World Journal of Emergency Medicine ›› 2024, Vol. 15 ›› Issue (1): 28-34.doi: 10.5847/wjem.j.1920-8642.2024.014
• Original Article • Previous Articles Next Articles
Yangyang Guo1, Yaqi Sun2, Hongxia Wu3, Jian Lu4, Yuan Lin5, Jiaqing Zhu1, Meihong Lai1, Meiqi Zhang1, Jun Wang5( ), Jungang Zheng5,6,7(
), Jungang Zheng5,6,7( )
)
Received:2023-07-18
Accepted:2023-11-26
Online:2023-12-31
Published:2024-01-01
Contact:
Jun Wang,Jungang Zheng
E-mail:0430wangjun0430@163.com;b2018064@zju.edu.cn
Yangyang Guo, Yaqi Sun, Hongxia Wu, Jian Lu, Yuan Lin, Jiaqing Zhu, Meihong Lai, Meiqi Zhang, Jun Wang, Jungang Zheng. Protective effect and mechanism of nanoantimicrobial peptide ND-C14 against Streptococcus pneumoniae infection[J]. World Journal of Emergency Medicine, 2024, 15(1): 28-34.
Add to citation manager EndNote|Ris|BibTeX
URL: http://wjem.com.cn/EN/10.5847/wjem.j.1920-8642.2024.014

Figure 1.
Amino acid composition of ND-C14 and its bactericidal effects on S. pneumoniae. A: amino acid composition of natural HD5 and ND-C14 with three pairs of disulfide bonds (cysteines 1-6, cysteines 2-4, cysteines 3-5). B-D: bactericidal effects of HD5 and ND-C14 on S. pneumoniae in vitro; growth curve of S. pneumoniae after treatment with HD5 (B) and ND-C14 (C) ranging from 0.78 μg/mL to 25.00 μg/mL for 2 h; HD5 and ND-C14 completely inhibited the growth of S. pneumoniae at 6.25, 12.50, and 25.00 μg/mL; bacterial survival in the HD5 and