INTRODUCTION
Upper gastrointestinal bleeding (UGIB) is bleeding from the esophagus, stomach, or duodenum, and is the most commonly characterized by black stools or vomiting blood.[1,2] UGIB has an incidence of 47/100000, with a mortality of 2%-10%.[1,3] In critically ill patients, the incidence of UGIB reaches 4.7%, leading to a prolonged length of intensive care unit (ICU) stay of approximately 4-8 d, increased ICU mortality of approximately 1-4 times, and an overall 90-day mortality of 26.2%.[4,5] Endoscopy is an important modality to uncover the etiology of UGIB, and early endoscopy (<24 h) has been proven to be beneficial for patients with UGIB.[2,6⇓-8] Endoscopy is often complicated with cardiopulmonary outcomes, with an incidence of approximately 50%. They mainly included dyspnea, pulmonary edema, hypotension, arrhythmias, myocardial infarction, and cardiac arrest. Although cardiac arrest and acute myocardial infarction are rare, they can be life-threatening when they occur.[9,10]
No difference was observed in the incidence of cardiopulmonary complications, ICU length of stay, or mortality whether UGIB patients received prophylactic endotracheal intubation (PEI) before endoscopy, but PEI was associated with fewer in-hospital cardiac arrests and massive aspiration.[11] In contrast, previous studies showed that PEI or selective intubation prior to upper endoscopy might lead to a higher incidence of pneumonia.[12,13] There is controversy over the protective role of PEI in reducing harmful cardiopulmonary outcomes.[11,14] Therefore, this real-world study aimed to examine the association between PEI and cardiopulmonary outcomes in critically ill patients with UGIB.
METHODS
Study design and participants
This was a retrospective, observational study. Patients who underwent diagnostic/therapeutic endoscopy for UGIB were enrolled in the eICU Collaborative Research Database (eICU-CRD). eICU-CRD is a high-quality public multicenter ICU database that incorporates electronic medical records from 208 hospital ICUs in the USA from 2014 to 2015.[15,16] The study population was critically ill patients diagnosed with UGIB undergoing endoscopy. The definition of PEI mainly includes the following points: no indications for tracheal intubation of the respiratory system or nervous system. The specific manifestation was as follows during intubation: (1) blood oxygen saturation ≥90%; (2) respiratory rate 12-30 breaths/min; (3) no significant aspiration; and (4) no acute respiratory distress syndrome (ARDS). The exclusion criteria were as follows: (1) extubation before endoscopy; (2) airway intubation for therapeutic purposes other than airway protection; (3) indications of emergency endotracheal intubation and mechanical ventilation; and (4) time of airway intubation beyond 48 h prior to endoscopy.
The primary endpoint was the composite cardiopulmonary outcome, including aspiration, pneumonia, pulmonary edema, shock or hypotension, cardiac arrest, myocardial infarction, and arrhythmia occurring within 48 h of endoscopy.[17] The secondary endpoints were in-hospital mortality, hospital length of stay (hospital LOS), ICU length of stay (ICU LOS), post-endoscopy vasopressor use within 48 h, radiology within 48 h, red blood cell infusion within 48 h, and post-endoscopy antibiotic use within 48 h. All cardiopulmonary complications before endoscopy were excluded.
Data collection
After gaining access to the database, we used Structured Query Language (SQL) with PostgreSQL (version 9.6 UC Berkeley, USA) to extract demographic and vital signs, treatment, laboratory tests, and relevant past medical history data. Cardiopulmonary outcomes were collected via ICD-9 codes in the eICU-CRD. Baseline variables were extracted at the most recent time before endoscopy. Intervention radiology and red blood cell infusion were identified from the description of the treatment in the eICU-CRD. The ratios of ongoing active hematemesis, agitation, and encephalopathy before endoscopy were described in baseline characteristics, and data extraction was performed by the diagnosis of acute blood loss anemia, encephalopathy, and mental state change (supplementary Table 1).
In the absence of atrial fibrillation, the shock index was calculated using the most recent pulse and systolic blood pressure before endoscopy.[18] Patients whose ages were greater than 89 were substituted with 90.[19] The AIMS65,[20] Charlson Comorbidity Index (CCI),[21] and MELD score[22] were calculated to determine the severity of illness and comorbidity in critically ill patients (supplementary Tables 2 and 3).
Statistical analysis
The PEI and non-PEI groups were matched 1:2 using propensity score matching. The propensity score was estimated using a logistic regression model adjusted for age, CCI, Acute Physiology and Chronic Health Evaluation (APACHE) IV score, Glasgow Coma Scale (GCS), AIMS65 score, history of heart or lung disease, history of cirrhosis, use of sedatives during endoscopy, and use of vasopressor 24 h before endoscopy, and nearest neighbor matching was used (match tolerance=0.2).
Baseline continuous variables were expressed as the mean±standard deviation (SD) or median (interquartile range) and were compared using the independent samples t-test or Wilcoxon rank sum test regarding the distribution. Categorical variables were expressed as numbers (percentages) and were compared using Fisher’s exact test, Yates’s corrected Chi-square tests, or Pearson’s Chi-square test where appropriate. Logistic regression was performed in population and subgroups to examine the association between PEI and cardiopulmonary outcomes. Subgroups included patients with/without ongoing active hematemesis, agitation, or encephalopathy before endoscopy, and patients with/without a history of cirrhosis. The performance of multivariate logistic regression model was evaluated using area under the curve (AUC) of the receiver operating characteristic (ROC) curve. Restricted cubic spline (RCS) functions with three nodes were used to examine the relationship between composite cardiopulmonary outcomes (yes/no) and continuous variables and indicate the objective reference values where odds ratios (ORs) were equal to 1, with a P-value of less than 0.05 to consider the fitted association. Missing data were imputed using the median. All analyses were performed using IBM SPSS Statistics software (version 22.0; IBM SPSS Inc., USA), R version 3.6.2 (R Foundation, Austria), and GraphPad Prism (version 8.0.0, GraphPad Software, USA). All probability values were two-tailed, and a P-value <0.05 indicated statistical significance.
