Intramyocardial dissecting hematoma (IDH) is an uncommon complication of myocardial infarction that can potentially lead to cardiac rupture.[1] Two distinguishable types of cardiac rupture have been identified: a simple tear, which is the most common case and is characterized by a lineal or tortuous tear along the myocardial wall, and complex hemorrhagic dissection. IDH is generally classified as complex hemorrhagic dissection, in which blood infiltrates into the myocardial wall.[2] IDH is characterized by a serpiginous tract within the myocardium, which is also known as subepicardial aneurysm. The mechanisms of IDH are multifactorial and contribute to the rupture of intramyocardial vessels, diminished tensile strength of the infarcted myocardium, abrupt increases in perfusion pressure during the perfusion phase, or in rare cases, iatrogenesis.[3] Furthermore, the anatomical substrate of IDH is the spiral helical structure of the heart that favors hemorrhage spreading along the spiral myocardial fibers.[4] IDH is often a challenging diagnosis and prone to misdiagnosis because of its rarity and various clinical presentations. Here, we report a patient with IDH.
CASE
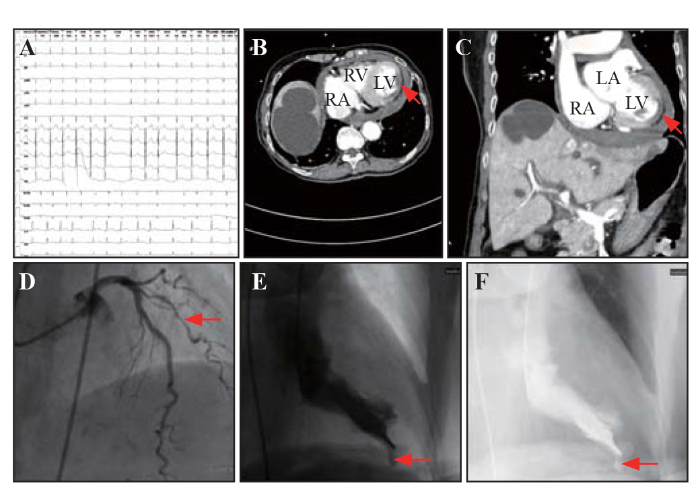
An 84-year-old woman with a history of hypertension and atrial fibrillation was admitted to our hospital because of sudden-onset, progressively worsening chest and upper abdominal pain that had lasted for 5 h. She was referred to us from another medical center with unexplained shock. The patient complained of severe chest and upper abdominal pain, which was accompanied by vomiting of suspicious coffee-like contents. Her vital signs included body temperature 36.2 ℃, blood pressure 77/47 mmHg (1 mmHg=0.133 kPa), and heart rate 98 beats/min. Physical examination revealed cyanosis of the lips, humid limbs, low cardiac sound, irregular heart rhythm, and upper abdominal tension. An electrocardiogram showed atrial fibrillation with slight ST-segment elevation from V4 to V9 compatible with acute anterior-lateral myocardial infarction (Figure 1A). Cardiac troponin-T was 531 ng/L, and N-terminal pro-B-type natriuretic peptide was 3,939 pg/mL. A contrast-enhanced computed tomography (CT) scan was performed to differentiate myocardial infarction from aortic dissection, acute necrotizing pancreatitis, and massive gastrointestinal bleeding. The findings delineated a low-enhanced segment in the anterior-lateral wall of the left ventricle (LV), left ventricular enlargement, atherosclerosis of multiple aortae and coronary arteries, and a moderate amount of pericardial effusion without leak of contrast into the pericardial cavity (Figures 1 B and C). In quick succession, the patient underwent emergency coronary angiography, which revealed 70%-80% stenosis in in the middle diagonal branch of the left anterior descending coronary artery (LADD), 20% stenosis in the caudal left main (LM) coronary artery, 20%-30% stenosis in the proximal left anterior descending (LAD) coronary artery, and 20%-30% stenosis in the middle of right coronary artery (RCA) (Figure 1D). Left ventricular angiography revealed a left ventricular subepicardial aneurysm characterized by systolic expansion of the pulsatile cavity with a narrow neck and abrupt interruption of the myocardium (Figures 1 E and F, video S1). As a result, percutaneous coronary intervention (PCI) was performed in the LADD. The patient reported consistent symptoms and refractory low blood pressure 4 h after the PCI. Progression to transmural rupture was subsequently suspected and further validated by bedside echocardiography, which revealed moderate left ventricular dysfunction with anterior-lateral wall dyskinesis and remarkable enlargement of pericardial effusion concomitant with blood clots. Echocardiography was not recorded due to the acute worsening of the patient’s hypotension and emergency situation. Unfortunately, considering the patient’s advanced age and poor prognosis, her next of kin refused further invasive treatment or surgery. The patient died within 12 h of onset.
Figure 1.
Figure 1.
