INTRODUCTION
Acute respiratory distress syndrome (ARDS) represents an important public health problem worldwide, leading to a high mortality rate of approximately 40%.[1] ARDS is associated with excess inflammation contributing to increased endothelial and epithelial permeability and leading to the accumulation of protein-rich alveolar oedema fluid in the lung interstitium.[2] During the process of ARDS, immune effector cells have key activities in defence of the normal lung.
Eosinophils (EOSs) are key innate immune cells in host defence,[3] and they have been found to be associated with mortality in patients with chronic obstructive pulmonary disease (COPD)[4,5] and asthma.[6,7] The blood EOS counts are considered as a potential biomarker for identifying COPD patients at risk and as a reference for the usage of inhaled corticosteroids.[8] For ARDS, EOSs have been considered as an important immune response contributor, and they may be a diagnostic biomarker.[9,10] The accumulation of EOSs in ARDS patients was documented to be a prognostic indicator of patient survival.[11] Recently, a retrospective analysis of 112 patients with ARDS found that ARDS surviving patients have higher blood EOS counts than non-survivors and that EOSs may play a protective role in ARDS independent of corticosteroid use.[12] The prognosis of ARDS patients is closely related to factors such as tidal volume.[13,14] The relationship between blood EOSs and mortality in patients with ARDS needs to be further evaluated with a large sample size after full consideration of confounders.
The purpose of our study is to detect the relationship between blood EOSs and 28-day mortality in patients with ARDS after adjusting for possible confounding factors by Cox regression and propensity score matching (PSM). We also aim to investigate whether this relationship varies by corticosteroid use.
METHODS
Database introduction
Our data source was the Medical Information Mart for Intensive Care III (MIMIC-III, version 1.4), an open international database. The MIMIC-III database includes deidentified health-related data associated with over forty thousand patients who stayed in critical care units (ICUs) of the Beth Israel Deaconess Medical Center between 2001 and 2012. Data were extracted by the author HTC (certification number: 37147539).
Inclusion and exclusion criteria
Patients with ARDS who were 16 years or older, used mechanical ventilation during the ICU stay, and stayed in the ICU for at least 48 consecutive hours, were selected for inclusion. To screen the patients with ARDS accurately, the diagnostic information recorded in the MIMIC-III database and the Berlin criteria[15] were considered simultaneously, and the following condition was proposed: the onset of ARDS was acute, patients must have partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio (P/F) ratio ≤300 mmHg (1 mmHg=0.133 kPa) when positive end expiratory pressure (PEEP) was at least 5 cmH2O (1 cmH2O=0.098 kPa) and the free-text radiology reports mentioned bilateral opacities/infiltrates in the first 24 hours after ICU admission. The patients with COPD or asthma and patients without EOS data within the first 72 hours after ICU admission were excluded.
Data extraction
Structured query language (SQL) was used to extract the following data: age, sex, weight, body mass index (BMI), heart rate (HR), mean arterial pressure (MAP), P/F ratio, comorbidities (diabetes, sepsis), disease severity score (Simplified Acute Physiology Score II [SAPS II]), laboratory outcomes (white blood cell [WBC] count, red blood cell [RBC] count, platelet [PLT], blood lactate, pH, EOS count), mechanical ventilation (tidal volume), minute ventilation (L/minute), and drugs (corticosteroid, vasopressor, and antibiotics). The extracted data were obtained within 72 hours after ICU admission.
Grouping and definition
According to the cut-off value of 2%, the maximum value of EOS counts within 72 hours after ICU admission were used to divide the patients into EOS counts ≥2% and EOS counts <2% groups. ARDS severity was classified based on P/F ratio: 200 mmHg<P/F≤300 mmHg (mild), 100 mmHg<P/F≤200 mmHg (moderate), and P/F≤100 mmHg (severe). Corticosteroids can decrease blood EOS t least 50% at the first four hours after administration and then return to baseline within 24 hours.[16] Therefore, we excluded the patients who used corticosteroid 24 hours before the EOS count recording time. In the subgroup analysis, all patients were assigned to two subgroups based on the usage of any corticosteroid drugs except the external administration route within 72 hours after ICU admission, including dexamethasone, hydrocortisone, and methylprednisolone. Vasopressor included norepinephrine, epinephrine, dobutamine, dopamine, vasopressin, and phenylephrine. The mean tidal volume ≤6 mL/kg predicted body weight (PBW) within 72 hours after ICU admission was adopted to define adherence to the target of low tidal volume. The primary endpoint was the 28-day mortality, defined as death within 28 days from ICU admission. The secondary endpoints included ICU mortality, hospital mortality, length of ICU stay, and length of hospital stay. For patients with ICU stay more than one time, only the first ICU stay of the first hospitalization was considered.
Statistical analysis
Continuous variables were summarized as the mean±standard deviation or median (upper and lower quartiles) when appropriate, and categorical data were summarized as proportions. The characteristics of patients with ARDS were compared using Student’s t-test, Wilcoxon rank-sum test, and Chi-square test according to the distribution of the data. The Kaplan-Meier method and log-rank tests were used to compare 28-day mortality among the EOS counts ≥2% and EOS counts <2% groups. Cox regression models were used to assess the relationship between EOS counts and 28-day mortality. A backward stepwise method with P <0.05 was used to build the model. Sixteen potential confounders with a P-value <0.10 in the univariate analyses were included in the Cox regression models: age, BMI, weight, HR, P/F ratio, sepsis, ARDS severity, SAPS II, WBC, EOS counts, lactate, pH, tidal volume, minute ventilation, low tidal volume, and vasopressor use. The variance inflation factor (VIF) was used to test multicollinearity, and VIF≥10 indicated multicollinearity between variables. The proportional hazards assumption was tested using Schoenfeld residuals, with P<0.05 constituting evidence for non-proportionality. Subgroup analyses were also performed separately in patients who used corticosteroids and those who did not. PSM was used to balance the cofounders between the EOS counts ≥2% and EOS counts <2% groups. A multivariable logistic regression model was used to evaluate the propensity score by the variables that entered the Cox regression model and that were essential to ARDS prognosis (sepsis and low tidal volume). A 1:1 nearest-neighbour matching algorithm was used with a calliper of 0.05. All P-values were two-tailed, and P<0.05 was considered statistically significant. Statistical analyses were performed using STATA (Version 16; Stata Corp., College Station, TX, USA).
RESULTS
Characteristics of patients
A total of 2,567 patients were included, and the 28-day mortality rate was 24.19% (621/2,567). The baseline characteristics of enrolled patients are shown in Table 1.
Table 1 Comparisons of baseline characteristics between survivors and non-survivors
| Variables | Total (n=2,567) | Survivors (n=1,946) | Non-survivors (n=621) | P |
|---|---|---|---|---|
| Age (years) | 63.35 (51.39-75.98) | 62.14 (50.38-74.55) | 68.02 (55.20-79.44) | <0.001 |
| Male, n (%) | 1,479 (57.62) | 1,127 (57.91) | 352 (56.68) | 0.589 |
| Weight (kg) | 81.1 (68.0-97.1) | 82.3 (70.0-98.2) | 77.9 (64.1-92.3) | <0.001 |
| BMI (kg/m2) | 27.91 (24.13-32.91) | 28.23 (24.41-33.09) | 27.08 (22.79-31.98) | <0.001 |
| Heart rate (beats per minute) | 88.37 (78.89-98.47) | 88.05(78.96-97.69) | 89.68 (78.90-100.89) | 0.028 |
| MAP (mmHg) | 77.25 (71.92-84.17) | 77.79 (72.30-84.17) | 75.85 (70.74-84.51) | 0.073 |
| PaO2/FiO2 ratio | 237.83 (193.89-289.95) | 239.17 (194.67-291.77) | 232.50 (191.42-279.47) | <0.001 |
| Comorbidities, n (%) | ||||
| Diabetes | 728 (28.36) | 562 (28.88) | 166 (26.73) | 0.301 |
| Sepsis | 1,584 (61.71) | 1,165 (59.87) | 419 (67.47) | 0.001 |
| Severity of illness | ||||
| SAPS II | 44 (35-55) | 42 (33-52) | 53(44-64) | <0.001 |
| ARDS severity, n (%) | ||||
| Mild | 379 (14.76) | 305 (15.67) | 74 (11.92) | <0.001 |
| Moderate | 1,023 (39.85) | 816 (41.93) | 207 (33.33) | |
| Severe | 1,165 (45.38) | 825 (42.39) | 340 (54.75) | |
| Laboratory data | ||||
| WBC (×109/L) | 12.8 (8.8-18.1) | 12.5 (8.8-17.5) | 14.0 (8.9-19.4) | 0.003 |
| RBC (×109/L) | 3.58 (3.10-4.10) | 3.59 (3.11-4.11) | 3.54 (3.06-4.07) | 0.133 |
| PLT (×109/L) | 201 (139-276) | 202 (144-270) | 197 (118-294) | 0.056 |
| Lactate (mmol/L) | 2.1 (1.4-2.7) | 2.0 (1.3-2.5) | 2.5 (1.7-3.8) | <0.001 |
| EOS counts initial (×109/L) | 0.2 (0-1.0) | 0.3 (0-1.0) | 0.1 (0-0.6) | <0.001 |
| EOS counts minimum (×109/L) | 0.2 (0-0.9) | 0.2 (0-1.0) | 0.1 (0-0.4) | <0.001 |
| EOS counts maximum (×109/L) | 0.4 (0-1.2) | 0.5 (0.1-1.3) | 0.2 (0-1.0) | <0.001 |
| pH | 7.35 (7.28-7.42) | 7.36 (7.29-7.42) | 7.33 (7.26-7.41) | <0.001 |
| Mechanical ventilation | ||||
| Tidal volume (mL/kg PBW) | 6.65 (5.41-8.04) | 6.56 (5.35-7.95) | 6.85 (5.63-8.21) | 0.001 |
| Minute ventilation (L/min) | 9.8 (8.0-12.4) | 9.6 (7.9-12.0) | 10.4 (8.3-13.8) | <0.001 |
| Low tidal volume, n (%) | 1,167 (45.46) | 910 (46.76) | 257 (41.38) | 0.019 |
| Drug usage, n (%) | ||||
| Corticosteroid use | 436 (16.98) | 276 (14.18) | 160 (25.76) | <0.001 |
| Vasopressor use | ||||
| No vasopressor | 1,765 (68.76) | 1,442 (74.10) | 323 (52.01) | <0.001 |
| One vasopressor | 512 (19.95) | 338 (17.37) | 174 (28.02) | |
| Two vasopressors | 290 (11.30) | 166 (8.53) | 124 (19.97) | |
| Antibiotics use | 2,208 (86.01) | 1,677 (86.18) | 531 (85.51) | 0.308 |
Values are shown as the median (interquartile range) unless otherwise indicated; BMI: body mass index; MAP: mean arterial pressure; PaO2/FiO2: oxygen (PaO2)/fraction of inspired oxygen (FiO2); SAPS II: Simplified Acute Physiology Score II; WBC: white blood cell; RBC: red blood cell; PLT: platelet; pH: hydrogen ion concentration; EOS: eosinophil; PBW: predicted body weight.
Clinical outcomes
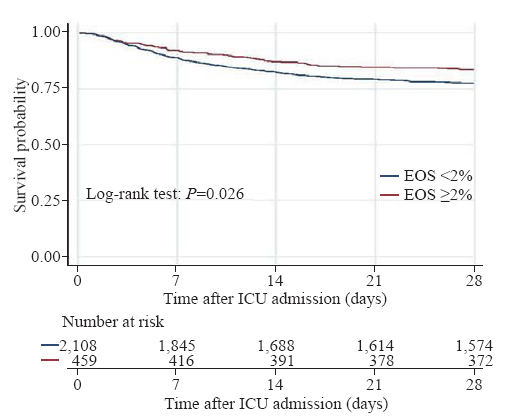
Without adjusting for covariates, the EOS counts ≥2% group had a significantly lower 28-day mortality rate, ICU mortality rate, and hospital mortality rate than the EOS counts <2% group (Table 2). In patients who did not use corticosteroids, the result was similar to the crude outcome, but this result was not observed in patients who used corticosteroids. The differences in the median length of ICU stay and length of hospital stay were not significant between the EOS counts ≥2% group and the EOS counts <2% group. Kaplan-Meier survival curves depicting the 28-day survival distributions of patients with EOS counts ≥2% or EOS counts <2% are presented in Figure 1, and the comparison between the two groups showed that patients with EOS counts ≥2% had a significantly higher survival rate (log-rank test, P=0.026).
Table 2 Comparisons of outcome characteristics between the EOS counts <2% and EOS counts ≥2% groups
| Variables | Total (n=2,567) | Patients who used corticosteroids (n=436) | Patients who did not use corticosteroids (n=2,131) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EOS counts <2% (n=2,110) | EOS counts ≥2% (n=457) | P | EOS counts <2% (n=389) | EOS counts ≥2% (n=47) | P | EOS counts <2% (n=1,721) | EOS counts ≥2% (n=410) | P | |
| 28-day mortality, n (%) | 536 (25.40) | 85 (18.60) | 0.002 | 139 (35.73) | 21 (44.68) | 0.229 | 397 (23.07) | 64 (15.61) | 0.001 |
| ICU mortality, n (%) | 448 (21.23) | 71 (15.54) | 0.006 | 125 (32.13) | 22 (46.81) | 0.144 | 323 (18.77) | 49 (11.95) | 0.001 |
| Hospital mortality, n (%) | 527 (24.98) | 81 (17.72) | 0.001 | 140 (35.99) | 21 (44.68) | 0.244 | 387 (22.49) | 60 (14.63) | <0.001 |
| Length of ICU stay (days) | 6.17 (3.67-11.92) | 6.21 (3.58-12.38) | 0.918 | 7.29 (4.33-14.13) | 7.33 (3.71-12.13) | 0.538 | 5.96 (3.46-11.33) | 6.08 (3.46-12.63) | 0.629 |
| Length of hospital stay (days) | 12.00 (7.25-20.25) | 11.92 (7.38-21.83) | 0.340 | 14.17 (8.25-23.83) | 12.21 (6.88-16.92) | 0.094 | 11.75 (7.16-19.00) | 11.89 (7.38-22.88) | 0.392 |
EOS: eosinophil; ICU: intensive care unit.
Figure 1.
Figure 1.
Kaplan-Meier survival curve of the study population
EOS: eosinophil; ICU: intensive care unit.
Relationship between EOS counts and 28-day mortality
To assess the relationship between EOS counts and 28-day mortality and to test whether the relationship varied by corticosteroid used, three models were developed using Cox regression analyses (Table 3). Model 1 used all patients in our study, and EOS counts ≥2% showed a significant association with a decreased 28-day mortality rate (hazard ratio [HR] 0.731; 95% confidence interval [CI] 0.581-0.921, P=0.008) after adjustment for SAPS II, lactate, minute ventilation, vasopressor use, ARDS severity, BMI, age, and P/F ratio. Model 2 used patients who did not use corticosteroids, and the results were similar to those in Model 1, with HR of 0.697 (95% CI 0.535-0.909, P=0.008). Model 3 included patients who used corticosteroids, and EOS counts were not included into the model because the P-value was 0.860. We also detected an interactive effect of EOS counts and corticosteroids on the 28-day mortality with an odd ratio of 2.585 (95% CI 1.444-4.627, P=0.001).
Table 3 Association between EOS counts and 28-day mortality
| Variables | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| EOS counts | 0.731 | 0.581-0.921 | 0.008 | 0.697 | 0.535-0.909 | 0.008 | / | / | / |
| SAPS II | 1.029 | 1.024-1.035 | <0.001 | 1.032 | 1.026-1.038 | <0.001 | 1.025 | 1.014-1.035 | <0.001 |
| Lactate | 1.110 | 1.081-1.140 | <0.001 | 1.111 | 1.077-1.147 | <0.001 | 1.131 | 1.072-1.195 | <0.001 |
| Minute ventilation | 1.033 | 1.011-1.055 | 0.003 | 1.036 | 1.010-1.062 | 0.005 | / | / | / |
| Vasopressor use | 1.288 | 1.159-1.431 | <0.001 | 1.368 | 1.208-1.548 | <0.001 | / | / | / |
| ARDS severity | 1.563 | 1.252-1.952 | <0.001 | 1.787 | 1.497-2.134 | <0.001 | / | / | / |
| BMI | 0.984 | 0.974-0.995 | 0.004 | 0.980 | 0.967-0.992 | 0.002 | / | / | / |
| Age | 1.002 | 1.001-1.004 | 0.003 | 1.002 | 1.001-1.004 | 0.003 | / | / | / |
| PaO2/FiO2 ratio | 0.998 | 0.996-0.999 | 0.018 | / | / | / | 0.991 | 0.988-0.993 | <0.001 |
Model 1 used all patients included in our study. The P-value of the proportional hazards assumption was 0.145, and the mean VIF=6.69. Model 2 used patients who did not use corticosteroids. The P-value of proportional hazards assumption was 0.166, and mean VIF=5.90. Model 3 used patients who used corticosteroids. The P-value of the proportional hazards assumption was 0.121, and the mean VIF=5.33. “/” indicates that the variable was not included into the model. EOS: eosinophil; SAPS II: Simplified Acute Physiology Score II; ARDS: acute respiratory distress syndrome; BMI: body mass index; PaO2/FiO2: oxygen (PaO2)/fraction of inspired oxygen (FiO2); VIF: variance inflation factor.
Outcomes after PSM
A total of 457 matched pairs were obtained after PSM. No significant difference was observed in any confounders between the two matched groups, indicating excellent matching among all pairs. Compared with the EOS counts <2% group, the EOS counts ≥2% group had significantly lower 28-day mortality (18.60% [85/457] vs. 26.70% [122/457], P=0.003), ICU mortality (15.54% [71/457] vs. 23.19% [106/457], P=0.003), and hospital mortality (17.72% [81/457] vs. 26.26% [120/457], P=0.002) after matching. The differences in the median length of ICU stay and length of hospital stay were not significant between the EOS counts ≥2% group and the EOS counts <2% group after matching.
DISCUSSION
In our large-sample study, we demonstrated that increased blood EOS counts were related to a significantly decreased risk of 28-day mortality after ICU admission in patients with ARDS. After adjustment for covariates, this result remained consistent in the PSM analysis. However, an interaction was observed between blood EOS counts and corticosteroid use. The relationship between blood EOSs and 28-day mortality was detected only in patients who did not use corticosteroid drugs, whereas this relationship was non-existent in patients who used corticosteroids.
The ARDS is related to innate immune response. Neutrophil-dependent lung injury is the key pathway. The inflammatory factors released from endothelial cells can recruit neutrophils and dramatically increase the number of neutrophils migrating to lungs.[17] Neutrophils may cause alveolar damage by forming extracellular traps in response to endothelial injury and histone release and further lead to multiple organ failure or death.[18] Recently, Zhu et al[12] found that EOSs can be grouped into CD101+ and CD101- subtypes by the CD101 marker. CD101+ EOSs may play a pro-inflammatory role by overexpressing alarmins. CD101- EOSs, the EOS subtype mostly elevated in patients with ARDS, might play a protective role in the inflammatory process by preventing neutrophil recruitment and stimulating clean-up of neutrophil debris through the production of protectin D1. Our study suggests that EOSs play a possible protective role in ARDS patients, which has rarely been demonstrated previously.
Corticosteroids may improve oxygenation and shorten mechanical ventilation times in ARDS.[19] However, no consistent result has been reported regarding whether corticosteroids should be routinely used in ARDS patients. Meduri et al[20] found that methylprednisolone can significantly improve pulmonary and extrapulmonary organ dysfunction in ARDS patients and reduce ICU mortality by downregulating systemic inflammation. Guidelines for corticosteroid insufficiency (CIRCI) also suggest that corticosteroids should be used in early moderate-to-severe ARDS.[21] However, in a randomized controlled trial including 180 patients with ARDS, no benefit of corticosteroids was found in hospital survival; moreover, using methylprednisolone two weeks after the onset of ARDS can significantly increase the 60-day and 180-day mortality rates.[22] A similar result[23] was also detected in patients with sepsis-associated ARDS. In our study, 28-day non-survivors had a higher ratio of corticosteroid use when compared with survivors, and the relationship between EOS counts and 28-day mortality was non-existent in patients who used corticosteroids; this suggests that the potential protective role of EOSs can be counteracted by corticosteroid use. Although corticosteroids improve clinical symptoms to some extent, the clinical use of corticosteroids in ARDS should be considered with caution, taking into account both the negative effects and the use time.
The large sample size from the MIMIC III database was our study strength, and it allowed a more in-depth analysis under full consideration of confounding variables and ensured robust results; however, the study also has limitations. First, patients in our study were divided based on the maximum value of blood EOS counts within 72 hours after ICU admission. However, the EOS fluctuation and variation tendency may also affect patients’ prognoses, and this needs further study. Second, the best cut-off value of EOSs has yet to be determined. A cut-off value of 2% has been used in a previous study of COPD;[24] therefore, we used 2% for our group standard, but this cannot avoid related bias. Third, there were many important data missed in the MIMIC-III database. Inflammatory markers, such as C-reaction protein, are important indicators of prognosis for ARDS patients. However, the proportion of missed C-reaction protein data was higher than 20%, and thus we did not include it in this study. Fourth, the subgroup analysis was conducted only according to whether corticosteroids were used within 24 hours before ICU admission to 72 hours after. Whether the dose of corticosteroids and the time courses of the corticosteroid treatment affect the relationship between EOS counts and the outcome of patients with ARDS needs to be explored in future studies. Finally, the present study was a retrospective study which only allowed us to deduce the relationships between the blood EOS counts, corticosteroids, and mortality, and a definite causal relationship cannot be established. Further studies, such as randomized controlled trials (RCTs), are needed to verify this relationship.
CONCLUSIONS
Higher EOS counts are related to lower mortality in patients with ARDS. This relationship is not influenced by confounders, such as the characteristics of mechanical ventilation or the disease severity. However, this result is significant only in patients who do not use corticosteroids. To definitively assess the protective role of blood EOS counts in ARDS, larger RCTs are needed.
Funding: None.
Ethical approval: The right of this database was approved by the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA) and consent was obtained for the original data collection. Patients’ information in the MIMIC-III database was anonymized; therefore, informed consent was not required.
Conflicts of interests: The authors declare that they have no competing interests.
Contributors: HTC and JFX contributed equally to this study. YM conceived and designed the study. HTC extracted data and performed all statistical analyses together with JFX. XXH and NYZ were involved in drafting the manuscript and the interpretation of the data. YKW was also involved in interpretation of the data and made critical revisions to the discussion section. All the authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work regarding questions related to the accuracy or integrity of any part of the work.
Reference
Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries
DOI:10.1001/jama.2016.0291
URL
PMID:26903337
[Cited within: 1]