RESULTS
A total of 946 eligible patients were enrolled in the study and 11.4% (108/946) underwent PEI (Figure 1). Compared to non-PEI patients, PEI patients were more likely to be sedated (23.1% vs. 7.9%, P<0.001), to receive vasopressors within 24 h prior to the endoscopy (29.6% vs. 9.3%, P<0.001), and to receive red blood cells infusion prior to endoscopy (63.0% vs. 52.7%, P=0.045). The prevalence of cirrhosis was higher (36.1% vs. 20.9%, P<0.001). Also, higher APACHE IV score (79.50 vs. 54.00, P<0.001), AIMS65 score (2.00 vs. 1.00, P<0.001), MELD score (11.26 vs. 7.58, P=0.001), INR (1.30 vs. 1.30, P=0.001), total bilirubin (0.80 vs. 0.80, P<0.001), and lower systolic blood pressure (113.00 vs. 119.00, P=0.029) and GCS (12.00 vs. 15.00, P<0.001) were observed in the PEI group. On the contrary, cardiac comorbidity (14.8% vs. 24.3%, P=0.027) was more common in non-PEI patients. Baseline characteristics were compared in Table 1. In the PEI group, endoscopy was performed at the median of 6 h after intubation, and 86.1% (93/108) of patients were intubated 24 h after intubation.
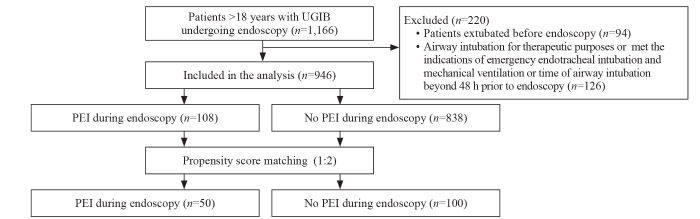
Figure 1.
Figure 1.
Patient selection flowchart. PEI: prophylactic endotracheal intubation; UGIB: upper gastrointestinal bleeding.
Table 1. Baseline characteristics of the PEI group and the non-PEI group—crude and adjusted
| Variables | Unmatched data | Matched data | |||||
|---|---|---|---|---|---|---|---|
| PEI (n=108) | Non-PEI (n=838) | P-value | PEI (n=50) | Non-PEI (n=100) | P-value | ||
| Age, year | 63.00 (52.00-74.00) | 65.00 (53.00-76.00) | 0.087a | 64.00 (56.75-74.25) | 64.00 (56.75-76.00) | 0.952a | |
| Male | 63 (58.3) | 494 (58.9) | 0.902a | 28 (56.0) | 50 (50.0) | 0.488a | |
| BMI, kg/m2 | 28.30 (23.92-33.49) | 26.83 (23.02-31.60) | 0.168a | 28.41 (24.46-33.19) | 26.81 (24.43-30.93) | 0.195a | |
| Heart rate, beat/min | 94.00 (79.25-107.25) | 92.00 (82.00-104.00) | 0.450a | 95.00 (77.00-111.00) | 92.00 (80.25-104.75) | 0.825a | |
| Systolic pressure, mmHg | 113.00 (103.00-131.00) | 119.00 (107.00-133.00) | 0.029a | 114.50 (102.75-129.70) | 119.00 (110.25-125.75) | 0.472a | |
| Caucasian | 76 (70.4) | 646 (77.1) | 0.353 | 34 (68.0) | 76 (76.0) | 0.716 | |
| Use of sedative during endoscopy | 25 (23.1) | 66 (7.9) | <0.001 | 13 (26.0) | 27 (27.0) | 0.896 | |
| Use of vasopressors before endoscopy | 32 (29.6) | 78 (9.3) | <0.001 | 10 (20.0) | 13 (13.0) | 0.262 | |
| Red blood cells infusion before endoscopy | 68 (63.0) | 442 (52.7) | 0.045 | 30 (60.0) | 43 (43.0) | 0.050 | |
| APACHE Ⅳ score | 79.50 (66.25-91.75) | 54.00 (45.00-65.00) | <0.001a | 68.99±2.58 | 66.63±1.99 | 0.319b | |
| CCI <4 | 98 (90.7) | 796 (95.0) | 0.068 | 47 (94.0) | 91 (91.0) | 0.750 | |
| AIMS65 score | 2.00 (2.00-3.00) | 1.00 (1.00-2.00) | <0.001a | 2.00 (1.00-2.00) | 1.00 (1.00-2.00) | 0.529a | |
| Glasgow Coma Scale | 12.00 (9.00-15.00) | 15.00 (15.00-15.00) | <0.001a | 15.00 (15.00-15.00) | 15.00 (14.25-15.00) | 0.616a | |
| MELD score | 11.26 (6.37-18.90) | 7.58 (4.35-13.06) | 0.001a | 10.46 (6.57-14.47) | 11.13 (5.48-17.09) | 0.739a | |
| Ongoing active hematemesis, agitation, or encephalopathy before endoscopy | 25 (23.1) | 238 (28.4) | 0.251 | 11 (22.0) | 36 (36.0) | 0.081 | |
| INR | 1.30 (1.20-1.67) | 1.30 (1.10-1.40) | 0.001a | 1.35 (1.19-1.50) | 1.35 (1.10-1.63) | 0.848a | |
| Total billirubin, mg/dL | 0.80 (0.80-2.47) | 0.80 (0.50-1.20) | <0.001a | 1.10 (0.67-1.30) | 1.10 (0.50-1.87) | 0.615a | |
| Creatinine, mg/dL | 0.95 (0.77-1.34) | 0.90 (0.71-1.27) | 0.231a | 1.03 (0.83-1.33) | 1.03 (0.80-1.94) | 0.851a | |
| Hemoglobin, g/dL | 8.80 (7.80-9.9) | 8.60 (7.80-9.60) | 0.226a | 9.14±0.27 | 8.72±0.17 | 0.168b | |
| History of cirrhosis | 39 (36.1) | 175 (20.9) | <0.001 | 15 (30.0) | 34 (34.0) | 0.622 | |
| History of heart disease a | 16 (14.8) | 204 (24.3) | 0.027 | 9 (18.0) | 21 (21.0) | 0.665 | |
| History of lung disease b | 17 (15.7) | 159 (19.0) | 0.416 | 10 (20.0) | 20 (20.0) | 1.000 | |
| Position of bleeding | |||||||
| Varices | 26 (24.1) | 119 (14.2) | 0.007 | 11 (22.0) | 14 (14.0) | 0.215 | |
| Ulcer | 17 (15.7) | 98 (11.7) | 0.226 | 6 (12.0) | 13 (13.0) | 0.862 | |
Data are expressed as median (interquartile range), or numbers (percentages). PEI: prophylactic endotracheal intubation; BMI: body mass index; APACHE IV: Acute Physiology and Chronic Health Evaluation IV; CCI: Charlson Comorbidity Index; MELD: model for end-stage liver disease; INR: international normalized ratio; a including congestive heart failure, arrhythmias, coronary artery bypass grafting; b including asthma, respiratory failure, restrictive pulmonary disease, lung transplant, pulmonary embolism, chronic obstructive pulmonary disease, pulmonary diffusion dysfunction. aP for the Wilcoxon rank sum test; bP for the t-test, P for Pearson’s Chi-square tests.