Acute myocardial infarction complicated with intramyocardial dissecting hematoma. A: electrocardiogram showed atrial fibrillation with slight ST-segment elevation from V4 to V9; contrast-enhanced computed tomography (CT) scan of transverse section (B) and coronal section (C) revealed a low-enhanced segment in the anterior-lateral wall of the left ventricle (red arrows); D: coronary angiography found 70%-80% stenosis in the middle diagonal branch of the left coronary artery (red arrows); E and F: left ventricular angiography delineated a left ventricular subepicardial aneurysm characterized by an abrupt interruption of the myocardium (red arrow). LV: left ventricle; RV: right ventricle; RA: right atrium; LA: left atrium.
DISCUSSION
Given the persistent ST elevation, dynamic change in troponin-T, and coronary angiography findings, acute anterior-lateral myocardial infarction was considered to be the cause of IDH in this patient. It is noteworthy that the culprit artery, LADD, was not completely occluded by a thrombus. According to the classical pathological understanding, transmural infarction contributes to complete obstruction of the culprit vessel. Based on the findings of the contrast-enhanced CT scan, we speculated that the patient’s coronary thrombus was lysed. Unfortunately, the exact etiology and mechanism cannot be concluded due to a lack of autopsy data.
IDH may manifest symptoms such as severe chest pain, progressive dyspnea, and extreme fatigue. However, the patient was asymptomatic, and her imaging findings during left ventricular angiography unexpectedly identified the IDH. Then, transthoracic echocardiography (TTE) validated that the IDH had progressed to transmural rupture. In view of the patient’s severe chest pain, ST elevation on the electrocardiogram, and increased troponin-T, acute myocardial infarction (AMI) with cardiogenic shock was considered the first possible differential diagnosis. Another possible differential diagnosis was obstructive shock by cardiac tamponade, which was also true in our case. TTE is considered to be a non-invasive technique and is the first choice for diagnosing IDH at the bedside.[5] TTE is an effective tool for differentiating AMI from aortic dissection, pulmonary embolism, and pericardial tamponade. Moreover, TTE provides additional information regarding the infarcted area, mechanical complications, hemodynamic status, and patient risk stratification. A wide body of recent research suggests that implementation of TTE within emergency departments could help improve diagnostic workflow, similar to our case. Echocardiographic features of IDH include myocardial neocavitation with an echo lucent center in the suspected segment, a pulsatile cavity with systolic expansion accompanied by an endocardial flap, and intact epicardium or outer border of the myocardium.[6] Moreover, while cardiovascular magnetic resonance (CMR) is currently considered the imaging modality to definitively and quantitatively assess the presence of IDH,[7] CMR was deemed inappropriate in this case because of her compromised hemodynamics. Alternatively, a contrast-enhanced CT scan was performed to differentiate IDH from the above-mentioned differential diagnoses. TTE has several limitations in terms of IDH diagnosis. For example, inexperienced echocardiographers might have difficulty in identifying IDH from prominent trabeculations and intra-ventricular thrombus. A CT scan may be an alternative technique for use in combination with echocardiography.
The prognosis of IDH patients depends on various factors, including age, hemodynamic stability, size and location of the hematoma, presence of a ventricular septal defect, LV function, and pericardial effusion.[8] LV free wall IDH has a higher possibility of evolving into complete cardiac rupture, while IDH limited to the apex has a high probability of spontaneous re-absorption and should be treated conservatively.[9] Nonetheless, IDH has a high mortality in both conservative treatment and surgical settings. Late coronary intervention has no chance of improving prognosis and avoiding progression to cardiac rupture. Since AMI with cardiogenic shock was considered the initial diagnosis in this case 12 h after the onset of symptoms, we still performed emergency coronary angiography and subsequent PCI without delay, aiming for initial reperfusion therapy rather than diagnosis of IDH or preventing progression.
CONCLUSION
IDH is a rare complication of post-myocardial infarction that can potentially lead to cardiac rupture. However, IDH is often a challenging diagnosis and misdiagnosed because of its rarity and various clinical presentations. The prognosis and treatment of IDH depends on prompt and accurate diagnosis.
Funding: This study was supported by a grant from the Traditional Chinese Medicine Bureau of Guangdong Province (20211073).
Ethical approval: Not needed.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Contributors: YYZ designed the study, analyzed the data, interpreted the results, and drafted the manuscript. All authors have read and approved the manuscript.
All the supplementary files in this paper are available at http://wjem.com.cn.