IMPORTANCE: Limited information exists about the epidemiology, recognition, management, and outcomes of patients with the acute respiratory distress syndrome (ARDS). OBJECTIVES: To evaluate intensive care unit (ICU) incidence and outcome of ARDS and to assess clinician recognition, ventilation management, and use of adjuncts-for example prone positioning-in routine clinical practice for patients fulfilling the ARDS Berlin Definition. DESIGN, SETTING, AND PARTICIPANTS: The Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure (LUNG SAFE) was an international, multicenter, prospective cohort study of patients undergoing invasive or noninvasive ventilation, conducted during 4 consecutive weeks in the winter of 2014 in a convenience sample of 459 ICUs from 50 countries across 5 continents. EXPOSURES: Acute respiratory distress syndrome. MAIN OUTCOMES AND MEASURES: The primary outcome was ICU incidence of ARDS. Secondary outcomes included assessment of clinician recognition of ARDS, the application of ventilatory management, the use of adjunctive interventions in routine clinical practice, and clinical outcomes from ARDS. RESULTS: Of 29,144 patients admitted to participating ICUs, 3022 (10.4%) fulfilled ARDS criteria. Of these, 2377 patients developed ARDS in the first 48 hours and whose respiratory failure was managed with invasive mechanical ventilation. The period prevalence of mild ARDS was 30.0% (95% CI, 28.2%-31.9%); of moderate ARDS, 46.6% (95% CI, 44.5%-48.6%); and of severe ARDS, 23.4% (95% CI, 21.7%-25.2%). ARDS represented 0.42 cases per ICU bed over 4 weeks and represented 10.4% (95% CI, 10.0%-10.7%) of ICU admissions and 23.4% of patients requiring mechanical ventilation. Clinical recognition of ARDS ranged from 51.3% (95% CI, 47.5%-55.0%) in mild to 78.5% (95% CI, 74.8%-81.8%) in severe ARDS. Less than two-thirds of patients with ARDS received a tidal volume 8 of mL/kg or less of predicted body weight. Plateau pressure was measured in 40.1% (95% CI, 38.2-42.1), whereas 82.6% (95% CI, 81.0%-84.1%) received a positive end-expository pressure (PEEP) of less than 12 cm H2O. Prone positioning was used in 16.3% (95% CI, 13.7%-19.2%) of patients with severe ARDS. Clinician recognition of ARDS was associated with higher PEEP, greater use of neuromuscular blockade, and prone positioning. Hospital mortality was 34.9% (95% CI, 31.4%-38.5%) for those with mild, 40.3% (95% CI, 37.4%-43.3%) for those with moderate, and 46.1% (95% CI, 41.9%-50.4%) for those with severe ARDS. CONCLUSIONS AND RELEVANCE: Among ICUs in 50 countries, the period prevalence of ARDS was 10.4% of ICU admissions. This syndrome appeared to be underrecognized and undertreated and associated with a high mortality rate. These findings indicate the potential for improvement in the management of patients with ARDS. TRIAL REGISTRATION: clinicaltrials.gov Identifier: NCT02010073.
Acute respiratory distress syndrome
DOI:10.1038/s41572-019-0069-0
URL
PMID:30872586
[Cited within: 1]

