INTRODUCTION
Although modern cardiopulmonary resuscitation (CPR) and intensive care are continuously developing, after return of spontaneous circulation (ROSC), patients still experience a low discharge survival rate and poor prognosis. This is closely associated with whole-body ischemia and reperfusion injury (IRI) after resuscitation, followed by a systemic inflammatory response.[1] Strategies capable of limiting the high levels of pro-inflammatory mediators and thus improving outcomes after CPR are of great interest.
Vagus nerve stimulation (VNS) was approved for clinical use in refractory partial epileptic seizure, resistant depression, and heart failure.[2] Its therapeutic potential in other applications is still being explored. Brief VNS showed benefits to cardiac and cerebral IRI.[3,4] This shows that VNS has potentially beneficial effects on cardiovascular, cerebrovascular, metabolic, and other diseases associated with inflammation. In our previous study, right-sided VNS (RVNS) effectively prolonged survival after CPR and attenuated the inflammatory response to post-resuscitation syndrome via the cholinergic anti-inflammatory pathway (CAP).[5]
The left and right vagus nerves have asymmetric innervation of the heart, with the atrioventricular node predominantly influenced by the left vagus nerve, while the sinoatrial node is mainly influenced by the right vagus nerve, and this leads to the speculation that right-sided stimulation could induce more pronounced bradycardia or other cardiac responses.[6] Low-amplitude left-sided VNS (LVNS) markedly reduces ventricular dysfunction and infarct size during acute IRI without heart rate (HR) alteration, supporting that the advantage of VNS could be independent of HR changes.[7] Furthermore, autonomic regulation therapy with the use of chronic low-amplitude VNS, on either the left or the right side, is feasible and well tolerated in patients with heart failure (HF).[8] However, whether LVNS could achieve similar effects as RVNS post-CPR remains unknown. Therefore, we hypothesized that LVNS might be as therapeutically effective as RVNS on improving post-CPR outcomes.
METHODS
Experimental protocol
All 41 rats were randomly assigned to four groups: (1) sham group (n=5), the rats were submitted to surgical preparation without CPR or VNS; (2) CPR group (n=12), the rats underwent untreated cardiac arrest (CA) for six minutes followed by CPR and defibrillation only; (3) LVNS group (n=12), the left vagus nerve was electrically stimulated 30 minutes before CA; (4) RVNS group (n=12), the right vagus nerve was electrically stimulated 30 minutes before CA.
Animal models
The 14-week-old male Sprague-Dawley rats with an average weight of 350±20 g were supplied by the Experimental Animal Center of Hubei (Wuhan, China) (SCXK: 2015-0018). Prior to the experiment, the animals fasted for 12 hours with free access to water. The rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (45 mg/kg), administered an additional dose (10 mg/kg) per hour, and then underwent endotracheal intubation and mechanical ventilation (ALC-V8S; Shanghai Alcott Biotechnology, China). Furthermore, the left femoral artery and venous catheterizations were performed to monitor arterial pressure and provide intravenous access. A conventional lead II electrocardiogram (ECG) was recorded. The rectal temperature was maintained at 36.9±0.2 ˚C throughout the experiment.
The bilateral cervical parts of the vagus nerve were dissected and carefully isolated from the surrounding tissue. The left or right vagus nerve was hooked by a pair of platinum wire electrodes that were connected to the stimulation device of BL420F Biological Function Experiment System (Chengdu Taimeng Software, China). The stimulation parameters were set at a pulse width of 1.2 ms, a frequency of 4 Hz, an intensity of 6 V, and a duration of 30 minutes with crude voltage and continuous single stimulation.
A rat model of CA was established using modified percutaneous epicardial electrical stimulation to induce ventricular fibrillation (VF).[9] After CA for six minutes without any treatment, CPR was commenced. The frequency of the mechanical ventilation was adjusted to 100 breaths/minute, while the inhaled oxygen concentration was increased to 100%. The examiner performed manual chest compressions using two fingers at a frequency of 200 beats/minute. During compressions, the coronary artery perfusion pressure was maintained at 20-25 mmHg (1 mmHg=0.133 kPa). Adrenaline (20 μg/kg) and defibrillation (biphasic, 4J Zoll M-series defibrillator; Zoll Medical Systems, USA) were administered simultaneously every two minutes. ROSC was considered when the mean arterial pressure (MAP) ≥60 mmHg for 10 minutes during resuscitation. This study was approved by the Medical Ethics Committee of Huazhong University of Science and Technology (S2196), and the procedures complied with the animal care and use guidelines of the Ministry of Science and Technology of the People’s Republic of China.