After propensity score matching, the baseline variables were essentially similar between the two groups. The mean age of patients in both groups was 64.00 years. Compared to the non-PEI patients, those with PEI had a higher incidence of cardiopulmonary outcomes (58.0% vs. 30.3%, P=0.001). The incidence of shock and hypotension (44.0% vs. 11.0%, P<0.001), pulmonary edema (6.0% vs. 0%, P=0.010), cardiac arrest (6.0% vs. 0.0%, P=0.010), and in-hospital mortality (18.0% vs. 2.0%, P=0.002) were higher in the PEI group. Additionally, the number of patients receiving vasopressors (22.0% vs. 10.0%, P=0.046) and the number of patients receiving red blood cell infusion (62.0% vs. 44.0%, P=0.038) was higher in the PEI group. Moreover, the ICU LOS (3.57 d vs. 1.90 d, P<0.001) and the hospital LOS (9.71 d vs. 5.50 d, P=0.001) were longer in the PEI group (Table 2).
Table 2. Outcomes of the PEI group and the non-PEI group—crude and adjusted
| Outcomes | Unmatched data | Matched data | |||||
|---|---|---|---|---|---|---|---|
| PEI (n=108) | Non-PEI (n=838) | P-value | PEI (n=50) | Non-PEI (n=100) | P-value | ||
| Cardiopulmonary outcomes | 57 (52.8) | 248 (29.6) | <0.001 | 29 (58.0) | 30 (30.3) | 0.001 | |
| Cardiac arrest | 4 (3.7) | 2 (0.2) | <0.001a | 3 (6.0) | 0 (0.0) | 0.010c | |
| Myocardial infarction | 1 (0.9) | 17 (2.0) | 0.678a | 1 (2.0) | 5 (5.0) | 0.659a | |
| Arrhythmia | 17 (15.7) | 103 (12.3) | 0.311 | 11 (22.0) | 11 (11.0) | 0.073 | |
| Shock/hypotension | 45 (41.7) | 146 (17.4) | <0.001 | 22 (44.0) | 11 (11.0) | <0.001 | |
| Aspiration | 1 (0.9) | 7 (0.8) | 1.000 | 1 (2.0) | 1 (1.0) | 1.000a | |
| Pneumonia | 6 (5.6) | 24 (2.9) | 0.133 | 5 (10.0) | 3 (3.0) | 0.158a | |
| Pulmonary edema | 5 (4.6) | 1 (0.1) | <0.001a | 3 (6.0) | 0 (0.0) | 0.010c | |
| Post-endoscopy vasopressor use within 48 h | 35 (32.4) | 55 (6.6) | <0.001 | 11 (22.0) | 10 (10.0) | 0.046 | |
| Post-endoscopy antibacterial use within 48 h | 28 (25.9) | 131 (15.6) | 0.007 | 16 (32.0) | 25 (25.0) | 0.364 | |
| Radiology within 48 h | 16 (14.8) | 85 (10.1) | 0.139 | 7 (14.0) | 8 (8.0) | 0.248 | |
| Red blood cells infusion within 48 h | 72 (66.7) | 453 (54.1) | 0.013 | 31 (62.0) | 44 (44.0) | 0.038 | |
| Hospital LOS, d | 9.62 (5.42-16.41) | 4.89 (3.11-8.06) | <0.001b | 9.71 (5.63-15.16) | 5.50 (3.40-10.53) | 0.001b | |
| ICU LOS, d | 3.44 (1.80-6.19) | 1.81 (0.96-2.74) | <0.001b | 3.57 (1.69-6.49) | 1.90 (1.02-2.98) | <0.001b | |
| In-hospital mortality | 16 (14.8) | 23 (2.7) | <0.001 | 9 (18.0) | 2 (2.0) | 0.002a | |
Data are expressed as median (interquartile range), or numbers (percentages). PEI: prophylactic endotracheal intubation; ICU LOS: intensive care unit length of stay. aP for Yates’s corrected Chi-square tests; bP for the Wilcoxon rank sum test; cP for Fisher’s exact tests; otherwise, P for Pearson’s Chi-squared tests.
Univariate logistic regression analysis showed that patients undergoing PEI had a 2.6-fold higher risk of cardiopulmonary outcomes than those in the non-PEI group (OR 2.659, 95% confidence interval [95% CI] 1.772-3.990, P<0.001). After matching for age, the CCI, APACHE IV score, GCS, AIMS65 score, history of heart or lung disease, history of cirrhosis, use of sedative during endoscopy, and use of vasopressors 24 h before endoscopy, the predictive value of PEI persisted (OR 3.176, 95% CI 1.567-6.438, P=0.001) (Table 3). In addition, analysis of the subgroups with no or only ongoing active hematemesis, agitation, or encephalopathy before endoscopy suggested that the likelihood of cardiopulmonary outcomes was 2.130 times (OR 2.130, 95% CI 1.328-3.418, P=0.002) higher in the PEI group and 5.597 times (OR 5.597, 95% CI 2.153-14.547, P<0.001) higher in the non-PEI group. Further analysis of patients with or without a history of cirrhosis showed that the likelihood of cardiopulmonary outcomes was 3.987 times (OR 3.987, 95% CI 1.861-8.542, P<0.001) higher in the PEI group and 2.909 times (OR 2.909, 95% CI 1.752-4.829, P<0.001) higher in the non-PEI group.