The acute respiratory distress syndrome (ARDS) is a common cause of respiratory failure in critically ill patients and is defined by the acute onset of noncardiogenic pulmonary oedema, hypoxaemia and the need for mechanical ventilation. ARDS occurs most often in the setting of pneumonia, sepsis, aspiration of gastric contents or severe trauma and is present in ~10% of all patients in intensive care units worldwide. Despite some improvements, mortality remains high at 30-40% in most studies. Pathological specimens from patients with ARDS frequently reveal diffuse alveolar damage, and laboratory studies have demonstrated both alveolar epithelial and lung endothelial injury, resulting in accumulation of protein-rich inflammatory oedematous fluid in the alveolar space. Diagnosis is based on consensus syndromic criteria, with modifications for under-resourced settings and in paediatric patients. Treatment focuses on lung-protective ventilation; no specific pharmacotherapies have been identified. Long-term outcomes of patients with ARDS are increasingly recognized as important research targets, as many patients survive ARDS only to have ongoing functional and/or psychological sequelae. Future directions include efforts to facilitate earlier recognition of ARDS, identifying responsive subsets of patients and ongoing efforts to understand fundamental mechanisms of lung injury to design specific treatments.
Lung-resident eosinophils represent a distinct regulatory eosinophil subset.
[J]DOI:10.1172/JCI85664 URL [Cited within: 1]
Peripheral blood eosinophil as a biomarker in outcomes of acute exacerbation of chronic obstructive pulmonary disease. Int
[J]
Blood eosinophil count as a prognostic biomarker in COPD. Int
[J]
The biomarkers suPAR and blood eosinophils are associated with hospital readmissions and mortality in asthma - a retrospective cohort study
DOI:10.1186/s12931-019-1234-4
URL
PMID:31730462
[Cited within: 1]

