Dear editor,
Henoch-Schönlein purpura (HSP) is the commonest vasculitis in children, typically affecting children aged three to ten years.[1] It is a multi-systemic vasculitis mediated by type III hypersensitivity with deposition of immunoglobulin. An immune complex-mediated vasculitis affects small vessels of the skin, joints, kidneys, and gastrointestinal (GI) tracts. HSP is usually a self-limiting condition and resolves within six to eight weeks.[1]
HSP tends to involve the GI tract and symptoms may occur before skin manifestations. Proximal small bowel and distal ileum are the sites that usually involved.[2,3] Colicky abdominal pain is the predominant GI manifestation and can be debilitating. Severe GI complications such as massive GI bleeding, intussusception, protein losing enteropathy, and pancreatitis can uncommonly occur.[2,4]
Imaging is often required in the evaluation of HSP with GI involvement. However, there is little pediatric literature on the role of point-of-care ultrasound (POCUS) by pediatric emergency physicians in the identification of pneumatosis intestinalis (PI) in HSP.
CASE
A five-year-old boy presented to the children’s emergency with severe abdominal pain and non-bilious, non-projectile vomiting of a day’s duration. He was previously hospitalized for HSP with GI involvement in the previous week and was discharged well on oral steroid therapy. The patient and his family verified that they were compliant to the oral steroids prescribed.
On examination, he was afebrile and was well-perfused with a stable blood pressure. His abdomen was soft and non-distended, and had no palpable masses. Palpable purpura was present on both lower limbs.
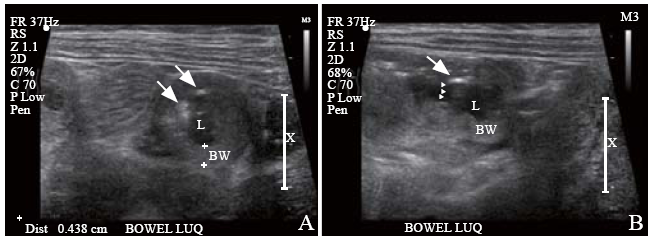
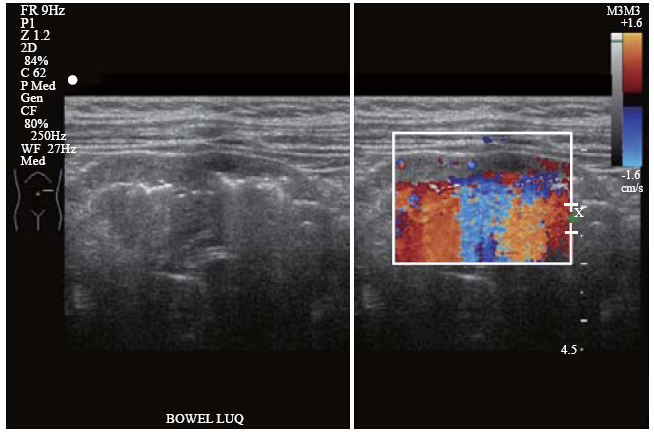
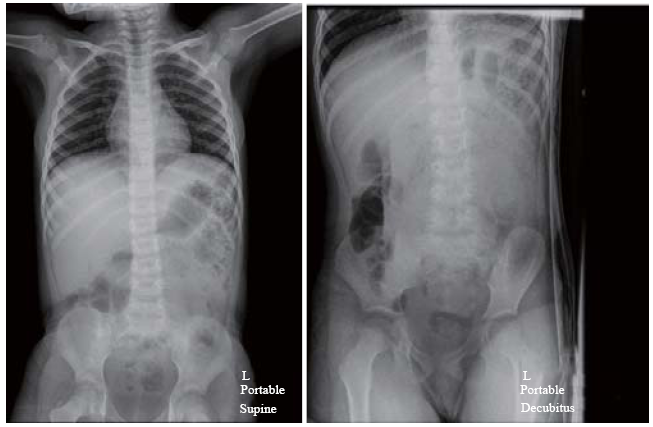
A pediatric emergency physician performed an abdominal POCUS to evaluate his abdominal pain and rule out intussusception. This, however, revealed dilated thickened bowel loops in the left upper quadrant of the abdomen. Detailed sonological assessment of the area revealed sub-serosal echogenic foci in the intestinal wall. This sub-serosal echogenic focus was suggestive of sonographic intramural air (Figure 1). A color Doppler study of the affected bowel loops also revealed significantly reduced blood flow (Figure 2). Supine and left lateral decubitus abdominal radiographs (X-rays) showed air-fluid levels and mottled shadowing over the left hemi-abdomen. The mottling was formally reported as possible fecal contents in the large bowel (Figure 3).