Table 3. Association between cardiopulmonary outcomes and PEI
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Matched | 3.176 (1.567-6.438) | 0.001 |
| Unmatched | 2.659 (1.772-3.990) | <0.001 |
| Subgroup analysis | ||
| Matched1 | 3.103 (1.322-7.287) | 0.009 |
| Non-matched1 | 2.130 (1.328-3.418) | 0.002 |
| Matched2 | 3.733 (0.847-16.452) | 0.082 |
| Non-matched2 | 5.597 (2.153-14.547) | <0.001 |
| Matched3 | 7.250 (1.476-35.611) | 0.015 |
| Non-matched3 | 3.987 (1.861-8.542) | <0.001 |
| Matched4 | 2.621 (1.135-6.054) | 0.024 |
| Non-matched4 | 2.909 (1.752-4.829) | <0.001 |
PEI: prophylactic endotracheal intubation; OR: odds ratio; CI: confidence interval. 1 represents a new subgroup without patients with ongoing active hematemesis, agitation, or encephalopathy before endoscopy; 2 represents a new subgroup consisting only of patients with ongoing active hematemesis, agitation, or encephalopathy before endoscopy; 3 represents a new subgroup consisting only of patients with a history of cirrhosis; 4 represents a new subgroup without patients with a history of cirrhosis.
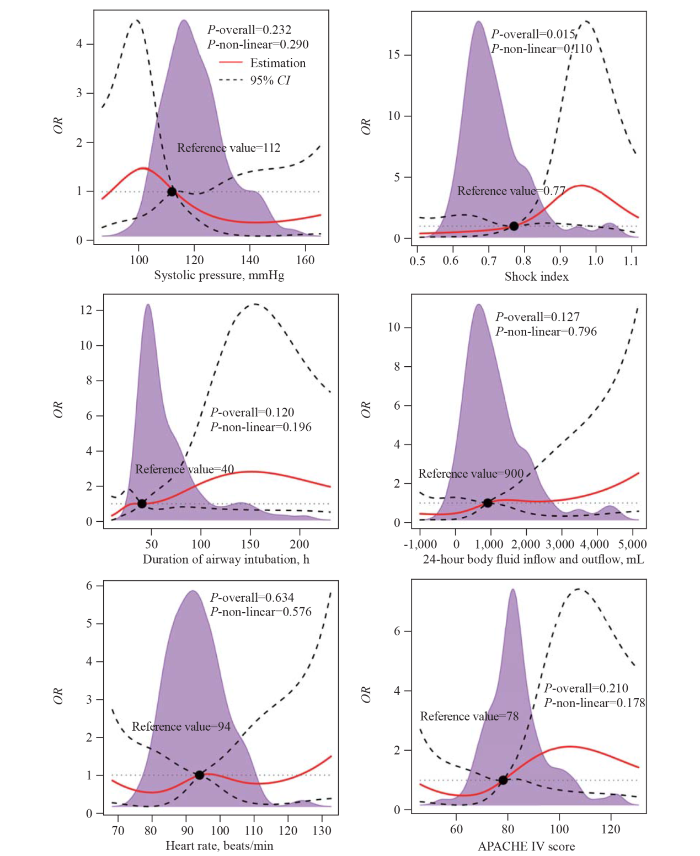
Restricted cubic spline plots were used to flexibly determine the relationship between clinical risk factors and cardiopulmonary outcomes (Figure 2). In patients undergoing PEI, the shock index was associated with cardiopulmonary outcomes (P<0.05), with a nadir of 0.77.
Figure 2.
Figure 2.
Relationship between clinical risk factors and cardiopulmonary outcomes determined by restricted cubic spline plots. OR: odds ratio; 95% CI: 95% confidence interval; APACHE IV: Acute Physiology and Chronic Health Evaluation IV. The purple density curve represents the distribution of each continuous factor. The solid red line represents the univariate odds ratio, while the dashed line represents the 95% confidence interval derived from restricted cubic spline regression. The black dashed line represents a reference line with a ratio of 1.0.
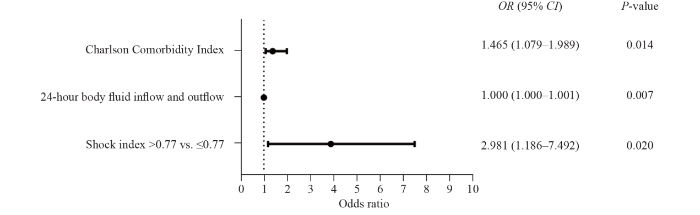
In Figure 3, the multivariable logistic analysis showed that cardiopulmonary outcomes after endoscopy were strongly correlated with the CCI (OR 1.465, 95% CI 1.079-1.989, P=0.014) and the shock index >0.77 (compared with shock index ≤0.77 [OR 2.981, 95% CI 1.186-7.492, P=0.020, AUC=0.764]).
Figure 3.
Figure 3.
Multivariable logistical analysis of cardiopulmonary outcomes in patients undergoing prophylactic endotracheal intubation.
DISCUSSION
This study is a real-world study of the relationship between PEI and cardiopulmonary outcomes after endoscopy in elderly and critically ill patients with UGIB. Approximately 32.2% (305/946) of critically ill patients with UGIB developed cardiopulmonary outcomes after endoscopy. Patients who underwent PEI had more cardiopulmonary outcomes, more use of vasopressor after endoscopy, longer hospital LOS, longer ICU LOS, and higher in-hospital mortality. PEI is associated with a higher risk of cardiopulmonary outcomes in patients with UGIB. It is well known that patients with cirrhosis develop complications from the heart and lungs more often than the non-cirrhotic population. Considering the complications in cirrhotic patients due to the natural course of the disease, we conducted a subgroup analysis for cirrhotic and non-cirrhotic patients to evaluate the association between cardiopulmonary outcomes and PEI. In addition, the European Society of Gastrointestinal Endoscopy (ESGE) only recommends intubation in very particular situations, such as ongoing active hematemesis, agitation, or encephalopathy with the inability to adequately control the airway.[23] With this in mind, we performed the same subgroup analysis. This association was consistent across multiple subgroup analyses, and it further illustrates that PEI is associated with a high risk of cardiopulmonary outcomes in patients with UGIB. In patients undergoing PEI, a shock index greater than 0.77 served as a predictor of cardiopulmonary outcomes.