Arrhythmia score
Ischemia-induced ventricular arrhythmia, including ventricular ectopic beat (VEB), ventricular tachycardia (VT), and VF, was determined according to the Lambeth Convention criteria,[10] and the severity of arrhythmia was quantified using a dedicated scoring system.[11] The arrhythmia scores were calculated based on the ECG within one hour after ROSC in each group.
Echocardiography
Echocardiography was performed at baseline and one hour after ROSC in each group. Images were obtained from the left parasternal short-axis view at the papillary muscle level using two-dimensional targeted M-mode tracing in an echocardiographic system equipped with an ultrasound probe (12S, Vivid E9; GE Healthcare, USA). At least three consecutive cardiac cycles were measured. The evaluation of myocardial function was presented using left ventricular ejection fraction (EF) and left ventricular fractional shortening (FS).
Hematoxylin and eosin (H&E) staining
The heart was harvested at 72 hours after ROSC and was divided into three equal parts perpendicular to its long axis, with the middle part being immersed in 4% paraformaldehyde overnight. The tissue was gradually dehydrated, and then transitioned to xylene for tissue transparency. Thereafter it was embedded in paraffin wax and then divided into sections of a thickness of 5 μm. After being dewaxed, rehydrated, stained, and dehydrated, the sections were transitioned to xylene for transparency and sealed with neutral gum.
Western blotting (WB)
Total protein was extracted from the remaining heart sample, and the concentration was measured by BCA assay kit based on the Lowry method (Beyotime Institute of Biotechnology, China). Antibodies against α-7 nicotinic acetylcholine receptor (α7nAchR) (1:500; sc-58607; Santa Cruz Biotechnology, Inc., USA) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:3,000; ANT012; Antgene, China) were used. A Bio-Rad gel imaging system was applied to obtain and analyze the chemiluminescent signals (Bio-Rad Laboratories, Inc., USA).
Myocardial tissue and serum tumor necrosis factor-alpha (TNF-α) assay
Blood samples were obtained at 1 hour (1 mL), 4 hours (1 mL), and 72 hours (5 mL) after ROSC, while myocardial tissues were collected at 72 hours after ROSC in each group. The levels of TNF-α in the supernatant of the myocardium homogenate and serum were measured using an enzyme-linked immunosorbent assay (ELISA) kit (RTA00; R&D Systems, Wiesbaden, Germany) according to the manufacturer’s instructions.
Immunofluorescence
The slices of myocardium were incubated with diluted primary antibody against α7nAchR (1:50; sc-58607; Santa Cruz Biotechnology, Inc., USA) and with tetramethyl rhodamine isothiocyanate (TRITC)-conjugated goat anti-rat IgG (1:50; SA00007-7; ProteinTech Group, Inc., USA). Further incubation with 4’,6-diamidino-2-phenylindole (DAPI) (1 µg/mL; AS1075; Aspen, China) was performed in the dark. The samples were observed under a fluorescence microscope (Olympus FV3000; Olympus Corporation, Japan) with ×400 magnification.
Statistical analysis
All data analyses were performed by SPSS (17.0, IBM Inc., USA). The measurements were presented as the mean±standard error of mean (SEM). The analysis of variance (ANOVA) was used followed by a post-hoc Tukey’s test for parametric data, and comparison between groups was performed using unpaired Student’s t-test. The differences of arrhythmia scores among the groups were analyzed by Kruskal-Wallis with Mann-Whitney U-test. The survival rates were calculated by Kaplan-Meier analysis and log-rank test. The P-value <0.05 was considered as a significant difference.