ND-C14 groups (D). E: scanning electron microscopy images of S. pneumoniae 1 h after treatment with PBS, HD5 or ND-C14. Scale bars for 5,000× and 20,000× field images are 5 μm and 1 μm in length, respectively. Data from three independent experiments are shown as the mean±standard deviation. *P<0.05; ns: not significant.
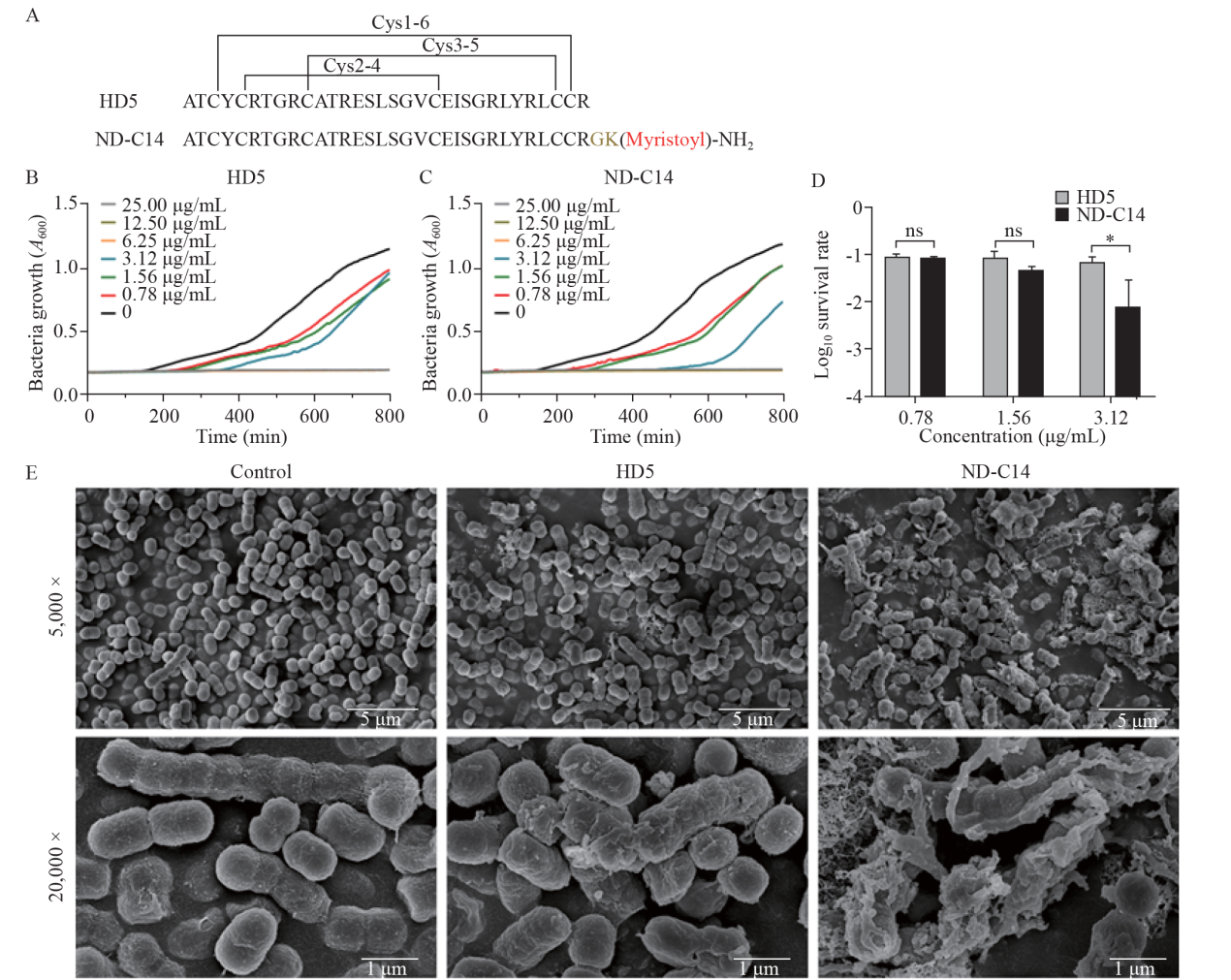
Figure 2.
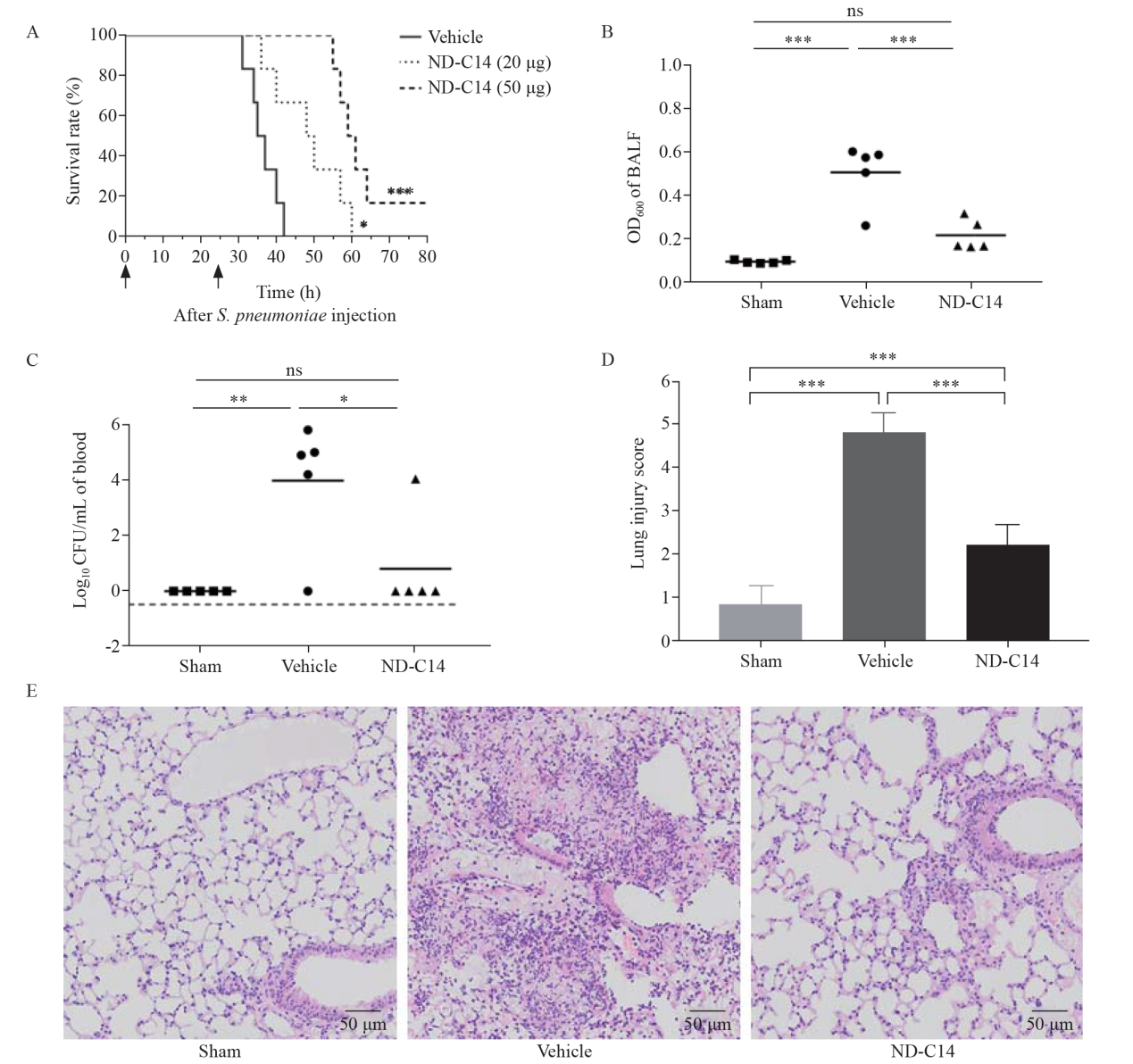
Protection of ND-C14 in mice infected with S. pneumoniae. A: survival analysis of S. pneumoniae pneumonia mice. Mice intratracheally injected with S. pneumoniae (5×106 CFU) and then administered vehicle (PBS) or ND-C14 (20 μg or 50 μg per mouse) (n=6). After 24 h, the mice were given the same dose again. The arrows indicates the time of administration of vehicle (PBS) or ND-C14. B, C: bacterial burden of infected mice (n=5); D, E (H&E staining): lung injury of infected mice (n=5). *P<0.05, **P<0.01, ***P<0.001; ns: not significant.
| 1 |
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 380(9859):2095-128.
doi: 10.1016/S0140-6736(12)61728-0 pmid: 23245604 |
| 2 |
Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009; 302(21):2323-9.
doi: 10.1001/jama.2009.1754 |
| 3 |
Wang X, Sun Y, Ni X, Zhang S. Development and validation of an emergency bloodstream infection score for predicting in-hospital mortality in patients with community-acquired bloodstream infections. World J Emerg Med. 2023; 14(4):280-286.