A case-control study by Rehman et al[14] in 2009 that included 307 critically ill patients with UGIB found no significant difference in cardiopulmonary outcomes between patients who received PEI and those who were not intubated, based on a composite of outcomes including pneumonia, pulmonary edema, myocardial infarction, ARDS, and cardiac arrest. It should be noted that the previous study did not report on the comorbidity of respiratory distress prior to intubation and was not adjusted for mental status or bleeding status. Mental status and bleeding status were adjusted in our study by GCS and AIMS65 score. The AIMS65 score is used to predict in-hospital mortality in patients with UGIB and has the advantages of being easy to calculate, highly accurate, and independent of ill-defined medical history criteria.[20] The GCS is critical in the assessment of consciousness in critically ill patients, as well as in the management of the entire clinical process.[23] Hayat et al[17] demonstrated that the overall incidence of cardiopulmonary outcomes in UGIB patients with a mean age of 59 years who received PEI and those who were not intubated was 13.0% after matching. The incidence of cardiopulmonary outcomes in our study was higher than that in the previous study (58.0% in the PEI group and 30.3% in the non-PEI group). One of the underlying reasons was that the median age of the patients in our study was older (64 years), and cardiopulmonary outcomes after endoscopy mainly occur in elderly patients.[24] All patients in the study by Hayat et al[17] underwent endoscopy within 24 h after admission, while the present study included patients who underwent endoscopy within 48 h, with a median duration of 16.7 h, and 69.7% (659/946) of patients completed endoscopy within 24 h after admission. However, it should be noted that patients in the present study had shock or hypotension (98/287, 34.1%), massive blood loss (116/287, 40.4%), and altered mental status (55/287, 19.2%) after admission, which might be the reason for not completing endoscopy within 24 h due to resusitation. In fact, because tracheal intubation and endoscopy are performed sequentially and ideally performed immediately after each other in clinical practice, complications of PEI should theoretically include complications of tracheal intubation and complications of endoscopy, which are often difficult to distinguish. It is reasonable that our observation of complications in the first 48 h after endoscopy captures the combined effects of this procedure.
The primary outcome of the study was adverse cardiopulmonary events after endoscopy, excluding adverse events before endoscopy. After propensity score matched, the observed difference between the two groups was whether PEI was performed. The discussion of the possible adverse factors of PEI is also consistent with previous literature reports and our clinical knowledge. One of the objectives of this study is to determine why PEI does not achieve the desired conservation purpose and to suggest possible complementary measures to make PEI more effective.
Among the 108 patients in the PEI group, the shock index was associated with cardiopulmonary outcomes, with a cut-off value of greater than 0.77 in patients who underwent PEI. Previous literature has shown that hemodynamic collapse is a common complication after endotracheal intubation.[25] The shock index was effective in evaluating hemodynamics with a normal range between 0.5 and 0.7.[26] In a retrospective cohort study conducted by Trivedi et al[27] in 2015, a pre-intubation shock index greater than 0.9 was identified as a risk factor for post-intubation hypotension and ICU mortality in adult intensive care patients. This suggested that hemodynamic instability, as indicated by an elevated shock index, might serve as a possible index of poor outcomes in intubated patients. Regarding patients with UGIB who underwent PEI before endoscopy, few practical predictors for cardiopulmonary outcomes have been recognized. We proposed a shock index greater than 0.77 as a possible predictor of cardiopulmonary outcomes after PEI.
The present study had several limitations. First, patients who were not intubated were classified as non-PEI because it was impossible to exclude patients with a contradiction of intubation. Second, because of the retrospective design of our study, we cannot determine the reason for intubation or whether intubation directly led to the observed outcomes. Third, our data are from the year of 2014 to 2015 and may have aging issues. Finally, because of the observational study design, only associations can be inferred, not causality. Meanwhile, the association of endoscopy with cardiopulmonary outcomes could not be demonstrated. Nevertheless, the present study was a real-world study that stemmed from a large-scale database. The current finding requires further validation in prospective studies.
CONCLUSION
The present study provides clinical evidence for airway protection in patients undergoing endoscopy. The study demonstrated that PEI was associated with cardiopulmonary outcomes in elderly and critically ill patients with UGIB undergoing endoscopy. Furthermore, a shock index greater than 0.77 could be used as a predictor of a worse prognosis in patients undergoing PEI.
Funding: This study was supported by grants from the Ministry of Science and Technology of the People’s Republic of China (2020AAA0109605), the National Natural Science Grant of China (82072225, 82272246), High-level Hospital Construction Project of Guangdong Provincial People’s Hospital (DFJHBF202104), Science and Technology Program of Guangzhou (202206010044), and Leading Medical Talents in Guangdong Province of Guangdong Provincial People’s Hospital (KJ012019425).
Ethical approval: This study was approved by the Ethics Committee of the Guangdong Provincial People's Hospital (KY2023-021-01).
Conflicts of interest: The authors do not have a financial interest or relationship to disclose regarding this research.
Contributors: YFL, FES, and WYZ contributed equally to this work. YFL, YCH, XL and XYC conceived and designed the experiments. YFL, XHC, and HXL collected and analyzed the data. YOY, FES, GXZ and WYZ contributed to the writing of the manuscript. FES, WYZ, RRW, and XKZ revised the manuscript. All authors approved the final version.
All the supplementary files are available at http://wjem.com.cn.