INTRODUCTION: Prognostic biomarkers in asthma are needed. The biomarker soluble urokinase plasminogen activator receptor (suPAR) has been associated with asthma control and with prognosis in acutely admitted medical patients. We investigated if suPAR and blood eosinophil counts at the time of admission for asthma are associated with readmission and mortality. METHODS: Our cohort comprised 1341 patients (median age 45.3, IQR 30.1-63.1) acutely admitted with a diagnosis of asthma to Hvidovre Hospital, Denmark (November 2013 to March 2017). Patients had suPAR and blood eosinophils measured at admission. Outcomes were 365-day readmission and all-cause mortality. Logistic regression analysis adjusted for age, sex, C-reactive protein, and Charlson comorbidity score was used to assess the association of the two biomarkers with readmission and all-cause mortality. RESULTS: Compared to event-free patients, patients who were either readmitted (n = 452, 42.3%) or died (n = 57, 5.3%) had significantly higher suPAR concentrations (p < 0.0001) and lower eosinophil counts (p = 0.0031) at admission. The highest odds of readmission or mortality were observed for patients in either the 4th suPAR quartile (p < 0.0001) or with eosinophil counts < 150 cells/muL at admission. Increasing levels of suPAR were associated with 365-day readmission (OR 1.3 [1.0-1.6]; p = 0.05) and mortality (OR 2.9 [1.7-5.1]; p = 0.0002). Eosinophil count > 300 cells/muL was significantly associated with lower odds of readmission (OR 0.64 [0.5-0.9]; p = 0.005) and lower mortality (OR 0.7 [0.6-0.9]; p = 0.0007). CONCLUSIONS: In patients acutely admitted with asthma, elevated suPAR concentrations together with blood eosinophil count < 150 cells/muL at the time of hospital admission were associated with both 365-day all-cause readmission and mortality.
Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study
DOI:10.1016/S2213-2600(15)00367-7
URL
PMID:26493938
[Cited within: 1]