RESULTS
Effects of LVNS and RVNS on hemodynamic data, resuscitation, and survival rate
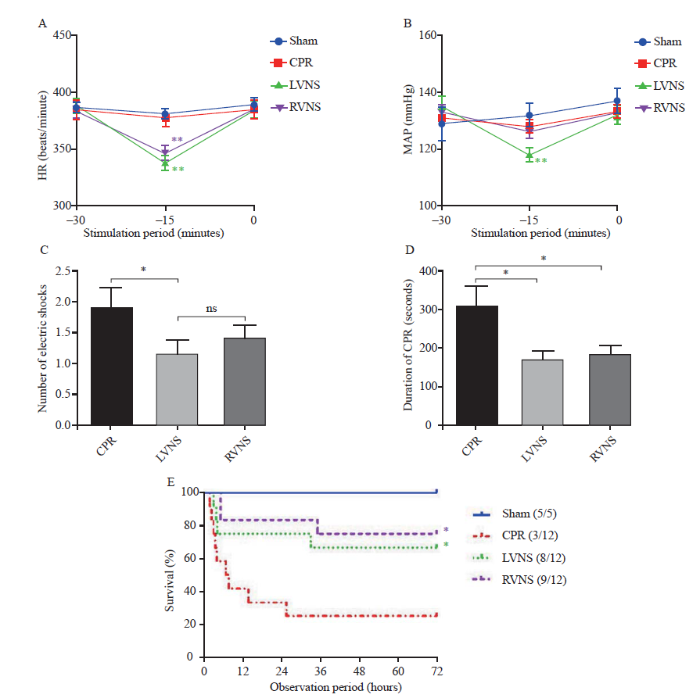
There were no differences in the basic physiological data (weight, basal HR, MAP, or rectal temperature [RT]) (Table 1). Both the LVNS and RVNS groups had a significant decrease in HR compared with that of their baseline status during VNS (Figure 1A). Concurrently, the MAP of the LVNS group decreased more significantly than that of the other groups (Figure 1B). Immediately upon terminating VNS, both HR and MAP in the VNS groups returned to the basal state, and there was no difference in hemodynamics among groups before the initiation of CA/CPR. There were fewer electrical defibrillation shocks (Figure 1C) and a shorter duration of CPR required to reach ROSC in the VNS groups compared with those in the CPR group, but no difference was found between the LVNS and RVNS groups (Figure 1D).
Table 1 Baseline characteristics
| Variables | Sham (n=5) | CPR (n=12) | LVNS (n=12) | RVNS (n=12) | P-value |
|---|---|---|---|---|---|
| Weight, g | 347±2 | 354±6 | 347±5 | 343±5 | 0.699 |
| HR, beats/minute | 387±5 | 385±8 | 388±7 | 383±8 | 0.966 |
| MAP, mmHg | 129±5 | 131±3 | 135±3 | 133±3 | 0.690 |
| RT, ℃ | 37.0 | 36.9 | 36.9 | 37.0 | 0.508 |
HR: heart rate; MAP: mean arterial blood pressure; RT: rectal temperature; CPR: cardiopulmonary resuscitation; LVNS: left-sided vagus nerve stimulation; RVNS: right-sided vagus nerve stimulation.
Figure 1.
Figure 1.
Effects of LVNS and RVNS on hemodynamic data. A: HR changes in each group during the VNS period (compared with sham, **P<0.01); B: MAP changes in each group during the VNS period (compared with baseline, **P<0.01); C: number of electric shocks in each group during resuscitation (*P<0.05); D: duration of CPR in each group (*P<0.05); E: survival rate curves in a 72-hour observation period of the sham, CPR, LVNS, and RVNS groups (compared with CPR, *P<0.05); ns: not significant; HR: heart rate; MAP: mean arterial pressure; VNS: vagus nerve stimulation; CPR: cardiopulmonary resuscitation; LVNS: left-sided vagus nerve stimulation; RVNS: right-sided vagus nerve stimulation.
There were no deaths in the sham group during the 72 hours (survival rate 100%). Improved survival rate was observed at 72 hours after ROSC in the VNS groups (LVNS 66.7%, RVNS 75.0%) compared with that of the CPR group (25.0%) (Figure 1E).