Reference
Intramyocardial dissecting hematoma and postinfarction cardiac rupture
DOI:10.1111/echo.12017
PMID:23167290
[Cited within: 1]

Potentially fatal cardiac rupture is a complication of myocardial infarction (MI), which can appear in the first hours of the acute event and during the course of the first week. The intramyocardial dissecting hematoma might appear as a component of the rupture during the evolution process. The description of the myocardium as a helical muscular band facilitates the explanation of the fiber dissection. With echocardiography, it is possible to diagnose intramyocardial dissecting hematomas (IDH), determine its location, progression, potential complications, and in some cases its reabsorption. It is necessary to search for neocavitations in the infarcted myocardium and identify the intramyocardial edge that surrounds the defect, as well as the flow inside the myocardial dissection, the pathway of the dissection, and its communication with ventricular cavities, and also to look for the complete or partial reabsorption of the cavitary image. The greater the myocardial dissection is, the worse the prognosis. If the dissecting hematoma is confined to the apical segments, it is more likely to reabsorb spontaneously. Tissue characterization with magnetic resonance during an acute myocardial infarction allows identification of reperfusion injuries with altered microcirculation and intramyocardial hemorrhage (IMH). It is necessary to search for IMH in reperfused patients with ventricular arrhythmias, stunned myocardium, and no reflow. These patients may develop an increased stiffness in the infarcted wall and a major likelihood to develop a parietal rupture. Everything seems to indicate that we are facing the same physiopathological process which can be characterized by 2 complementary imaging methods, echocardiography and magnetic resonance.© 2012, Wiley Periodicals, Inc.
Intramyocardial dissecting hematoma after acute myocardial infarction—echocardiographic features and clinical outcome
DOI:10.1111/echo.2016.33.issue-7 URL [Cited within: 1]
Intramyocardial dissecting hematoma: two case reports and a meta-analysis of the literature
DOI:10.1111/echo.13796
PMID:29315786
[Cited within: 1]

Until recently, diagnosis of intramyocardial dissecting hematoma (IDH) was performed during necropsy or at surgery. During the recent years, echocardiography has permitted clinical suspicion, which usually needed confirmation with magnetic resonance imaging (MRI). In this study, we tried to define clinical and imaging features of IDH and predictors of mortality. We searched the literature for proven cases of IDH and analyzed them together with 2 of our cases. A total of 40 cases of IDH (2 our original and 38 literature cases) were included. Mean age was 60. In 32 cases, IDH was a complication of myocardial infarction (MI), in 66% anterior, a mean time from symptoms to diagnosis was 9 days. Thirty-eight % underwent surgery. In-hospital mortality was 23%. Multivariate analysis showed that the strongest independent predictor of mortality (42%) was EF < 35%; in patients with age >60, mortality risk was 44%; and in the presence of MI or late diagnosis (>24 hours since symptoms started), mortality risk was 50%. In summary, IDH is a diagnostic challenge. A high level of suspicion is needed for prompt diagnosis. Management of these patients is based on individual clinical and imaging parameters. Low EF, age > 60, and late diagnosis, all are predictors of in-hospital mortality.© 2018 Wiley Periodicals, Inc.
Intramyocardial dissecting hematoma: fatal complication of reperfusion damage in myocardial infarction - an autopsy case report
Intramyocardial dissecting hematoma of right ventricle following a myocardial infarction
DOI:10.1007/s00134-018-5115-y PMID:29525850 [Cited within: 1]
Left ventricular intramyocardial dissecting hematomas
Intramyocardial dissecting hematoma in patients with ischemic cardiomyopathy: role of multimodality imaging in three patients treated conservatively
Intramyocardial dissecting hematoma in anterior wall ST elevation myocardial infarction: impact on left ventricular remodeling and prognosis
DOI:10.1007/s10554-017-1221-0
PMID:28766162
[Cited within: 1]

Intramyocardial dissecting hematoma is an uncommon complication of myocardial infarction potentially leading to cardiac rupture. The aim of the present study was to investigate coronary reperfusion results, left ventricular (LV) function recovery and remodeling and clinical outcomes in patients with anterior STEMI complicated by intramyocardial hematoma. We prospectively studied 87 patients (mean age 59 ± 10 years; 88% male) with anterior STEMI (42 with intramyocardial hematoma) in order to evaluate coronary reperfusion results, LV remodeling (≥15% increase in end-systolic volume) and clinical outcomes (cardiac death, non-fatal reinfarction, and hospitalization for congestive heart failure) at 24 months. Thrombolysis in myocardial infarction (TIMI) flow score and myocardial blush grade (MBG) were assessed both pre- and post-percutaneous coronary intervention (PCI) and speckle-tracking echocardiography was performed post PCI and at 6-month follow-up. Patients with hematoma had lower post-PCI TIMI score and MBG, higher heart rate, worse LV ejection fraction and longitudinal or rotational function than their counterparts. LV remodeling occurred in 33 (78.6%) patients with hematoma and 11 (24.4%) patients without (p < 0.001). Independent predictors of LV remodeling were heart rate (p = 0.018), MBG (p = 0.036) and presence of hematoma (p < 0.001). Hematoma (log-rank test, χ = 9.849; p = 0.002) and LV remodeling (log-rank test, χ = 13.770; p < 0.001) were associated to a higher rate of adverse events. Cox analysis identified LV remodeling as the only independent predictor of adverse events (hazard ratio = 3.912; 95% confidence interval, 1.429-10.714; p = 0.008). Intramyocardial dissecting hematoma complicating anterior STEMI is an independent determinant of LV remodeling and is associated to poor prognosis.
Progressive intramyocardial dissecting hematoma in the apex of the left ventricle after percutaneous coronary intervention
DOI:10.21037/qims URL [Cited within: 1]