Reference
Management of acute upper gastrointestinal bleeding
Upper gastrointestinal bleeding: etiologies and management
Changing epidemiology and etiology of upper and lower gastrointestinal bleeding
DOI:10.1016/j.bpg.2019.04.003 URL [Cited within: 1]
The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients
DOI:10.1186/cc1071
PMID:11737927
[Cited within: 1]

To estimate the mortality and length of stay in the intensive care unit (ICU) attributable to clinically important gastrointestinal bleeding in mechanically ventilated critically ill patients.Three strategies were used to estimate the mortality attributable to bleeding in two multicentre databases. The first method matched patients who bled with those who did not (matched cohort), using duration of ICU stay prior to the bleed, each of six domains of the Multiple Organ Dysfunction Score (MODS) measured 3 days prior to the bleed, APACHE II score, age, admitting diagnosis, and duration of mechanical ventilation. The second approach employed Cox proportional hazards regression to match bleeding and non-bleeding patients (model-based matched cohort). The third method, instead of matching, derived estimates based on regression modelling using the entire population (regression method). Three parallel analyses were conducted for the length of ICU stay attributable to clinically important bleeding.Sixteen Canadian university-affiliated ICUs.A total of 1666 critically ill patients receiving mechanical ventilation for at least 48 hours.We prospectively collected data on patient demographics, APACHE II score, admitting diagnosis, daily MODS, clinically important bleeding, length of ICU stay, and mortality. Independent adjudicators determined the occurrence of clinically important gastrointestinal bleeding, defined as overt bleeding in association with haemodynamic compromise or blood transfusion.Of 1666 patients, 59 developed clinically important gastrointestinal bleeding. The mean APACHE II score was 22.9 +/- 8.6 among bleeding patients and 23.3 +/- 7.7 among non-bleeding patients. The risk of death was increased in patients with bleeding using all three analytic approaches (matched cohort method: relative risk [RR]= 2.9, 95% confidence interval (CI)= 1.6-5.5; model-based matched cohort method: RR = 1.8, 95% CI = 1.1-2.9; and the regression method: RR = 4.1, 95% CI = 2.6-6.5). However, this was not significant for the adjusted regression method (RR = 1.0, 95% CI = 0.6-1.7). The median length of ICU stay attributable to clinically important bleeding for these three methods, respectively, was 3.8 days (95% CI = -0.01 to 7.6 days), 6.7 days (95% CI = 2.7-10.7 days), and 7.9 days (95% CI = 1.4-14.4 days).Clinically important upper gastrointestinal bleeding has an important attributable morbidity and mortality, associated with a RR of death of 1-4 and an excess length of ICU stay of approximately 4-8 days.
Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients
DOI:10.1007/s00134-015-3725-1
PMID:25860444
[Cited within: 1]

To describe the prevalence of, risk factors for, and prognostic importance of gastrointestinal (GI) bleeding and use of acid suppressants in acutely ill adult intensive care patients.We included adults without GI bleeding who were acutely admitted to the intensive care unit (ICU) during a 7-day period. The primary outcome was clinically important GI bleeding in ICU, and the analyses included estimations of baseline risk factors and potential associations with 90-day mortality.A total of 1,034 patients in 97 ICUs in 11 countries were included. Clinically important GI bleeding occurred in 2.6 % (95 % confidence interval 1.6-3.6 %) of patients. The following variables at ICU admission were independently associated with clinically important GI bleeding: three or more co-existing diseases (odds ratio 8.9, 2.7-28.8), co-existing liver disease (7.6, 3.3-17.6), use of renal replacement therapy (6.9, 2.7-17.5), co-existing coagulopathy (5.2, 2.3-11.8), acute coagulopathy (4.2, 1.7-10.2), use of acid suppressants (3.6, 1.3-10.2) and higher organ failure score (1.4, 1.2-1.5). In ICU, 73 % (71-76 %) of patients received acid suppressants; most received proton pump inhibitors. In patients with clinically important GI bleeding, crude and adjusted odds for mortality were 3.7 (1.7-8.0) and 1.7 (0.7-4.3), respectively.In ICU patients clinically important GI bleeding is rare, and acid suppressants are frequently used. Co-existing diseases, liver failure, coagulopathy and organ failures are the main risk factors for GI bleeding. Clinically important GI bleeding was not associated with increased adjusted 90-day mortality, which largely can be explained by severity of comorbidity, other organ failures and age.
Impact of the COVID-19 pandemic on endoscopic procedures: a single-center study in China
DOI:10.5847/wjem.j.1920-8642.2022.065 URL [Cited within: 1]
Timing of endoscopy for acute upper gastrointestinal bleeding: a territory-wide cohort study
Use of endoscopy for management of acute upper gastrointestinal bleeding in the UK: results of a nationwide audit
DOI:10.1136/gut.2008.174599
PMID:20357318
[Cited within: 1]

To examine the use of endoscopy in the UK for acute upper gastrointestinal bleeding (AUGIB) and compare with published standards. To assess the organisation of endoscopy services for AUGIB in the UK. To examine the relationship between outcomes and out of hours (OOH) service provision.Multi-centre cross sectional clinical audit.All UK hospitals accepting admissions with AUGIB.All adults (>or=16 yrs) presenting with AUGIB between 1st May and 30th June 2007.Collection A custom designed web-based reporting tool was used to collect data on patient characteristics, comorbidity and haemodynamic status at presentation to calculate the Rockall score, use and timing of endoscopy, treatment including endoscopic, rebleeding and in-hospital mortality. A mailed questionnaire was used to collect data on facilities and service organisation.Data on 6750 patients (median age 68 years) were analysed from 208 hospitals. 74% underwent inpatient endoscopy; of these 50% took place within 24 h of presentation, 82% during normal working hours and 3% between midnight and 8 am. Of patients deemed high-risk (pre-endoscopy Rockall score >or=5) only 55% were endoscoped within 24 h and 14% waited >or=72 h for endoscopy. Lesions with a high risk of rebleeding were present in 28% of patients of whom 74% received endoscopic therapy. Further bleeding was evident in 13% and mortality in those endoscoped was 7.4% (95% CI 6.7% to 8.1%). In 52% of hospitals a consultant led out of hours (OOH) endoscopy rota existed; in these hospitals 20% of first endoscopies were performed OOH compared with 13% in those with no OOH rota and endoscopic therapy was more likely to be administered (25% vs 21% in hospitals with no OOH rota). The risk adjusted mortality ratio was higher (1.21, p=0.10, (95%CI 0.96 to 1.51)) in hospitals without such rotas.This audit has found continuing delays in performing endoscopy after AUGIB and underutilisation of standard endoscopic therapy particularly for variceal bleeding. In hospitals with a formal OOH endoscopy rota patients received earlier endoscopy, were more likely to receive endoscopic therapy and may have a lower mortality.