Effects of LVNS and RVNS on arrhythmia severity and cardiac function after CA/CPR
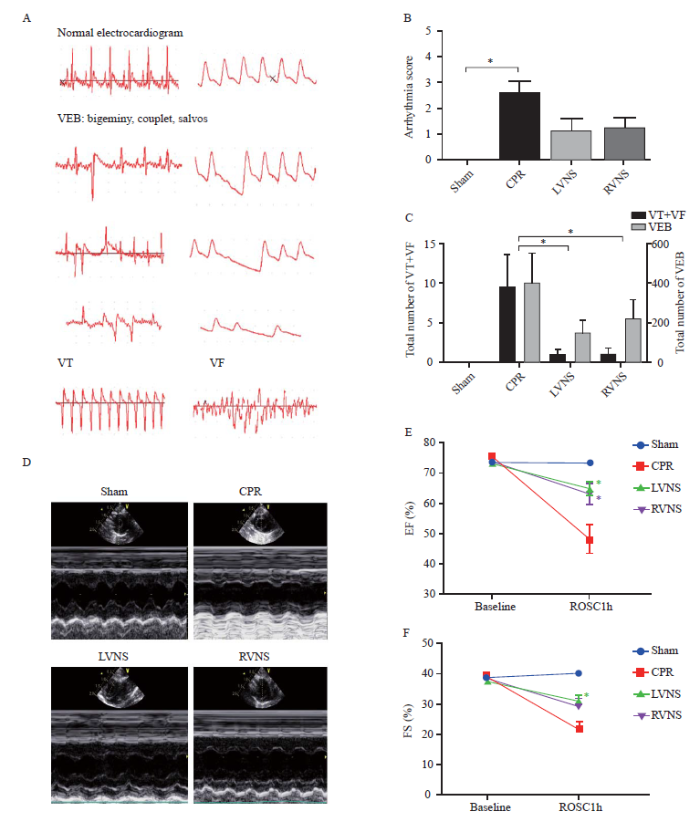
Representative tracings of VEB, VT, and VF are shown in Figure 2A. Compared with the sham group, the severity of ventricular arrhythmia was obviously aggravated in the CPR group. However, the arrhythmia scores were evidently lower in the LVNS and RVNS groups compared with those of the CPR group (Figure 2B). During the first 60 minutes after ROSC, the total number of VT+VF or VEB episodes was markedly decreased in the VNS groups compared with those in the CPR group (Figure 2C). Cardiac function, as measured by EF and FS, was impaired in all animals that had undergone CA at one hour after resuscitation. Pre-treatment with VNS markedly improved EF and FS compared with the CPR group (Figures 2E and 2F). However, there was no difference between the LVNS and RVNS groups.
Figure 2.
Figure 2.
Effects of LVNS and RVNS on arrhythmia severity and cardiac function after CA/CPR. A: representative instances of different types of ventricular arrhythmias during the first 60 minutes after ROSC; B: arrhythmia score during the first 60 minutes after ROSC in each group (compared with sham, *P<0.05); C: total number of VT+VF episodes and total number of VEB episodes during the first 60 minutes after ROSC in each group (*P<0.05); D: representative echocardiogram images obtained at one hour after ROSC; E: left ventricular EF of each group (compared with CPR, *P<0.05); F: left ventricular FS of each group (compared with CPR, *P<0.05); VEB: ventricular ectopic beat; VT: ventricular tachycardia; VF: ventricular fibrillation; CPR: cardiopulmonary resuscitation; LVNS: left-sided vagus nerve stimulation; RVNS: right-sided vagus nerve stimulation; ROSC: return of spontaneous circulation; EF: ejection fraction; FS: fractional shortening; CA: cardiac arrest.
Effects of LVNS and RVNS on myocardial inflammatory infiltration and TNF-α levels after CA/CPR
H&E staining revealed histopathological changes in the myocardial tissue of rats at 72 hours after ROSC. Myocardial cells were neatly arranged, the morphology was normal, and no neutrophil infiltration was found in the sham group, but disordered arrangement of the myocardium and obvious inflammatory cell infiltration in the muscles and perivascular tissues were observed in the CPR group. Treatment with either LVNS or RVNS protected hearts from structural damage and inflammatory infiltration caused by CA/CPR (supplementary Figure 1A). In order to explore whether LVNS could exert anti-inflammatory effects on rats exposed to CA/CPR similar to RVNS, the TNF-α levels were assessed by ELISA. LVNS and RVNS significantly reduced TNF-α levels in serum at 1 hour and 4 hours after ROSC, although there was no difference in serum TNF-α levels at 72 hours after ROSC among the groups (supplementary Figure 1B). Additionally, the TNF-α levels in myocardium homogenate at 72 hours after ROSC in the CPR group were higher than those in other groups (supplementary Figure 1C).
