INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a chronic disease of the respiratory system characterized by progressive respiratory decline and recurrent lower respiratory tract infections.[1,2] Respiratory failure is a common complication of COPD with high mortality.[3] A study reported that the bodies of patients with acute exacerbation of COPD (AECOPD), who were on mechanical ventilation (MV) and could not eat independently, were in a state of high decomposition, and there was a negative balance of energy and protein metabolism.[4] This greatly aggravates malnutrition and causes acute muscular atrophy. The disease condition was further complicated by muscle weakness, which led to difficulty in weaning, concurrent infection, and increased mortality. Growth differentiation factor-15 (GDF-15) is a divergent member of the transforming growth factor-β (TGF-β) superfamily, which is involved in developing muscle atrophy in various diseases, including COPD, cancer, and pulmonary hypertension.[5] The cross-sectional area of the erector spine muscle (ESMcsa) and plasma GDF-15 levels can be used to assess muscle loss. Therefore, this study aimed to compare the effects of early standardized enteral nutrition (EN) and conventional EN on preventing acute muscle loss in AECOPD patients with MV by assessing the ESMcsa and plasma GDF-15 levels.
METHODS
Study population
A total of 97 AECOPD patients, who were on invasive MV from April 2020 to May 2022 and had complications of acute respiratory failure, were sequentially screened and recruited in this study. According to the start time (May 2021 as the time node) of the continuous quality improvement project for EN in critically ill patients from the First People’s Hospital of Lianyungang, the study was divided into two stages, including stage I (from April 2020 to April 2021)and stage II (from May 2021 to May 2022). There was a training period from April 2021 to May 2021. The study protocol was approved by the Ethics Committee of the First People’s Hospital of Lianyungang (approval number: LCYJ2020032001). Written informed consent was obtained from the legal representative of each patient before the study. The patients’ clinical records/information were anonymized and deidentified before analysis. The study was registered in the China Clinical Trial Center under registration number ChiCTR1900025382. The study was reported according to the STROBE checklist (supplementary Table 1).[6]
Inclusion criteria
All patients were diagnosed based on the Guidelines for the Diagnosis and Management of Chronic Obstructive Pulmonary Disease (Revised in 2021) and had respiratory failure.[7] Other inclusion criteria included indications for invasive MV, ages ranging from 18 to 80 years, consciousness, Glasgow Coma Scale (GCS) score ≥12, estimated MV ≥5 d, and ICU length of stay ≥7 d.
Exclusion criteria
The exclusion criteria were spinal cord injury, acute stroke, lower limb fracture, history of cognitive dysfunction or neuromuscular disease, previously bedridden for a long time and already having muscle atrophy, muscle relaxant treatment, acute and chronic heart failure, pulmonary fibrosis, pulmonary vascular disease, severe edema, intestinal perforation, peritonitis, intestinal fistula, necrosis, other EN contraindications, severe hemodynamic instability, or severe coagulopathy on admission.
EN feeding protocols
In stage I (conventional EN group), the physicians provided EN according to their judgment or experience and did not uniformly set the start time, EN preparation, or energy goals. Before the initiation of stage II (early standardized EN group), all physicians, nurses, and nutritionists involved in the study received a one-month training on the early standardized EN feeding protocol to ensure that they received sufficient training and knowledge on the EN feeding protocol. In addition, a researcher was appointed to monitor and promote compliance with the EN feeding protocol. From May 2021, patients were given early standardized EN according to the feeding protocol.
Based on the clinical practice summary of the American Society for Parenteral and Enteral Nutrition (ASPEN) Guidelines (2020), a process-based nutrition treatment strategy was developed.[8] EN tolerance and complications were evaluated using the EN tolerance scale and EN intolerance algorithm, respectively. The nutritional risk screening (NRS) score 2002 and acute gastrointestinal injury (AGI) score were calculated immediately after admission to the ICU. Before the start of EN treatment, the hemodynamics were stable, the mean arterial pressure (MAP) was >65 mmHg (1 mmHg=0.133 kPa), the lactic acid was <4 mmol/L, and the dose of vasopressor was reduced. Gastrointestinal function was evaluated using the AGI score. For patients with AGI of grades I and II-III, EN and predigestion EN were started at 25 mL/h and 10-15 mL/h, respectively. EN was reserved for those with AGI of grade IV.[9] For patients with a high risk of malnutrition, parenteral nutrition (PN) was recommended, while for other patients, PN was reserved for 7-10 d. EN patients were evaluated every four hours using the tolerance score. When the EN tolerance score was greater than 5, EN was discontinued.
The EN used in this study consisted of the whole protein Nengquanli (enteral nutritional suspension, Nutricia Pharmaceuticals, the Netherlands) and short peptide Baipuli (enteral nutritional suspension, Nutricia Pharmaceuticals, the Netherlands).