Figure 1.
Figure 1.
Left upper quadrant longitudinal (A) and transverse (B) abdominal ultrasound scans showing a loop of thickened bowel wall. Presence of intramural air was identified by sub-serosal echogenic foci (arrows), with posterior echogenic shadows (arrowheads); BW: bowel wall; L: lumen (bowel).
Figure 2.
Figure 2.
Left upper quadrant ultrasound scan showing loops of thickened bowel with pneumatosis intestinalis (sub-serosal echogenic foci) and color flow Doppler study demonstrating significantly reduced blood flow in the bowel wall. Color flow Doppler artefacts can be observed arising from the intramural air.
Figure 3.
Figure 3.
Abdominal X-rays (supine and decubitus) demonstrating mottled shadowing over the left hemi-abdomen which was formally reported to be suggestive of fecal masses.
Intravenous (IV) morphine was used to manage his severe abdominal pain. He was fasted and rehydrated with IV fluids. The IV ondansetron was given for his emesis.
Despite his hemodynamic stability, in view of the concerns of the severity of his gut ischemia with the finding of PI on bedside ultrasound, the child was admitted to the high dependency unit (HDU) for closer monitoring. Complete blood count showed marked leukocytosis with neutrophilia; the white blood cell count was 35.66×109/L (normal age-based range [5.00-14.50]×109/L) with 68% neutrophils and left shift (myelocytes 1%). His inflammatory indicators were not raised; C-reactive protein was 0.6 mg/L (normal range 0.0-5.0 mg/L), and erythrocyte sedimentation rate was 3 mm/hour (normal range 1-10 mm/hour). Coagulation studies, renal and liver function tests were normal. Empirical IV antibiotics (IV ceftriaxone and metronidazole) were started. Blood cultures and stool cultures were obtained (both negative). Subsequently, while being closely monitored in the HDU, the child had massive hematochezia within a few hours of admission. He had episodes of tachycardia and developed hypotension, requiring fluid resuscitation. He was then transferred to the intensive care unit (ICU). Inotropes were avoided in view of the concerns of the gut ischemia. He received 60 mL/kg of fluid resuscitation and blood product support in the ICU and was subsequently hemodynamically stable. His condition was significantly improved with IV steroids. A surgical referral was made but his pneumatosis was managed conservatively. Serial abdomen radiographs and formal radiology ultrasounds done to monitor the pneumatosis showed no progression. He was gradually improved and discharged home with oral steroids after eight days’ hospitalization.
DISCUSSION
There is an increased interest in using bedside ultrasound for early detection of intra-abdominal pediatric pathologies in the emergency department setting.[4] There is only one published pediatric case study on PI associated with HSP gut vasculitis.[5] This was detected by computed tomography (CT). There was an adult case report with HSP terminal ileitis and presented with symptoms mimicking appendicitis.[6] Pneumatosis was also detected on his abdominal CT. In our case study, we presented a case report on the early detection of PI in a child with HSP using bedside ultrasound in the emergency department.
About 15% of patients with PI may not have specific underlying etiology identified.[7,8] Recognized secondary causes of PI include necrotizing enterocolitis (in infants), congenital heart disease, instrumentation of GI tract, infectious colitis, ischemic colitis, inflammatory bowel disease, immunocompromised states, and chronic lung disease. It has also been rarely associated in patients with gut vasculitis such as primary amyloidosis, systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, and other autoimmune connective tissue diseases.[7,8]
PI is the presence of gas in the bowel wall. Classically, this is diagnostic of necrotizing enterocolitis in the neonatal population. Intramural gas can also be seen in intestinal ischemia which may eventually lead to bowel infarction. Traditionally, the diagnosis of PI can be made through abdominal X-rays or abdominal CT scans.[9] The presence of PI is not in itself a diagnosis but a radiographic finding with clinical significance. It can be due to wide range of abdominal and non-abdominal conditions.[9] In abdominal X-rays, PI will be shown as linear or transmural gas pattern. Abdominal X-rays can detect approximate two-thirds of the patients with PI. However, for our patient, the X-ray findings were inconclusive (Figure 3). Ultrasound may potentially be able to pick up early stages of pneumatosis before X-ray findings are apparent.