Effects of LVNS and RVNS on α7nAchR expression in myocardial tissue after CA/CPR
The expression of α7nAchR in myocardial tissue at 72 hours after ROSC was determined by immunofluorescence staining. Compared with the sham and CPR groups, a prominent upregulation of α7nAchR was observed in the VNS groups (supplementary Figure 2A). To further verify this tendency, WB was performed (supplementary Figure 2B). Consistent with the immunofluorescence results, the expression of α7nAchR was increased in the LVNS and RVNS groups to a similar degree (supplementary Figure 2C).
DISCUSSION
This study investigated the effects of LVNS on CA/CPR outcomes in rats, and compared its efficacy to that of RVNS. In this study, both LVNS and RVNS significantly improved survival rate, attenuated acute cardiac dysfunction, reduced the occurrence of malignant arrhythmia events after ROSC, mitigated inflammatory responses induced by IRI, increased the serum TNF-α levels, and elevated the expression of α7nAchR in myocardial tissue. However, there was no difference between the LVNS and RVNS groups.
A previous study noted that VNS exerted its cardiovascular protective properties mainly through the sinus node.[12] VNS-induced bradycardia is an important mechanism. A reduced HR could improve the oxygen supply and demand mismatch in a canine I/R model.[13] However, the reduced HR is not always necessary or is a prerequisite for VNS to play a cardioprotective role. In our study, compared with the observations in the sham group, both LVNS and RVNS groups experienced a decrease in HR during stimulation. Notably, the LVNS group had a more pronounced hemodynamic fluctuation, which was attributed to factors such as stimulation voltage, frequency, and pulse duration, as well as animal species and anatomical differences.[14]
The balance of the cardiac autonomic nervous system is crucial for preserving normal cardiac electrophysiology and function in cardiovascular diseases,[8] and vagal innervation protects against malignant ventricular arrhythmia.[15] Our results indicated that both LVNS and RVNS reduced the duration of CPR, alleviated arrhythmia severity, and improved heart function. One possible mechanism is that VNS may modulate sympathetic nervous activity. A study showed that VNS applied after the onset of VF improved defibrillation efficacy by decreasing the defibrillation threshold.[16] LVNS and RVNS have similar effects on ventricular activation recovery interval.[17] Both the right and left vagus nerves anatomically emit substantial projections to all chambers of the heart and are mediated by multiple ganglionated plexuses of the intrinsic cardiac nervous system (ICNS).[8] Due to the ICNS, the neuronal hierarchy aimed at cardiac control can maintain cardiac stability when faced with stress in an intact state.[18] The interactions may contribute to the result that no difference on defibrillation efficacy or arrhythmogenic properties between LVNS and RVNS.
The anti-inflammatory effect may be another possible protective mechanism of VNS.[5] After CA, a systemic inflammatory response occurs, a large number of circulating inflammatory cytokines were released, and one of them is TNF-α in the early phase post-ROSC in a swine model of CA.[19] This mediates IRI, where inflammatory activation can cause inflammatory cell infiltration and cytokine expression in the myocardium, thus resulting in myocardial damage.[20] Our results indicated that both LVNS and RVNS suppressed the expression of TNF-α after resuscitation, and inhibited inflammatory cell infiltration of the myocardium, as demonstrated by H&E staining.
To further explore the mechanism of the cardioprotective effects of VNS, we examined the expression of α7nAChR in myocardium tissue after CA/CPR, and found it to be higher in animals exposed to VNS. α7nAChR is an essential molecule in the CAP induced by VNS, which can regulate the level of cytokines during inflammation.[21] VNS improvement of cardiac function after resuscitation is dependent on α7nAChR expression, since the effect is eliminated when applying the α7nAChR antagonist methyllycaconitine citrate (MLA).[5] Consistent with a previous study, the upregulation of α7nAChR might be a compensatory response to IRI induced by CA/CPR.[22] The enhancement of α7nAChR expression induced by VNS might be one of the molecular mechanisms related to the cardioprotective effects of VNS against IRI after CA/CPR.