A national study of cardiopulmonary un-planned events after GI endoscopy
DOI:10.1016/j.gie.2006.12.040 URL [Cited within: 1]
High risk of post-myocardial infarction cardiac arrest in young adults
DOI:10.1016/j.jacasi.2022.06.008 PMID:36624796 [Cited within: 1]
Endotracheal intubation for airway protection during endoscopy for severe upper GI hemorrhage
Prophylactic tracheal intubation for upper GI bleeding: a meta-analysis
Risk of aspiration pneumonia in suspected variceal hemorrhage: the value of prophylactic endotracheal intubation prior to endoscopy
DOI:10.1007/s10620-006-9616-0 URL [Cited within: 1]
Prophylactic endotracheal intubation in critically ill patients undergoing endoscopy for upper GI hemorrhage
DOI:10.1016/j.gie.2009.03.002 URL [Cited within: 2]
The eICU Collaborative Research Database, a freely available multi-center database for critical care research
Brief introduction of medical database and data mining technology in big data era
Association of prophylactic endotracheal intubation in critically ill patients with upper GI bleeding and cardiopulmonary unplanned events
DOI:S0016-5107(16)30838-0
PMID:28011279
[Cited within: 3]

Prophylactic endotracheal intubation (PEI) is often advocated to mitigate the risk of cardiopulmonary adverse events in patients presenting with brisk upper GI bleeding (UGIB). However, the benefit of such a measure remains controversial. Our study aimed to compare the incidence of cardiopulmonary unplanned events between critically ill patients with brisk UGIB who underwent endotracheal intubation versus those who did not.Patients aged 18 years or older who presented at Cleveland Clinic between 2011 and 2014 with hematemesis and/or patients with melena with consequential hypovolemic shock were included. The primary outcome was a composite of several cardiopulmonary unplanned events (pneumonia, pulmonary edema, acute respiratory distress syndrome, persistent shock/hypotension after the procedure, arrhythmia, myocardial infarction, and cardiac arrest) occurring within 48 hours of the endoscopic procedure. Propensity score matching was used to match each patient 1:1 in variables that could influence the decision to intubate. These included Glasgow Blatchford Score, Charleston Comorbidity Index, and Acute Physiology and Chronic Health Evaluation scores.Two hundred patients were included in the final analysis. The baseline characteristics, comorbidity scores, and prognostic scores were similar between the 2 groups. The overall cardiopulmonary unplanned event rates were significantly higher in the intubated group compared with the nonintubated group (20% vs 6%, P =.008), which remained significant (P =.012) after adjusting for the presence of esophageal varices.PEI before an EGD for brisk UGIB in critically ill patients is associated with an increased risk of unplanned cardiopulmonary events. The benefits and risks of intubation should be carefully weighed when considering airway protection before an EGD in this group of patients.Copyright © 2017 American Society for Gastrointestinal Endoscopy. Published by Elsevier Inc. All rights reserved.
Shock index and early recognition of sepsis in the emergency department: pilot study
DOI:10.5811/westjem.2012.8.11546 URL [Cited within: 1]
E-CatBoost: an efficient machine learning framework for predicting ICU mortality using the eICU Collaborative Research Database
DOI:10.1371/journal.pone.0262895
URL
[Cited within: 1]

Improving the Intensive Care Unit (ICU) management network and building cost-effective and well-managed healthcare systems are high priorities for healthcare units. Creating accurate and explainable mortality prediction models helps identify the most critical risk factors in the patients’ survival/death status and early detect the most in-need patients. This study proposes a highly accurate and efficient machine learning model for predicting ICU mortality status upon discharge using the information available during the first 24 hours of admission. The most important features in mortality prediction are identified, and the effects of changing each feature on the prediction are studied. We used supervised machine learning models and illness severity scoring systems to benchmark the mortality prediction. We also implemented a combination of SHAP, LIME, partial dependence, and individual conditional expectation plots to explain the predictions made by the best-performing model (CatBoost). We proposed E-CatBoost, an optimized and efficient patient mortality prediction model, which can accurately predict the patients’ discharge status using only ten input features. We used eICU-CRD v2.0 to train and validate the models; the dataset contains information on over 200,000 ICU admissions. The patients were divided into twelve disease groups, and models were fitted and tuned for each group. The models’ predictive performance was evaluated using the area under a receiver operating curve (AUROC). The AUROC scores were 0.86 [std:0.02] to 0.92 [std:0.02] for CatBoost and 0.83 [std:0.02] to 0.91 [std:0.03] for E-CatBoost models across the defined disease groups; if measured over the entire patient population, their AUROC scores were 7 to 18 and 2 to 12 percent higher than the baseline models, respectively. Based on SHAP explanations, we found age, heart rate, respiratory rate, blood urine nitrogen, and creatinine level as the most critical cross-disease features in mortality predictions.
Risk stratification in acute upper GI bleeding: comparison of the AIMS 65 score with the Glasgow-Blatchford and Rockall scoring systems
DOI:10.1016/j.gie.2015.10.021
PMID:26515955
[Cited within: 2]

The American College of Gastroenterology recommends early risk stratification in patients presenting with upper GI bleeding (UGIB). The AIMS65 score is a risk stratification score previously validated to predict inpatient mortality. The aim of this study was to validate the AIMS65 score as a predictor of inpatient mortality in patients with acute UGIB and to compare it with established pre- and postendoscopy risk scores.ICD-10 (International Classification of Diseases, Tenth Revision) codes identified patients presenting with UGIB requiring endoscopy. All patients were risk stratified by using the AIMS65, Glasgow-Blatchford score (GBS), pre-endoscopy Rockall, and full Rockall scores. The primary outcome was inpatient mortality. Secondary outcomes were a composite endpoint of inpatient mortality, rebleeding, and endoscopic, radiologic, or surgical intervention; blood transfusion requirement; intensive care unit (ICU) admission; rebleeding; and hospital length of stay. The area under the receiver-operating characteristic curve (AUROC) was calculated for each score.Of the 424 study patients, 18 (4.2%) died and 69 (16%) achieved the composite endpoint. The AIMS65 score was superior to both the GBS (AUROC, 0.80 vs 0.76, P <.027) and the pre-endoscopy Rockall score (0.74, P =.001) and equivalent to the full Rockall score (0.78, P =.18) in predicting inpatient mortality. The AIMS65 score was superior to all other scores in predicting the need for ICU admission and length of hospital stay. AIMS65, GBS, and full Rockall scores were equivalent (AUROCs, 0.63 vs 0.62 vs 0.63, respectively) and superior to pre-endoscopy Rockall (AUROC, 0.55) in predicting the composite endpoint. GBS was superior to all other scores for predicting blood transfusion.The AIMS65 score is a simple risk stratification score for UGIB with accuracy superior to that of GBS and pre-endoscopy Rockall scores in predicting in-hospital mortality and the need for ICU admission.Crown Copyright © 2016. Published by Elsevier Inc. All rights reserved.