Observation indicators
2.5.1 Baseline data
The patient’s gender, age, weight, body mass index (BMI), Glasgow Coma Scale (GCS), Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, underlying medical history, smoking history, mechanical ventilation parameters, and arterial blood gas analysis were recorded in detail.
2.5.2 ESMcsa
All patients in this study received a chest and abdominal computed tomography (CT) scan before or on the first day of admission. CT re-examination was performed at 7 d after treatment. The mediastinal window of the chest and abdominal CT was used for the ESMcsa analysis. The inferior margin of the T-12 vertebra was the target of the ESM analysis. The detailed results of ESMcsa were recorded. The right and left ESMs were identified in the CT images, and the pseudocolor images of the ESMs were delineated manually.
As shown in supplementary Figure 1, the ESMcsa on each side and the total ESMcsa on both sides of the spine were calculated. All measurements were performed by two trained physicians. Each physician obtained three measurements each time from each patient. First, to determine the coefficient of variation (CV), two additional measurements were taken (3×3 measurements for the first 15 patients). The CV of the first 15 patients was controlled within 8.1% and that of other patients was 8.3%, showing good inter-observer reliability.
2.5.3 Plasma GDF-15
Blood samples were collected into ethylene diamine tetra-acetic acid (EDTA) tubes on days 1 and 7. Plasma was separated from the blood samples by centrifugation at 3,500 r/min for 10 min within 2 h of collection. The plasma samples were stored at -80 ℃ until they were processed. The plasma GDF-15 concentration was determined using an enzyme-linked immunosorbent assay (ELISA) kit (Life-tech, USA) according to the manufacturer’s instructions.
2.5.4 Muscle strength
All sedatives and analgesics were discontinued on days 1 and 7 of ICU admission. All patients were awake and responsive during the examination. Muscle strength was evaluated with the Medical Research Council (MRC) sum score. This score appoints a value between 0 (no contraction at all) and 5 (normal muscle strength) for six muscle groups, including shoulder abduction, elbow flexion, wrist extension, hip flexion, knee extension, and dorsiflexion of the ankle, all scored bilaterally. The sum score ranges between 0 and 60, and intensive care unit-acquired weakness (ICU-AW) is diagnosed with a total score of <48. In this study, the MRC scores were given simultaneously by the two trained physicians.
2.5.5 ICU management indicators
The incidence of ICU-AW, sepsis, and ventilator-associated pneumonia (VAP), the time of invasive mechanical ventilation, ICU and hospital length of stay, and 28-day mortality in the two groups were statistically analyzed in detail.
Statistical analyses
The statistical data were analyzed using SPSS 22.0 statistical software and GraphPad Prism version 6.0. All the continuous variables were expressed as the mean±standard deviation (SD) and compared using two independent sample t-tests. The comparisons of the data counts were performed using the Chi-square test. A P-value <0.05 was considered statistically significant.
RESULTS
Comparison of the general data
Among the 143 enrolled patients, 32 patients were excluded based on the exclusion criteria. For the remaining 111 patients, 10 patients were excluded due to <7 d of their stay time, against-advice discharge, or death, and four patients were excluded for not completing the chest CT re-examination and ESMcsa measurements on day 7. Ultimately, a total of 97 patients, including 61 males and 36 females, were enrolled in this study. Among these 97 patients, 46 patients were in stage I (conventional EN group), and 51 patients were in stage II (early standardized EN group) (supplementary Figure 2). There were no significant differences in sex, age, weight, body mass index (BMI), basic medical history, smoking history, MV parameters, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, or other baseline levels (all P>0.05) between the two groups, as listed in Table 1.