doi: 10.5847/wjem.j.1920-8642.2023.067 pmid: 37425085 |
| 4 |
Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021; 398(10300):622-37.
doi: 10.1016/S0140-6736(21)00439-6 pmid: 34217425 |
| 5 |
Engholm DH, Kilian M, Goodsell DS, Andersen ES, Kjærgaard RS. A visual review of the human pathogen Streptococcus pneumoniae. FEMS Microbiol Rev. 2017; 41(6):854-79.
doi: 10.1093/femsre/fux037 pmid: 29029129 |
| 6 |
Laxminarayan R, Matsoso P, Pant S, Brower C, Røttingen JA, Klugman K, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016; 387(10014):168-75.
doi: 10.1016/S0140-6736(15)00474-2 pmid: 26603918 |
| 7 |
Gan BH, Gaynord J, Rowe SM, Deingruber T, Spring DR. The multifaceted nature of antimicrobial peptides: current synthetic chemistry approaches and future directions. Chem Soc Rev. 2021; 50(13):7820-80.
doi: 10.1039/d0cs00729c pmid: 34042120 |
| 8 | Mhlongo JT, Waddad AY, Albericio F, de la Torre BG. Antimicrobial peptide synergies for fighting infectious diseases. Adv Sci (Weinh). 2023; 10(26):e2300472. |
| 9 |
Svenson J, Brandsdal BO, Stensen W, Svendsen JS. Albumin binding of short cationic antimicrobial micropeptides and its influence on the in vitro bactericidal effect. J Med Chem. 2007; 50(14):3334-9.
pmid: 17569519 |
| 10 |
Lai ZH, Yuan XJ, Chen HY, Zhu YH, Dong N, Shan AS. Strategies employed in the design of antimicrobial peptides with enhanced proteolytic stability. Biotechnol Adv. 2022; 59:107962.
