Ritodrine hydrochloride (ritodrine) is a β2-adrenoceptor stimulant that has been effectively prescribed for the prevention of premature labor.[1] In clinical practice, one of the biggest drawbacks of β-mimetics is their adverse effects, including arrhythmias, myocardial ischemia, and pulmonary edema, due to their β-sympathomimetic effects.[2] Here, we report a rare pregnant case of spontaneous coronary artery dissection (SCAD) after intravenous infusion of ritodrine.
CASE
A 29-year-old woman visited the local hospital at gestational age of 32 weeks and 3 days with frequent uterine contractions for 2 d. She was in good general health with no abnormalities in regular obstetric examinations or family history of genetic diseases. Physical examination and obstetrical ultrasound showed that her cervix was shortened, and irregular contractions were observed during fetal heart rate monitoring. In addition, myocardial enzyme, troponin, brain natriuretic peptide (BNP), electrocardiogram (ECG), and echocardiography on admission were normal. Subsequently, she only received intravenous infusion of ritodrine to inhibit uterine contraction. The initial dose of ritodine was 100 μg/min, increased by 50 μg per 10 min, and finally maintained at 250 μg/min. After 3 h of ritodrine infusion, the patient developed chest tightness and chest pain, and myocardial enzyme, troponin, BNP, ECG and echocardiography were immediately tested (these results were normal). Then, the ritodine dose was reduced to 100 μg/min, and these symptoms were relieved, with stable blood pressure and heart rate 90-100 beats/min. After the disappearance of chest pain, ritodrine was gradually increased to 250 μg/min, as she still had contractions. Chest tightness and chest pain occurred again after 30 min of infusion at 250 μg/min. After reducing the dose to 100 μg/min, the symptoms were relieved and tolerable. After maintaining this dose for 2 h, the patient suddenly developed severe chest pain and radiated to the left upper limb, left shoulder, and left back. The ritodrine infusion was immediately stopped, and her chest pain and radiation pain were relieved. ECG showed acute anterior myocardial infarction (MI). The patient was then transferred to our hospital.
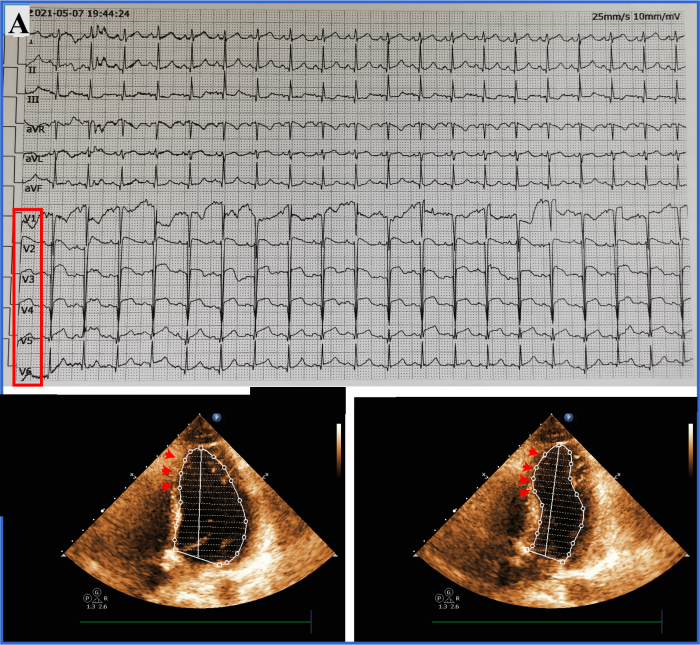
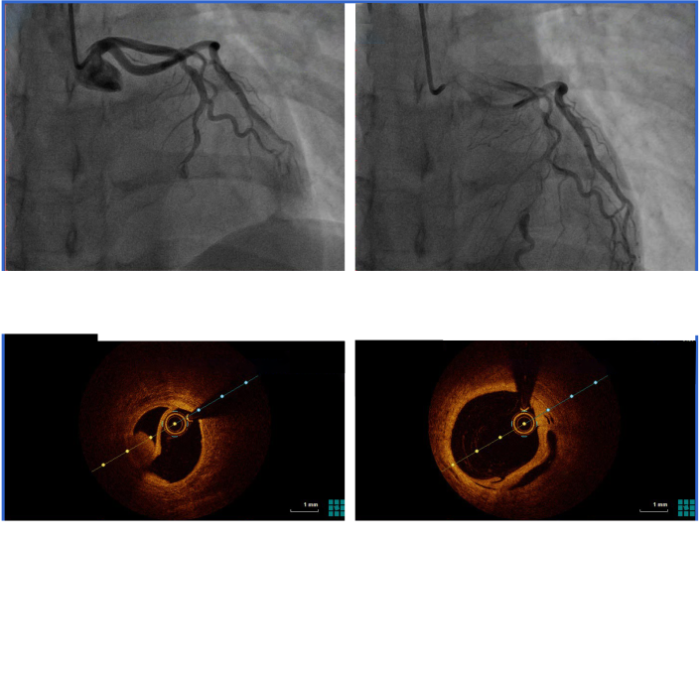
An ECG re-examination at our hospital showed sinus rhythm, ST-segment elevation in leads V1 to V6, and T wave inversion in leads V1 to V5 (Figure 1A). Laboratory tests showed that creatine kinase (CK) was 1,628 U/L (normal range 18-198 U/L), creatine kinase isoenzyme (CK-MB) 162 U/L (normal range <20 U/L), myoglobin 133 mg/L (normal range 10-70 mg/L), and troponin 34.996 ng/mL (normal range 0-0.020 ng/mL). Other serum biochemical indexes were normal. Transthoracic echocardiography showed that her left ventricular wall had abnormal phasic motion (Figures 1B, C). Based on these findings, her initial diagnosis was suspected to be acute extensive anterior ST-elevation MI. After multidisciplinary consultation and discussion, she received a cesarean section on the second day after admission. Later, coronary angiography (CAG) and