BACKGROUND: Elevated sputum eosinophil counts predict asthma exacerbations and responsiveness to inhaled corticosteroids but are impractical to measure in primary care. We investigated the relation between blood eosinophil count and prospective annual asthma outcomes for a large UK cohort. METHODS: This historical cohort study used anonymised medical record data to identify primary care patients with asthma aged 12-80 years with 2 years of continuous records, including 1 year before (baseline) and 1 year after (outcome) their most recent eosinophil count. Negative binomial regression was used to compare outcome exacerbation rates and logistic regression to compare odds of asthma control for patients with blood eosinophil counts of 400 cells per muL or less versus greater than 400 cells per muL, adjusting for age, sex, body-mass index, smoking status, and Charlson comorbidity index. The study is registered at ClinicalTrials.gov, number NCT02140541. FINDINGS: Overall, 20 929 (16%) of 130 248 patients had blood eosinophil counts greater than 400 cells per muL. During the outcome year, these patients experienced significantly more severe exacerbations (adjusted rate ratio [RR] 1.42, 95% CI 1.36-1.47) and acute respiratory events (RR 1.28, 1.24-1.33) than those with counts of 400 cells per muL or less. They also had significantly lower odds of achieving overall asthma control (OR 0.74, 95% CI 0.72-0.77), defined as limited reliever use and no asthma-related hospital attendance or admission, acute course of oral corticosteroids, or prescription for antibiotics. Exacerbation rates increased progressively with nine ascending categories of blood eosinophil count as compared with a reference category of 200 cells per muL or less. INTERPRETATION: Patients with asthma and blood eosinophil counts greater than 400 cells per muL experience more severe exacerbations and have poorer asthma control. Furthermore, a count-response relation exists between blood eosinophil counts and asthma-related outcomes. Blood eosinophil counts could add predictive value to Global Initiative for Asthma control-based risk assessment. FUNDING: Teva Pharmaceuticals.
Global strategy for the diagnosis, management, prevention of COPD update 2019
Available at https://goldcopd.org/.
The predictive and discriminative value of biologically active products of eosinophils, neutrophils and complement in bronchoalveolar lavage and blood in patients with adult respiratory distress syndrome
DOI:10.1016/0300-9572(86)90116-4
URL
PMID:3027805
[Cited within: 1]