doi: 10.1016/j.biotechadv.2022.107962 |
| 11 |
Gao X, Ding J, Liao C, Xu J, Liu X, Lu W. Defensins: the natural peptide antibiotic. Adv Drug Deliv Rev. 2021; 179:114008.
doi: 10.1016/j.addr.2021.114008 |
| 12 |
Lei R, Hou J, Chen Q, Yuan W, Cheng B, Sun Y, et al. Self-assembling myristoylated human α-defensin 5 as a next-generation nanobiotics potentiates therapeutic efficacy in bacterial infection. ACS Nano. 2018; 12(6):5284-96.
doi: 10.1021/acsnano.7b09109 pmid: 29856606 |
| 13 | Luo G, Zhang J, Wang HB, Sun YQ, Cheng BL, Xu ZP, et al. Human defensin-inspired discovery of peptidomimetic antibiotics. Proc Natl Acad Sci USA. 2022; 119(10):e2117283119. |
| 14 |
Raj VS, Barman TK, Kalia V, Purnapatre K, Dube S, Ramkumar G, et al. A novel ketolide, RBx 14255, with activity against multidrug-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 2014; 58(8):4283-9.
doi: 10.1128/AAC.01589-13 |
| 15 |
Hu ZH, Zhang CL, Sifuentes-Dominguez L, Zarek CM, Propheter DC, Kuang Z, et al. Small proline-rich protein 2A is a gut bactericidal protein deployed during helminth infection. Science. 2021; 374(6568):eabe6723.
doi: 10.1126/science.abe6723 |
| 16 |
Guha S, Ghimire J, Wu E, Wimley WC. Mechanistic landscape of membrane-permeabilizing peptides. Chem Rev. 2019; 119(9):6040-85.
doi: 10.1021/acs.chemrev.8b00520 pmid: 30624911 |
| 17 |
Chileveru HR, Lim SA, Chairatana P, Wommack AJ, Chiang IL, Nolan EM. Visualizing attack of Escherichia coli by the antimicrobial peptide human defensin 5. Biochemistry. 2015; 54(9):1767-77.
doi: 10.1021/bi501483q pmid: 25664683 |
| 18 |
Zhu Y, Hao W, Wang X, Ouyang J, Deng X, Yu H, et al. Antimicrobial peptides, conventional antibiotics, and their synergistic utility for the treatment of drug-resistant infections. Med Res Rev. 2022; 42(4):1377-422.
doi: 10.1002/med.21879 pmid: 34984699 |
| 19 |
Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018; 16(6):355-67.
doi: 10.1038/s41579-018-0001-8 pmid: 29599457 |
| 20 |
GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022; 400(10369):2221-48.
doi: 10.1016/S0140-6736(22)02185-7 pmid: 36423648 |
| 21 |
Martín-Cerezuela M, Aseginolaza-Lizarazu M, Boronat-García P, Asensio-Martín MJ, Alamán-Laguarda G, Álvarez-Lerma F, et al. Severe community-acquired Streptococcus pneumoniae bacterial meningitis: clinical and prognostic picture from the intensive care unit. Crit Care. 2023; 27(1):72.
doi: 10.1186/s13054-023-04347-3 |
| 22 |
Yu VL, Chiou CCC, Feldman C, Ortqvist A, Rello J, Morris AJ, et al. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin Infect Dis. 2003; 37(2):230-7.
doi: 10.1086/377534 |
| 23 |
van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009; 374(9700):1543-56.