optical coherence tomography (OCT) were performed on the 7th day after admission. The CAG revealed approximately 60% eccentric stenosis in the middle of the left anterior descending (LAD) branch and approximately 80% localized stenosis in the distal segment with a linear spontaneous dissection obturating the true arterial lumen (Figures 2A, B). OCT showed distal intimal detachment of the LAD, accompanied by intramural hematoma, thrombus, and a few fibrous plaques (Figures 2C, D). On the 8th day after admission, she was discharged home and took 90 mg of tegrenol orally twice a day for 12 months, 75 mg of aspirin, and 2.5 mg of bisoprolol once a day. At 6 months of follow-up, she was clinically asymptomatic and refused further consultation.
Figure 1.
Figure 1.
Examination at our hospital. A: admission ECG; B, C: transthoracic echocardiography showed segmental weakening of left ventricular wall motion (arrows).
Figure 2.
Figure 2.
Images of CAG and OCT. A, B: CAG showed an intimal tear (A, arrow) with persistent distal filling defects and a linear dissection (B, arrow) in the distal segment of LAD with false lumen filled with contrast agent; C, D: OCT indicated distal intimal detachment of LAD with intramural hematoma and thrombus (arrows). CAG: coronary angiography; OCT: optical coherence tomography; LAD: left anterior descending.
DISCUSSION
SCAD is an acute noniatrogenic tear in the coronary arterial wall, leading to disruption of coronary blood flow and MI.[3] SCAD is an uncommon but increasingly recognized cause of acute MI among young and middle-aged women and is an important cause of pregnancy-associated MI. Pregnancy-related SCAD (P-SCAD) accounts for <5% of SCAD cases.[4] Ritodrine is a selective β2 receptor agonist that strongly inhibits uterine contractions and is widely used clinically to prevent preterm birth.[5] A previous study found that the incidence of adverse reactions in ritozine tablets was approximately 3.9%, and in injections, it was approximately 18.3%.[5]
In this case, SCAD occurred after ritodrine infusion. The reason may be as follows: (1) after exclusion of other causes or triggers of SCAD during pregnancy, a clinically relevant fact was clearly observed, that is, SCAD-related symptoms disappeared with ritodrine withdrawal or dose reduction and recurred with dose increase; (2) the pathological changes in vascular wall structure related to progesterone in the third trimester of pregnancy could provide a prerequisite for the formation of dissection;[6] and (3) due to the relatively large dose of ritodrine used in the clinic to inhibit premature labor, ritodrine may have lost its β-receptor selectivity, leading to increased adverse cardiac effects such as increased cardiac output, stroke volume, or heart rate.
CONCLUSIONS
This case highlights that in addition to other previously reported complications, ritodrine may also cause SCAD. Therefore, we suggest that pregnant women should be cautious in using ritodrine. In addition, when acute coronary syndrome occurs in the context of environmental stress factors (such as ritodrine treatment), CAG and OCT should be performed for final diagnosis.