To determine whether biologically active products of eosinophils, neutrophils and complement contribute to the development of adult respiratory distress system (ARDS) we measured eosinophil cationic protein (ECP), lactoferrin (LF) and C3a in bronchoalveolar lavage (BAL) and blood by means of radioimmunoassays. Seventeen patients served as controls. Fifteen patients were studied before and after major surgery to evaluate the influence of the surgical procedure, and 12 patients with ARDS were investigated 4-12 h after the onset of the disease. Major surgery per se significantly increased ECP in BAL, LF in serum and C3a in BAL and plasma. ECP, LF and C3a levels in BAL and blood were all significantly higher in ARDS patients as compared with levels in controls and those observed after major surgery. The higher ECP levels in BAL were associated with the more severe ARDS as was also the case for C3a in BAL and plasma and LF in serum. One out of 15 patients subjected to major surgery developed ARDS postoperatively and had very high levels of ECP, LF and C3a in BAL and blood at sampling 3 h prior to onset of ARDS, and these levels were similar to those observed in ARDS patients. One out of 12 ARDS patients died from the disease and this patient had the highest level of ECP in BAL and serum. Our results strongly support the role of activated polymorphonuclears, and notably the activated eosinophils, in the pathogenesis of ARDS. Evidence is also presented that ECP can be used as a predictor of impending ARDS.
Signs of neutrophil and eosinophil activation in adult respiratory distress syndrome
DOI:10.1097/00003246-198401000-00004
URL
PMID:6690198
[Cited within: 1]

Circulating levels of lactoferrin, a specific granule protein of neutrophilic leukocytes, and eosinophil cationic protein (ECP), a specific granule protein of eosinophilic leukocytes, were serially measured in 19 patients at risk for adult respiratory distress syndrome (ARDS). Those patients who developed ARDS had significantly higher concentrations of both proteins than the patients without signs of ARDS. High ECP levels were observed in spite of peripheral eosinopenia. The lactoferrin levels were also increased in relation to circulating numbers of neutrophils. These findings are consistent with an enhanced turnover and/or activity of eosinophils and neutrophils in ARDS and thereby support other clinical and experimental observations suggesting a central pathophysiologic role for granulocytes in ARDS. No relation was found between ARDS or serum concentrations of lactoferrin or ECP and degree of complement consumption, suggesting that other mechanisms besides complement activation may underlie granulocyte activation in ARDS.
Immunodetection of occult eosinophils in lung tissue biopsies may help predict survival in acute lung injury
DOI:10.1186/1465-9921-12-116
URL
PMID:21871108
[Cited within: 1]