CT scans have often been performed to rapidly evaluate intra-abdominal pathologies associated with HSP.[10] However, radiation exposure to pediatric patients in CT scans needs to be considered due to their increased vulnerability, in keeping with the principles of as low as reasonably achievable (ALARA).[11] In our case study, with the early diagnosis, serial abdominal radiographs and formal ultrasound scans were used to monitor our patient’s condition without the need for CT scans. In addition, there are potential benefits of serial ultrasound examination instead of serial abdominal radiographs, especially in children and young adults, as this would avoid repeated radiation exposure completely.
The use of ultrasound can help in identifying intussusception, pneumoperitoneum, and bowel ischemia, which are known risk complications of HSP. Intramural air can be detected by ultrasound as echogenic foci within the circumference of the bowel (Figure 1). If they coalesce, they may cast “A-lines” posteriorly. If extensive, “circle sign”, a continuous echogenic ring[12] may be seen on ultrasound. PI may be better appreciated on ultrasound if the bowels are edematous and thickened.[13] It is relatively more difficult to detect sub-serosal air especially if intraluminal air is prominent.[6] Thus, both long-axis and short-axis views of the bowel are used to confirm the presence of intramural and sub-serosal air (Figure 1). For our patient, the edematous bowel loops were fluid filled and intramural air was easily appreciated.
The early detection of PI by bedside ultrasound in the emergency department allowed for risk-stratification of its severity of his condition despite lack of significant X-ray findings. Despite initial hemodynamic stability, the patient while closely monitored in HDU developed massive hematochezia and required ICU monitoring.
CONCLUSIONS
We present a case report on the use of bedside abdominal ultrasound by a pediatric emergency physician in identifying PI in a child with HSP. Ultrasound may provide for earlier identification of PI in HSP gut vasculitis and this finding may be of prognostic value. Further studies are required to evaluate the clinical value of this finding.
Funding: None.
Ethical approval: The study was approved by Central Institutional Review Board, SingHealth.
Conflicts of interest: The authors declare that there are no conflicts of interest regarding the publication of this paper.
Contributors: SWT, VJ, and AW prepared and drafted the manuscript, and revised the work for critically important intellectual content. GYKO conceived the study, obtained the images, contributed to the manuscript writing, and revised the work for critically important intellectual content. All authors read and approved the final manuscript.
Reference
Clinical practice: Diagnosis and management of Henoch-Schönlein purpura
URL PMID:20012647 [Cited within: 2]
Gastrointestinal manifestations and complications of Henoch-Schönlein purpura
URL PMID:15148994 [Cited within: 2]
Gastrointestinal manifestations of Henoch-Schönlein purpura
DOI:10.1007/s10620-007-0147-0
URL
PMID:18351468
[Cited within: 1]

Henoch-Schonlein Purpura (HSP) is the most common systemic vasculitis in childhood. The diagnostic criteria include palpable purpura with at least one other manifestation -- abdominal pain, IgA deposition, arthritis or arthralgia, or renal involvement. Immune complex deposits result in necrosis of the wall of small- and medium-sized arteries with infiltration of tissue by neutrophils and deposition of nuclear fragments, a process called leukocytoclastic vasculitis (LCV). It is often associated with infections, medications, or tumors. It may coexist with or mimic Crohn's disease. Periumbilical and epigastric pain worsens with meals, from bowel angina. Bleeding is usually occult or, less commonly, associated with melena. Intussusception, the most common surgical complication, is usually ileo-ileo or ileo-colic. Perforations, usually ileal, may occur spontaneously or be associated with intussusception. Ultrasound, recommended as the first diagnostic test, and CT scans may show intussusception and asymmetric bowel wall thickening mainly involving the jejunum and ileum. There are a range of endoscopic findings including gastritis, duodenitis, ulceration, and purpura, with the second portion of the duodenum characteristically being involved more than the bulb. Intestinal biopsies show IgA deposition and LCV in the submucosal vessels. Superficial biopsies may show inflammation, ulceration, edema, hemorrhage, and vascular congestion, presumably due to vasculitis-induced mucosal ischemia. The efficacy of corticosteroids in preventing severe complications or relapses is controversial. The majority of patients, however, improve spontaneously.