Funding: None.
Ethical approval: This study was approved by the Local Ethical Committee. Written informed consent for publication of their clinical details and clinical images was obtained from our patient.
Contributors: YQA and YLD contributed equally to this work. All authors made a significant contribution to the work reported.
Conflicts of interests: The authors declare no conflict of interest.
Reference
Effects of ritodrine hydrochloride, a beta 2-adrenoceptor stimulant, on uterine motilities in late pregnancy
PMID:6482092
[Cited within: 1]

Ritodrine hydrochloride (ritodrine) is a beta 2-adrenoceptor stimulant which has been effectively prescribed for the prevention of premature labor. The present studies were carried out to investigate the effects of ritodrine on uterine motility in rats and rabbits during gestation, as compared with those of isoproterenol and isoxsuprine. The results were as follows: 1) Spontaneous movements and evoked contractile responses of isolated rat uterus (19-20th days of gestation) were suppressed by 10(-9) - 10(-6) M ritodrine. The potency of ritodrine was approximately 10 times more than that of isoxsuprine and 100 - 1,000 times less than that of isoproterenol. 2) When these drugs were administered to pregnant rats or rabbits intravenously, the tocolytic potency was in the following order: isoproterenol greater than ritodrine greater than isoxsuprine. 3) Ritodrine induced hypotension and tachycardia, but these effects were less than those of isoproterenol and isoxsuprine. 4) The effects of isoproterenol and ritodrine were almost prevented by pretreatment with propranolol, but those of isoxsuprine were only partially or not affected. These results suggest that ritodrine is effective in preventing the uterine contractions in rats and rabbits and that it has less effect on the circulatory system than isoproterenol and isoxsuprine. It is also concluded that ritodrine produces these effects through activation of beta-adrenoceptors.
Deleterious genetic variants in ciliopathy genes increase risk of ritodrine-induced cardiac and pulmonary side effects
DOI:10.1186/s12920-018-0323-4
PMID:29368655
[Cited within: 1]

Background: Ritodrine is a commonly used tocolytic to prevent preterm labour. However, it can cause unexpected serious adverse reactions, such as pulmonary oedema, pulmonary congestion, and tachycardia. It is unknown whether such adverse reactions are associated with pharmacogenomic variants in patients.Methods: Whole-exome sequencing of 13 subjects with serious ritodrine-induced cardiac and pulmonary side-effects was performed to identify causal genes and variants. The deleterious impact of nonsynonymous substitutions for all genes was computed and compared between cases (n = 13) and controls (n = 30). The significant genes were annotated with Gene Ontology (GO), and the associated disease terms were categorised into four functional classes for functional enrichment tests. To assess the impact of distributed rare variants in cases with side effects, we carried out rare variant association tests with a minor allele frequency <= 1% using the burden test, the sequence Kernel association test (SKAT), and optimised SKAT.Results: We identified 28 genes that showed significantly lower gene-wise deleteriousness scores in cases than in controls. Three of the identified genes-CYP1A1, CYP8B1, and SERPINA7-are pharmacokinetic genes. The significantly identified genes were categorized into four functional classes: ion binding, ATP binding, Ca2+-related, and ciliopathies-related. These four classes were significantly enriched with ciliary genes according to SYSCILIA Gold Standard genes (P < 0.01), thus representing ciliary genes. Furthermore, SKAT showed a marginal trend toward significance after Bonferroni correction with Joubert Syndrome ciliopathy genes (P = 0.05). With respect to the pharmacokinetic genes, rs1048943 (CYP1A1) and rs1804495 (SERPINA7) showed a significantly higher frequency in cases than controls, as determined by Fisher's exact test (P < 0.05 and P < 0.01, respectively).Conclusions: Ritodrine-induced cardiac and pulmonary side effects may be associated with deleterious genetic variants in ciliary and pharmacokinetic genes.
Spontaneous coronary artery dissection: a call for consensus and research advancement
DOI:S0735-1097(19)35937-6 PMID:31488266 [Cited within: 1]
Postpartum multi-vessel spontaneous coronary artery dissection in the setting of cocaine and amphetamine use: a case report
Acute coronary syndrome associated with oral administration of ritodrine hydrochloride during pregnancy: a case report
Spontaneous coronary artery dissection- a need for raised awareness among healthcare professionals evaluating pregnant and post-partum women with chest pain
DOI:S0378-5122(17)30714-4 PMID:28789878 [Cited within: 1]