BACKGROUND: Acute lung injury (ALI) is a serious respiratory disorder for which therapy is primarily supportive once infection is excluded. Surgical lung biopsy may rule out other diagnoses, but has not been generally useful for therapy decisions or prognosis in this setting. Importantly, tissue and peripheral blood eosinophilia, the hallmarks of steroid-responsive acute eosinophilic pneumonia, are not commonly linked with ALI. We hypothesized that occult eosinophilic pneumonia may explain better outcomes for some patients with ALI. METHODS: Immunohistochemistry using a novel monoclonal antibody recognizing eosinophil peroxidase (EPX-mAb) was used to assess intrapulmonary eosinophil accumulation/degranulation. Lung biopsies from ALI patients (n = 20) were identified following review of a pathology database; 45% of which (i.e., 9/20) displayed classical diffuse alveolar damage (ALI-DAD). Controls were obtained from uninvolved tissue in patients undergoing lobectomy for lung cancer (n = 10). Serial biopsy sections were stained with hematoxylin and eosin (H&E) and subjected to EPX-mAb immunohistochemistry. RESULTS: EPX-mAb immunohistochemistry provided a >40-fold increased sensitivity to detect eosinophils in the lung relative to H&E stained sections. This increased sensitivity led to the identification of higher numbers of eosinophils in ALI patients compared with controls; differences using H&E staining alone were not significant. Clinical assessments showed that lung infiltrating eosinophil numbers were higher in ALI patients that survived hospitalization compared with non-survivors. A similar conclusion was reached quantifying eosinophil degranulation in each biopsy. CONCLUSION: The enhanced sensitivity of EPX-mAb immunohistochemistry uniquely identified eosinophil accumulation/degranulation in patients with ALI relative to controls. More importantly, this method was a prognostic indicator of patient survival. These observations suggest that EPX-mAb immunohistochemistry may represent a diagnostic biomarker identifying a subset of ALI patients with improved clinical outcomes.
Homeostatic and early-recruited CD101 eosinophils suppress endotoxin-induced acute lung injury
DOI:10.1183/13993003.02354-2019
URL
PMID:32527738
[Cited within: 2]

INTRODUCTION: Acute lung injury (ALI) is a fatal but undertreated condition with severe neutrophilic inflammation, although little is known about the functions of eosinophils in the pathogenesis of ALI. Our objectives were to investigate the roles and molecular mechanisms of eosinophils in ALI. METHODS: Pulmonary eosinophils were identified by flow cytometry. Mice with abundant or deficient eosinophils were used. Cellularity of eosinophils and neutrophils in bronchoalveolar lavage fluid, inflammatory assessment, and survival rate were determined. Human samples were also used for validating experimental results. RESULTS: Blood eosinophils were increased in surviving patients with acute respiratory distress syndrome (ARDS) independent of corticosteroid usage. There existed homeostatic eosinophils in lung parenchyma in mice and these homeostatic eosinophils, originating from the bone marrow, were predominantly CD101(-). More CD101(-) eosinophils could be recruited earlier than lipopolysaccharide (LPS)-initiated neutrophilic inflammation. Loss of eosinophils augmented LPS-induced pulmonary injury. Homeostatic CD101(-) eosinophils ameliorated, while allergic CD101(+) eosinophils exacerbated, the neutrophilic inflammation induced by LPS. Likewise, CD101 expression in eosinophils from ARDS patients did not differ from healthy subjects. Mechanistically, CD101(-) eosinophils exhibited higher levels of Alox15 and Protectin D1. Administration of Protectin D1 isomer attenuated the neutrophilic inflammation. CONCLUSIONS: Collectively, our findings identify an uncovered function of native CD101(-) eosinophils in suppressing neutrophilic lung inflammation and suggest a potential therapeutic target for ALI.
First tidal volume greater than 8 mL/kg is associated with increased mortality in complicated influenza infection with acute respiratory distress syndrome.
[J]
Interaction between low tidal volume ventilation strategy and severity of acute respiratory distress syndrome: a retrospective cohort study
DOI:10.1186/s13054-019-2530-6
URL
PMID:31300012
[Cited within: 1]

BACKGROUND: Although low tidal volume is strongly recommended for acute respiratory distress syndrome (ARDS), whether or not the benefit varies according to the severity of ARDS remains unclear. This study aimed to investigate whether or not there is an interaction between low tidal volume and severity of ARDS. METHODS: This was a secondary analysis from a randomized controlled trial. The patients were subgrouped according to whether the PaO2/FiO2 (P/F) was > 150 or 150 mmHg] and 491 in the low P/F subgroup [
Acute respiratory distress syndrome: the Berlin Definition
DOI:10.1001/jama.2012.5669
URL
PMID:22797452
[Cited within: 1]

The acute respiratory distress syndrome (ARDS) was defined in 1994 by the American-European Consensus Conference (AECC); since then, issues regarding the reliability and validity of this definition have emerged. Using a consensus process, a panel of experts convened in 2011 (an initiative of the European Society of Intensive Care Medicine endorsed by the American Thoracic Society and the Society of Critical Care Medicine) developed the Berlin Definition, focusing on feasibility, reliability, validity, and objective evaluation of its performance. A draft definition proposed 3 mutually exclusive categories of ARDS based on degree of hypoxemia: mild (200 mm Hg < PaO2/FIO2 /=10 cm H2O), and corrected expired volume per minute (>/=10 L/min). The draft Berlin Definition was empirically evaluated using patient-level meta-analysis of 4188 patients with ARDS from 4 multicenter clinical data sets and 269 patients with ARDS from 3 single-center data sets containing physiologic information. The 4 ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition. Using the Berlin Definition, stages of mild, moderate, and severe ARDS were associated with increased mortality (27%; 95% CI, 24%-30%; 32%; 95% CI, 29%-34%; and 45%; 95% CI, 42%-48%, respectively; P < .001) and increased median duration of mechanical ventilation in survivors (5 days; interquartile [IQR], 2-11; 7 days; IQR, 4-14; and 9 days; IQR, 5-17, respectively; P < .001). Compared with the AECC definition, the final Berlin Definition had better predictive validity for mortality, with an area under the receiver operating curve of 0.577 (95% CI, 0.561-0.593) vs 0.536 (95% CI, 0.520-0.553; P < .001). This updated and revised Berlin Definition for ARDS addresses a number of the limitations of the AECC definition. The approach of combining consensus discussions with empirical evaluation may serve as a model to create more accurate, evidence-based, critical illness syndrome definitions and to better inform clinical care, research, and health services planning.
Clinical studies on the activity of orally administered cortisone. N Engl
[J]
Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am
[J]DOI:10.1164/rccm.201603-0645OC URL [Cited within: 1]
Neutrophils in the activation and regulation of innate and adaptive immunity
DOI:10.1038/nri3024
URL
PMID:21785456
[Cited within: 1]