Pediatric emergency medicine point-of-care ultrasound: summary of the evidence
DOI:10.1186/s13089-016-0049-5
URL
PMID:27812885
[Cited within: 2]

The utility of point-of-care ultrasound is well supported by the medical literature. Consequently, pediatric emergency medicine providers have embraced this technology in everyday practice. Recently, the American Academy of Pediatrics published a policy statement endorsing the use of point-of-care ultrasound by pediatric emergency medicine providers. To date, there is no standard guideline for the practice of point-of-care ultrasound for this specialty. This document serves as an initial step in the detailed
Pneumatosis intestinalis associated with Henoch-Schönlein purpura
URL PMID:25157006 [Cited within: 1]
Terminal ileitis induced by Henoch-Schönlein purpura that presented as acute appendicitis: a case report
A systematic analysis of pneumatosis cystoids intestinalis
DOI:10.3748/wjg.v19.i30.4973
URL
PMID:23946603
[Cited within: 2]

AIM: To increase the understanding, diagnosis and treatment of pneumatosis cystoides intestinalis (PCI) and to find the characteristics and potential cause of the disease in China. METHODS: We report here one case of PCI in a 70-year-old male patient who received a variety of treatment methods. Then, we systematically searched the PCI eligible literature published from an available Chinese database from May 2002 to May 2012, including CBM, CBMDisc, CMCC, VIP, Wanfang, and CNKI. The key words were pneumatosis cystoides intestinalis, pneumatosis, pneumatosis intestinalis, pneumatosis coli and mucosal gas. The patients' information, histories, therapies, courses, and outcomes were reviewed. RESULTS: The study group consisted of 239 PCI cases (male:female = 2.4:1) from 77 reported incidents. The mean age was 45.3 +/- 15.6 years, and the median illness course was 6 mo. One hundred and sixty patients (66.9%) were in high altitude areas. In addition, 43.5% (104/239) of the patients had potential PCI-related disease, and 16.3% had complications with intestinal obstruction and perforation. The most common symptom was abdominal pain (53.9%), followed by diarrhea (53.0%), distention (42.4%), nausea and vomiting (14.3%), bloody stool (12.9%), mucous stool (12.0%) and constipation (7.8%). Most multiple pneumocysts developed in the submucosa of the colon (69.9%). The efficacy of the treatments by combined modalities, surgery, endoscopic treatment, conservative approach, oxygen, and antibiotics were 100%, 100%, 100%, 93.3%, 68.3% and 26.3%, respectively. CONCLUSION: PCI can be safely managed by conservative treatments, presents more frequently in males, in the large bowel and submucosa, than in females, in the small intestine and subserosa. High altitude residence maybe associated with the PCI etiology.
The spectrum of pneumatosis intestinalis
URL PMID:12511155 [Cited within: 2]
An approach to pneumatosis intestinalis: factors affecting your management
Gastrointestinal manifestations of Henoch-Schönlein purpura: a report of two cases
URL PMID:25825636 [Cited within: 1]
Clinical application of “Justification” and “Optimization” principle of ALARA in pediatric CT imaging: “How many children can be protected from unnecessary radiation?”
URL PMID:26072096 [Cited within: 1]
The “circle sign”: a new sonographic sign of pneumatosis intestinalis—clinical, pathologic and experimental findings
URL PMID:10398791 [Cited within: 1]
Pneumatosis intestinalis versus pseudo-pneumatosis: review of CT findings and differentiation
URL PMID:22347936 [Cited within: 1]