doi: 10.1016/S0140-6736(09)61114-4 pmid: 19880020 |
| [1] | Catherine V. Levitt, Quincy K. Tran, Hashem Hraky, Maryann Mazer-Amirshahi, Ali Pourmand. Emergency department approach to monkeypox [J]. World Journal of Emergency Medicine, 2023, 14(5): 341-348. |
| [2] | Xinlei Wang, Yao Sun, Xiaoyu Ni, Shu Zhang. Development and validation of an emergency bloodstream infection score for predicting in-hospital mortality in patients with community-acquired bloodstream infections [J]. World Journal of Emergency Medicine, 2023, 14(4): 280-286. |
| [3] | Chun Zhao, Mei-yun Xin, Jing Li, Jin-fang Zhao, Yu-juan Wang, Wei Wang, Qian Gao, Jie Chen, Qi-wei Wang, You-peng Jin. Comparing the precision of the pSOFA and SIRS scores in predicting sepsis-related deaths among hospitalized children: a multi-center retrospective cohort study [J]. World Journal of Emergency Medicine, 2022, 13(4): 259-265. |
| [4] | Li-wei Duan, Jin-long Qu, Jian Wan, Yong-hua Xu, Yi Shan, Li-xue Wu, Jin-hao Zheng, Wei-wei Jiang, Qi-tong Chen, Yan Zhu, Jian Zhou, Wen-bo Yu, Lei Pei, Xi Song, Wen-fang Li, Zhao-fen Lin. Effects of viral infection and microbial diversity on patients with sepsis: A retrospective study based on metagenomic next-generation sequencing [J]. World Journal of Emergency Medicine, 2021, 12(1): 29-35. |
| [5] | Yi-wen Fan, Shao-wei Jiang, Jia-meng Chen, Hui-qi Wang, Dan Liu, Shu-ming Pan, Cheng-jin Gao. A pulmonary source of infection in patients with sepsis-associated acute kidney injury leads to a worse outcome and poor recovery of kidney function [J]. World Journal of Emergency Medicine, 2020, 11(1): 18-26. |
| [6] | Genevieve Mazza, Carina Mireles Romo, Marlene Torres, Ali Duffens, Annasha Vyas, Katherine Moran, Joshua Livingston, Savannah Gonzales, Shadi Lahham, Inna Shniter, Maxwell Thompson, John Christian Fox. Assessment of clinical dehydration using point of care ultrasound for pediatric patients in rural Panama [J]. World Journal of Emergency Medicine, 2019, 10(1): 46-50. |
| [7] | Chun Tat Lui. Prescription practice of antihistamines for acute upper respiratory tract infections in pediatric patients in a local emergency department in Hong Kong [J]. World Journal of Emergency Medicine, 2017, 8(1): 47-54. |
| [8] | Atahar Jamal, Naveen Sankhyan, Murlidharan Jayashree, Sunit Singhi, Pratibha Singhi. Full Outline of Unresponsiveness score and the Glasgow Coma Scale in prediction of pediatric coma [J]. World Journal of Emergency Medicine, 2017, 8(1): 55-60. |
| [9] | Tomoya Okazaki, Toru Hifumi, Arisa Manabe, Hikari Matsumura, Satoshi Egawa, Hideyuki Hamaya, Nastuyo Shinohara, Koshiro Takano, Hajime Shishido, Yuko Abe, Kenya Kawakita, Masanobu Hagiike, Yasuhiro Kuroda. Invasive group B streptococcal infection in a patient with post splenectomy for hypersplenism secondary to liver cirrhosis and portal hypertension [J]. World Journal of Emergency Medicine, 2016, 7(1): 68-70. |
| [10] | Maria Barsky, Lauren Kushner, MeganAnsbro, Kate Bowman, Michael Sassounian, Kevin Gustafson, Shadi Lahham, Linda Joseph, John C Fox. A feasibility study to determine if minimally trained medical students can identify markers of chronic parasitic infection using bedside ultrasound in rural Tanzania [J]. World Journal of Emergency Medicine, 2015, 6(4): 293-298. |
| [11] | Gholamreza S Roodsari, Farhad Zahedi, Shahriar Zehtabchi. The risk of wound infection after simple hand laceration [J]. World Journal of Emergency Medicine, 2015, 6(1): 44-47. |
| [12] | Bao-chi Liu, Lei Zhang, Jin-song Su, Andy Tsun, Bin Li. Treatment of postoperative infectious complications in patients with human immunodeficiency virus infection [J]. World Journal of Emergency Medicine, 2014, 5(2): 103-106. |
| [13] | Min Chen, Ri-jin Zhu, Feng Chen, Xiao-pin Wang, Jun Ke. Clinical analysis of central venous catheter-related infections in patients in the emergency ICU [J]. World Journal of Emergency Medicine, 2013, 4(3): 196-200. |
| [14] | He-chen Zhu, Ruo-lan Cao. The relationship between serum levels of uric acid and prognosis of infection in critically ill patients [J]. World Journal of Emergency Medicine, 2012, 3(3): 186-190. |
| [15] | Rong-rong Song, Yan-ping Qiu, Yong-ju Chen, Yong Ji. Application of fiberoptic bronchscopy in patients with acute exacerbations of chronic obstructive pulmonary disease during sequential weaning of invasive-noninvasive mechanical ventilation [J]. World Journal of Emergency Medicine, 2012, 3(1): 29-34. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
