.02) to be a predictor of infarct growth, independent of mismatch volume (β = 0.27, p = 0.004) and minimum sodium (β = -0.21, p = 0.03), and adjusted to the non-predicting variables age, National Institute of Health Stroke Scale score, maximum leucocytes and C-reactive protein, blood glucose, and Hct decrease.Hb levels that decrease after admission independently predict infarct growth in thrombolyzed stroke patients. The clinical implications of this relationship remain to be investigated.
Silent brain infarcts and the risk of dementia and cognitive decline
DOI:10.1056/NEJMoa022066 URL [Cited within: 1]
Microembolism, silent brain infarcts and dementia
DOI:10.1016/j.jns.2012.02.021 URL [Cited within: 1]
Inter-ethnic differences in cardiovascular disease: impact on therapies and outcomes
DOI:10.1016/j.jacasi.2021.05.001 URL [Cited within: 1]