Finally, our results indicated that both LVNS and RVNS prolonged survival when compared with control animals. The favorable impacts of VNS are derived from various mechanisms, including restoring autonomic balance,[8] decreasing systemic inflammation,[5] improving coronary flow,[3] and exerting anti-apoptotic effects.[23] These mechanisms illustrate that VNS may play complex roles in improving the outcomes of CA/CPR. Although the survival rate at 72 hours in the RVNS group was slightly higher than that in the LVNS group, the data were not different enough to allow concluding that VNS applied to the right or left vagus nerve was more advantageous; thus, further evaluation is required.
CONCLUSIONS
The present results indicate that a significant improvement in outcomes after CPR can be induced by either LVNS or RVNS in rats. Furthermore, the protective effects of VNS are not different in terms of the laterality of the stimulation.
Funding: This work was supported by research grants from the National Natural Science Foundation of China (81571866, 82072137).
Ethical approval: The present study was approved by the Medical Ethics Committee of Huazhong University of Science and Technology (S2196).
Conflicts of interests: The authors declare that they have no competing interests.
Contributors: WJS, TTS, and SX contributed equally to this study. WJS, TTS, and SX conceived of the study, conducted the experiments, collected the data, and drafted the initial manuscript. LCL participated in the design of the study, while YC helped to perform the experiments. JMLG participated in the study design, contributed to refining ideas and helped editing the manuscript. YRZ and HH coordinated the data management, and participated in data acquisition and analysis. QZ and PS developed the study’s aims, interpreted the results and contributed to the completion of the manuscript. All authors have read and approved the final version of the manuscript.
Reference
Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome
DOI:10.1161/01.CIR.0000023891.80661.AD URL [Cited within: 1]
KenKnight BH. Electrical interaction between implantable vagus nerve stimulation device and implantable cardiac rhythm management device
DOI:10.1109/EMBC.2018.8513067
PMID:30441171
[Cited within: 1]

Autonomic regulation therapy via vagus nerve stimulation (VNS) was recently approved as a therapy for chronic heart failure, and will likely be utilized in patients who are also indicated for cardiac rhythm management device implantation. This study is designed to assess the degree to which VNS is likely to cause interference in the cardiac sensing of an implantable cardiac rhythm management device.A VNS stimulation lead and a cardiac sensing lead were placed in a simulated biological medium. A nonconductive carrier frame was used to position the leads at a precise electrode spacing. Stimulation was delivered through the VNS Therapy lead at a maximum output current and a variety of combinations of stimulation frequencies from 5-30 Hz and stimulation pulse widths from 130-1000 μs. The electrode spacing began at 0 cm and was increased in 1 cm increments until the measured signal dropped below the cardiac rhythm management device noise floor for sensing. The test was conducted with both bipolar and unipolar sensing.In the bipolar sensing configuration, the maximum sensed signal amplitude was 687 μV at an electrode separation of 0 cm, signal frequency of 30 Hz, pulse width of 1000 μs, and output current of 3.5 mA. In the unipolar sensing configuration, the maximum amplitude was 406 μV. In both configurations, the measured signal with maximum stimulation intensity decreased significantly with electrode separation, and dropped below the noise floor at an electrode spacing of 3.0 cm. The sensed signal amplitude was further attenuated at lower stimulation amplitudes and pulse widths.Even at maximum neural stimulation intensity of 3.5 mA, at an electrode separation of at least 3.0 cm, neural stimulation did not result in a detectable level of interference with either bipolar or unipolar sensing. Because this separation is significantly smaller than the minimum electrode separation of 15 cm in clinical practice, VNS Therapy is not expected to interfere with the function of implantable cardiac devices.