New ICD-10 version of the Charlson Comorbidity Index predicted in-hospital mortality
DOI:10.1016/j.jclinepi.2004.03.012
PMID:15617955
[Cited within: 1]

The ICD-9-CM adaptation of the Charlson comorbidity score has been a valuable resource for health services researchers. With the transition into ICD-10 coding worldwide, an ICD-10 version of the Deyo adaptation was developed and validated using population-based hospital data from Victoria, Australia.The algorithm was translated from ICD-9-CM into ICD-10-AM (Australian modification) in a multistep process. After a mapping algorithm was used to develop an initial translation, these codes were manually examined by the coding experts and a general physician for face validity. Because the ICD-10 system is country specific, our goal was to keep many of the translated code at the three-digit level for generalizability of the new index.There appears to be little difference in the distribution of the Charlson Index score between the two versions. A strong association between increasing index scores and mortality exists: the area under the ROC curve is 0.865 for the last year using the ICD-9-CM version and remains high, at 0.855, for the ICD-10 version.This work represents the first rigorous adaptation of the Charlson comorbidity index for use with ICD-10 data. In comparison with a well-established ICD-9-CM coding algorithm, it yields closely similar prevalence and prognosis information by comorbidity category.
The model for end-stage liver disease (MELD)
DOI:10.1002/hep.21563
PMID:17326206
[Cited within: 1]

The Model for End-stage Liver Disease (MELD) was initially created to predict survival in patients with complications of portal hypertension undergoing elective placement of transjugular intrahepatic portosystemic shunts. The MELD which uses only objective variables was validated subsequently as an accurate predictor of survival among different populations of patients with advanced liver disease. The major use of the MELD score has been in allocation of organs for liver transplantation. However, the MELD score has also been shown to predict survival in patients with cirrhosis who have infections, variceal bleeding, as well as in patients with fulminant hepatic failure and alcoholic hepatitis. MELD may be used in selection of patients for surgery other than liver transplantation and in determining optimal treatment for patients with hepatocellular carcinoma who are not candidates for liver transplantation. Despite the many advantages of the MELD score, there are approximately 15%-20% of patients whose survival cannot be accurately predicted by the MELD score. It is possible that the addition of variables that are better determinants of liver and renal function may improve the predictive accuracy of the model. Efforts at further refinement and validation of the MELD score will continue.
Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021
DOI:10.1055/a-1369-5274
PMID:33567467
[Cited within: 2]

1: ESGE recommends in patients with acute upper gastrointestinal hemorrhage (UGIH) the use of the Glasgow-Blatchford Score (GBS) for pre-endoscopy risk stratification. Patients with GBS ≤ 1 are at very low risk of rebleeding, mortality within 30 days, or needing hospital-based intervention and can be safely managed as outpatients with outpatient endoscopy.Strong recommendation, moderate quality evidence. 2: ESGE recommends that in patients with acute UGIH who are taking low-dose aspirin as monotherapy for secondary cardiovascular prophylaxis, aspirin should not be interrupted. If for any reason it is interrupted, aspirin should be re-started as soon as possible, preferably within 3-5 days.Strong recommendation, moderate quality evidence. 3: ESGE recommends that following hemodynamic resuscitation, early (≤ 24 hours) upper gastrointestinal (GI) endoscopy should be performed. Strong recommendation, high quality evidence. 4: ESGE does not recommend urgent (≤ 12 hours) upper GI endoscopy since as compared to early endoscopy, patient outcomes are not improved. Strong recommendation, high quality evidence. 5: ESGE recommends for patients with actively bleeding ulcers (FIa, FIb), combination therapy using epinephrine injection plus a second hemostasis modality (contact thermal or mechanical therapy). Strong recommendation, high quality evidence. 6: ESGE recommends for patients with an ulcer with a nonbleeding visible vessel (FIIa), contact or noncontact thermal therapy, mechanical therapy, or injection of a sclerosing agent, each as monotherapy or in combination with epinephrine injection. Strong recommendation, high quality evidence. 7 : ESGE suggests that in patients with persistent bleeding refractory to standard hemostasis modalities, the use of a topical hemostatic spray/powder or cap-mounted clip should be considered. Weak recommendation, low quality evidence. 8: ESGE recommends that for patients with clinical evidence of recurrent peptic ulcer hemorrhage, use of a cap-mounted clip should be considered. In the case of failure of this second attempt at endoscopic hemostasis, transcatheter angiographic embolization (TAE) should be considered. Surgery is indicated when TAE is not locally available or after failed TAE. Strong recommendation, moderate quality evidence. 9: ESGE recommends high dose proton pump inhibitor (PPI) therapy for patients who receive endoscopic hemostasis and for patients with FIIb ulcer stigmata (adherent clot) not treated endoscopically. (A): PPI therapy should be administered as an intravenous bolus followed by continuous infusion (e. g., 80 mg then 8 mg/hour) for 72 hours post endoscopy. (B): High dose PPI therapies given as intravenous bolus dosing (twice-daily) or in oral formulation (twice-daily) can be considered as alternative regimens.Strong recommendation, high quality evidence. 10: ESGE recommends that in patients who require ongoing anticoagulation therapy following acute NVUGIH (e. g., peptic ulcer hemorrhage), anticoagulation should be resumed as soon as the bleeding has been controlled, preferably within or soon after 7 days of the bleeding event, based on thromboembolic risk. The rapid onset of action of direct oral anticoagulants (DOACS), as compared to vitamin K antagonists (VKAs), must be considered in this context.Strong recommendation, low quality evidence.European Society of Gastrointestinal Endoscopy. All rights reserved.
The risks associated with GI endoscopy: how much do we really know?
PMID:17591471 [Cited within: 1]
Comparison of the outcome of emergency endotracheal intubation in the general ward, intensive care unit and emergency department
DOI:10.1016/j.bj.2020.07.006 URL [Cited within: 1]
Schock index
DOI:10.1055/s-0028-1106070 URL [Cited within: 1]
Evaluation of preintubation shock index and modified shock index as predictors of postintubation hypotension and other short-term outcomes