Neutrophils have long been viewed as the final effector cells of an acute inflammatory response, with a primary role in the clearance of extracellular pathogens. However, more recent evidence has extended the functions of these cells. The newly discovered repertoire of effector molecules in the neutrophil armamentarium includes a broad array of cytokines, extracellular traps and effector molecules of the humoral arm of the innate immune system. In addition, neutrophils are involved in the activation, regulation and effector functions of innate and adaptive immune cells. Accordingly, neutrophils have a crucial role in the pathogenesis of a broad range of diseases, including infections caused by intracellular pathogens, autoimmunity, chronic inflammation and cancer.
Corticosteroids for patients with acute respiratory distress syndrome: a systematic review and meta-analysis of randomized trials
DOI:10.20452/pamw.15239
URL
PMID:32186831
[Cited within: 1]

INTRODUCTION: Acute respiratory distress syndrome (ARDS) is a rapidly progressing, inflammatory lung disease with a high mortality rate and no specific pharmacological treatment available. OBJECTIVES: We conducted a systematic review and metaanalysis on corticosteroid use in ARDS. METHODS: We searched 4 medical literature databases and retained randomized controlled trials on the use of corticosteroids in hospitalized adults with ARDS, which could be found there until February 2020. Two reviewers identified eligible studies, independently extracted data, and evaluated the risk of bias. The authors assessed the certainty of evidence using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach. RESULTS: We included 7 randomized controlled trials involving 851 patients. They showed that corticosteroids reduced allcause mortality (risk ratio [RR], 0.75; 95% CI, 0.59-0.95; P = 0.02; moderate certainty) and the duration of mechanical ventilation (mean difference [MD], -4.93 days; 95% CI; -7.81 to -2.06; P <0.001; low certainty), and increased the number of ventilatorfree days (MD, 4.28 days; 95% CI, 2.67-5.88; P <0.001; moderate certainty), as compared with placebo. Corticosteroids also increased the risk of hyperglycemia (RR, 1.12%; 95% CI, 1.01-1.24; P = 0.03; moderate certainty), and the effect on neuromuscular weakness was unclear (RR, 1.3; 95% CI, 0.8-2.11; P = 0.28; low certainty). CONCLUSIONS: These results suggest that systemic corticosteroids may potentially improve mortality, shorten ventilation times, and increase the number of ventilatorfree days in patients with ARDS. However, the studies included different corticosteroid classes and initiated drug administration at different times, as well as used various dosing regimens. Thus, caution in the actual clinical application of these results is recommended.
Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial
DOI:10.1378/chest.06-2100
URL
PMID:17426195
[Cited within: 1]

OBJECTIVE: To determine the effects of low-dose prolonged methylprednisolone infusion on lung function in patients with early severe ARDS. DESIGN: Randomized, double-blind, placebo-controlled trial. SETTING: ICUs of five hospitals in Memphis. PARTICIPANTS: Ninety-one patients with severe early ARDS (
Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017
DOI:10.1007/s00134-017-4919-5
URL
PMID:28940011
[Cited within: 1]

OBJECTIVE: To update the 2008 consensus statements for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in adult and pediatric patients. PARTICIPANTS: A multispecialty task force of 16 international experts in Critical Care Medicine, endocrinology, and guideline methods, all of them members of the Society of Critical Care Medicine and/or the European Society of Intensive Care Medicine. DESIGN/METHODS: The recommendations were based on the summarized evidence from the 2008 document in addition to more recent findings from an updated systematic review of relevant studies from 2008 to 2017 and were formulated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology. The strength of each recommendation was classified as strong or conditional, and the quality of evidence was rated from high to very low based on factors including the individual study design, the risk of bias, the consistency of the results, and the directness and precision of the evidence. Recommendation approval required the agreement of at least 80% of the task force members. RESULTS: The task force was unable to reach agreement on a single test that can reliably diagnose CIRCI, although delta cortisol (change in baseline cortisol at 60 min of <9 microg/dl) after cosyntropin (250 microg) administration and a random plasma cortisol of <10 microg/dl may be used by clinicians. We suggest against using plasma free cortisol or salivary cortisol level over plasma total cortisol (conditional, very low quality of evidence). For treatment of specific conditions, we suggest using intravenous (IV) hydrocortisone <400 mg/day for >/=3 days at full dose in patients with septic shock that is not responsive to fluid and moderate- to high-dose vasopressor therapy (conditional, low quality of evidence). We suggest not using corticosteroids in adult patients with sepsis without shock (conditional recommendation, moderate quality of evidence). We suggest the use of IV methylprednisolone 1 mg/kg/day in patients with early moderate to severe acute respiratory distress syndrome (PaO2/FiO2 < 200 and within 14 days of onset) (conditional, moderate quality of evidence). Corticosteroids are not suggested for patients with major trauma (conditional, low quality of evidence). CONCLUSIONS: Evidence-based recommendations for the use of corticosteroids in critically ill patients with sepsis and septic shock, acute respiratory distress syndrome, and major trauma have been developed by a multispecialty task force.
Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl
[J]
Effects of fluid balance on prognosis of acute respiratory distress syndrome patients secondary to sepsis. World
[J]DOI:10.1046/j.1442-2026.1999.00072.x URL [Cited within: 1]
Eosinophils in COPD: just another biomarker?
DOI:10.1016/S2213-2600(17)30217-5
URL
PMID:28601554
[Cited within: 1]

Eosinophils are innate immune cells that, under certain conditions, can be recruited to the lungs, where they have an incompletely understood role in health and disease. Eosinophils have been found in the airways, tissues, and circulation of patients with COPD, during both stable disease and exacerbations. Epidemiological studies and post-hoc analyses of clinical trials of corticosteroid treatment for COPD have shown that the blood eosinophil count is associated with the risk of COPD exacerbations, mortality, decline in FEV1, and response to both inhaled and systemic corticosteroids. Further studies are urgently needed to explore the contribution of eosinophils to the mechanism of disease in COPD and to identify their association with levels of clinical risk. In this review, we explore the role of the eosinophil as a biomarker and mediator of disease in COPD.