Subthreshold vagal nerve stimulation and the controversial findings regarding the anti-infarct effect against myocardial ischaemia-reperfusion injury
DOI:10.1113/EP086183 PMID:28247476 [Cited within: 2]
Nicotinic acetylcholine receptor alpha7 subunit mediates vagus nerve stimulation-induced neuroprotection in acute permanent cerebral ischemia by a7nachr/jak2 pathway
DOI:10.12659/MSM.907628 URL [Cited within: 1]
Improved outcomes of cardiopulmonary resuscitation in rats treated with vagus nerve stimulation and its potential mechanism
DOI:10.1097/SHK.0000000000000962 URL [Cited within: 4]
Electrophysiological-anatomic correlates of ATP-triggered vagal reflex in dogs
DOI:10.1152/ajpheart.1993.265.2.H681 URL [Cited within: 1]
Low-amplitude, left vagus nerve stimulation significantly attenuates ventricular dysfunction and infarct size through prevention of mitochondrial dysfunction during acute ischemia-reperfusion injury
DOI:10.1016/j.hrthm.2013.08.009
PMID:23933295
[Cited within: 1]

Right cervical vagus nerve stimulation (VNS) provides cardioprotective effects against acute ischemia-reperfusion injury in small animals. However, inconsistent findings have been reported.To determine whether low-amplitude, left cervical VNS applied either intermittently or continuously imparts cardioprotection against acute ischemia-reperfusion injury.Thirty-two isoflurane-anesthetized swine (25-30 kg) were randomized into 4 groups: control (sham operated, no VNS), continuous-VNS (C-VNS; 3.5 mA, 20 Hz), intermittent-VNS (I-VNS; continuously recurring cycles of 21-second ON, 30-second OFF), and I-VNS + atropine (1 mg/kg). Left cervical VNS was applied immediately after left anterior descending artery occlusion (60 minutes) and continued until the end of reperfusion (120 minutes). The ischemic and nonischemic myocardium was harvested for cardiac mitochondrial function assessment.VNS significantly reduced infarct size, improved ventricular function, decreased ventricular fibrillation episodes, and attenuated cardiac mitochondrial reactive oxygen species production, depolarization, and swelling, compared with the control group. However, I-VNS produced the most profound cardioprotective effects, particularly infarct size reduction and decreased ventricular fibrillation episodes, compared to both I-VNS + atropine and C-VNS. These beneficial effects of VNS were abolished by atropine.During ischemia-reperfusion injury, both C-VNS and I-VNS provide significant cardioprotective effects compared with I-VNS + atropine. These beneficial effects were abolished by muscarinic blockade, suggesting the importance of muscarinic receptor modulation during VNS. The protective effects of VNS could be due to its protection of mitochondrial function during ischemia-reperfusion.© 2013 Heart Rhythm Society. All rights reserved.
Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial
DOI:10.1016/j.cardfail.2014.08.009 URL [Cited within: 4]
Model of cardiac arrest in rats established by modified transcutaneous electrical stimulation on epicardium
The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion
PMID:3252968
[Cited within: 1]

The Lambeth Conventions are guidelines intended to be of practical value in the investigation of arrhythmias induced by ischaemia, infarction, and reperfusion. They cover the design and execution of experiments and the definition, classification, quantification, and analysis of arrhythmias. Investigators are encouraged to adopt the conventions in the hope that this will improve uniformity and interlaboratory comparisons.
Noradrenaline reduces ischemia-induced arrhythmia in anesthetized rats: involvement of alpha1-adrenoceptors and mitochondrial K ATP channels
DOI:10.1111/jce.2008.19.issue-3 URL [Cited within: 1]
Vagus nerve stimulation therapy for seizures
DOI:10.1097/ANA.0b013e31815b7df1 URL [Cited within: 1]
Intravenous electrical vagal nerve stimulation prior to coronary reperfusion in a canine ischemia-reperfusion model markedly reduces infarct size and prevents subsequent heart failure
DOI:10.1016/j.ijcard.2016.10.074 URL [Cited within: 1]
Differential hemodynamic and respiratory responses to right and left cervical vagal nerve stimulation in rats
DOI:10.14814/phy2.13244 URL [Cited within: 1]
Vagal modulation of cardiac ventricular arrhythmia
DOI:10.1113/eph.2014.99.issue-2 URL [Cited within: 1]
Application of vagus nerve stimulation from the onset of ventricular fibrillation to post-shock period improves defibrillation efficacy
DOI:10.1016/j.ijcard.2014.07.302 PMID:25156835 [Cited within: 1]
Electrophysiological effects of right and left vagal nerve stimulation on the ventricular myocardium
DOI:10.1152/ajpheart.00279.2014 URL [Cited within: 1]
Central-peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function
DOI:10.1152/ajpheart.00557.2015 URL [Cited within: 1]
Tumor necrosis factor-alpha is associated with early postresuscitation myocardial dysfunction
PMID:15286554
[Cited within: 1]

Left ventricular dysfunction after successful cardiopulmonary resuscitation contributes to early death following resuscitation. The stress-induced proinflammatory cytokines, particularly tumor necrosis factor-alpha and interleukin-1beta, are known to depress myocardial function. We hypothesized that tumor necrosis factor-alpha and interleukin-1beta, synthesized and released in response to the stress of global ischemia accompanying cardiac arrest, play a role in development of postresuscitation left ventricular dysfunction.Hemodynamic variables, tumor necrosis factor-alpha, interleukin-1beta, interleukin-6 (enzyme-linked immunosorbent assay method), and ionized calcium were measured in ten anesthetized swine before and after 7 mins of cardiac arrest and during the early postresuscitation period (60-90 mins).Tumor necrosis factor-alpha increased three-fold within 15 mins of restoration of circulation and remained elevated throughout the observation period. A significant negative correlation was observed between tumor necrosis factor-alpha and left ventricular systolic change in pressure over time (r = -.54, p <.001). Interleukin-1beta was undetectable before and after resuscitation, and interleukin-6 was detectable in only two animals after resuscitation. Although a significant decline in ionized calcium was observed and correlated with left ventricular systolic change in pressure over time, an independent role for ionized calcium in postresuscitation left ventricular dysfunction was not demonstrated.Tumor necrosis factor-alpha increases during the early postresuscitation period and may play a role in postresuscitation myocardial dysfunction.
The role of inflammatory cytokines in cardiac arrest
DOI:10.1177/0885066618817518 URL [Cited within: 1]
Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation
DOI:10.1038/nature01339 URL [Cited within: 1]
Alterations of muscarinic acetylcholine receptors-2, 4 and α7-nicotinic acetylcholine receptor expression after ischaemia / reperfusion in the rat isolated heart
DOI:10.1111/j.1440-1681.2010.05448.x URL [Cited within: 1]
miR-210 mediates vagus nerve stimulation-induced antioxidant stress and anti-apoptosis reactions following cerebral ischemia/reperfusion injury in rats
DOI:10.1111/jnc.13097
PMID:25783636
[Cited within: 1]

Vagus nerve stimulation (VNS) exerts neuroprotective effects against cerebral ischemia/reperfusion (I/R) injury and modulates redox status, potentially through the activity of miR-210, an important microRNA that is regulated by hypoxia-inducible factor and Akt-dependent pathways. The aim of this study was to determine the mechanisms of VNS- and miR-210-mediated hypoxic tolerance. Male Sprague-Dawley rats were preconditioned with a miR-210 antagomir (A) or with an antagomir control (AC), followed by middle cerebral artery occlusion and VNS treatment. The animals were divided into eight groups: sham I/R, I/R, I/R+AC, I/R+A, sham I/R+VNS, I/R+VNS, I/R+VNS+AC, and I/R+VNS+A. Activation of the endogenous cholinergic a7 nicotinic acetylcholine receptor (a7nAchR) pathway was identified using double immunofluorescence staining. miR-210 expression was measured by PCR. Behavioral outcomes, infarct volume, and neuronal apoptosis were observed at 24 h following reperfusion. Markers of oxidative stress were detected using ELISA. Rats treated with VNS showed increased miR-210 expression as well as decreased apoptosis and antioxidant stress responses compared with the I/R group; these rats also showed increased p-Akt protein expression and significantly decreased levels of cleaved caspase 3 in the ischemic penumbra, as measured by western blot and immunofluorescence analyses, respectively. Strikingly, the beneficial effects of VNS were attenuated following miR-210 knockdown. In conclusion, our results indicate that miR-210 is a potential mediator of VNS-induced neuroprotection against I/R injury. Our study highlights the neuroprotective potential of VNS, which, to date, has been largely unexplored. Since approved by the FDA in 1997, vagus nerve stimulation (VNS) has proven to be a safe and effective treatment for refractory epilepsy and resistant depression. Recent studies have found that VNS also provided neuroprotective effects against ischemic injury in a rat stroke model. We showed that miR-210 played an important role in the antioxidant stress and anti-apoptosis responses induced by VNS. This is the first report showing the effects of VNS at the mRNA level. Therefore, VNS represents a promising candidate treatment for ischemic stroke patients. Schematic view of the role of miR210 mediated in the protective effects of the VNS on the acute cerebral ischemia. VNS acts to activate neuronal and astrocytes a7nAchR, inhibits the apoptosis and oxidant stress responses possibly associated with increased Akt phosphorylation and miR210 expression. © 2015 International Society for Neurochemistry.