Table 1. Comparison of the baseline data and mechanical ventilation variables between the two groups
| Indicators | Conventional EN group (n=46) | Early standardized EN group (n=51) | t/χ2 | P-value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, years | 65.49±9.28 | 64.32±10.23 | 0.587 | 0.558 |
| Male/total | 28/46 | 33/51 | 0.391 | 0.696 |
| Weight, kg | 61.22±10.41 | 62.87±9.68 | 0.809 | 0.431 |
| Body mass index, kg/m2 | 24.32±3.32 | 25.13±3.74 | 1.123 | 0.264 |
| Glasgow Coma Scale | 13.54±1.33 | 13.32±1.56 | 0.743 | 0.459 |
| Sequential Organ Failure Assessment | 8.95±3.56 | 8.79±3.73 | 0.216 | 0.830 |
| APACHE II | 15.75±3.43 | 16.94±4.23 | 1.512 | 0.134 |
| PaO2, mmHg | 108.24±24.32 | 103.54±21.53 | 1.010 | 0.315 |
| PaCO2, mmHg | 83.32±14.27 | 85.42±15.38 | 0.695 | 0.488 |
| Smoking history | 23/46 | 21/51 | 0.872 | 0.383 |
| Comorbidities | ||||
| Coronary artery disease | 8/46 | 7/51 | 0.499 | 0.618 |
| Diabetes mellitus | 6/46 | 7/51 | 0.098 | 0.922 |
| Hypertension | 10/46 | 8/51 | 0.766 | 0.444 |
| Liver cirrhosis | 3/46 | 2/51 | 0.578 | 0.563 |
| Mechanical ventilation | ||||
| Tidal volume, mL/kg | 7.54±2.34 | 7.32±2.29 | 0.468 | 0.641 |
| PEEP, cmH2O | 6.15±2.32 | 6.32±1.92 | 0.395 | 0.694 |
| Driving pressure, cmH2O | 13.12±3.19 | 13.23±3.31 | 0.166 | 0.868 |
| Plateau pressure, cmH2O | 24.63±3.23 | 24.35±3.76 | 0.391 | 0.696 |
| Static lung compliance, mL/cmH2O | 46.23±12.27 | 45.39±15.31 | 0.296 | 0.768 |
| Dynamic lung compliance, mL/cmH2O | 37.23±5.29 | 38.36±4.43 | 1.144 | 0.255 |
| FiO2 | 0.45±0.12 | 0.43±0.14 | 0.751 | 0.454 |
Data are expressed as mean±standard deviation or number/total. EN: enteral nutrition; APACHE II: Acute Physiology and Chronic Health Evaluation II; PaO2: partial pressure of oxygen; PaCO2: partial pressure of carbon dioxide; PEEP: positive end expiratory pressure; FiO2: fraction of inspired oxygen.
Comparison of ESMcsa, GDF-15, and MRC scores
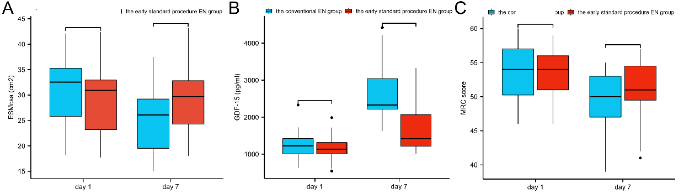
There were no significant differences in the ESMcsa, plasma GDF-15, or MRC scores between the two groups on day 1 (all P>0.05). On day 7, the plasma GDF-15 levels in the early standardized EN group were significantly lower than those in the conventional EN group, while the ESMcsa and MRC scores were significantly higher than those in the conventional EN group (all P<0.05) (Figure 1). The ESMcsa on day 7 in the two groups decreased compared to that on day 1, suggesting that both groups showed acute muscular atrophy with a significant decrease in the skeletal muscle content. This decline was more obvious in the conventional EN group (supplementary Figure 3).
Figure 1.
Figure 1.
Comparison of the ESMcsa, GDF-15 levels, and MRC scores between the two groups. EN: enteral nutrition; ESMcsa: cross-sectional area of erector spine muscle; GDF-15: growth differentiation factor-15; MRC score: Medical Research Council score; ns: not significant. *P<0.05.
Evaluation of the ICU-associated indicators
The incidence of ICU-AW, MV time, and ICU and hospital length of stay in the early standardized EN group were significantly lower than those in the conventional EN group (all P<0.05). The first-time success rate of the spontaneous breathing test (SBT) was significantly higher in the early standardized EN group (P<0.05). However, there were no significant differences in the incidence of sepsis or VAP, or re-intubation rate between the groups (P>0.05, supplementary Table 2).
Correlation analysis of the decrease in the ESMcsa with the GDF-15 level and MRC score on day 7
The correlation analysis showed that on day 7, the ESMcsa loss was significantly positively and negatively correlated with the plasma GDF-15 level and MRC score, respectively (r=0.452 and -0.328, all P<0.001) (supplementary Figure 4).
Evaluation of the 28-day prognosis
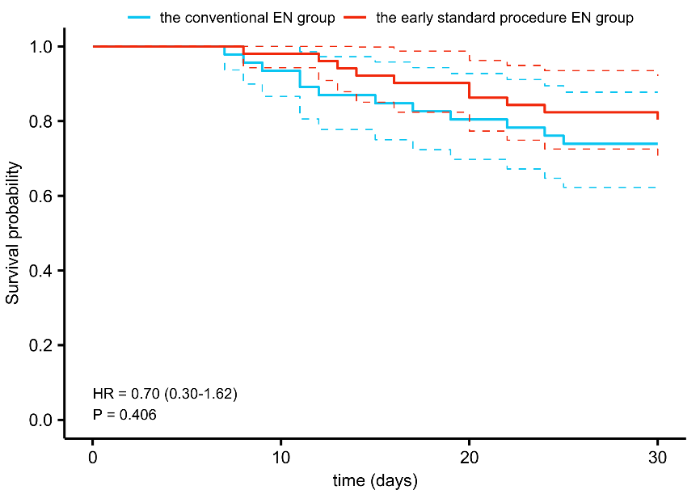
The 28-day survival rate of the enrolled patients was assessed. The Kaplan-Meier survival curve analysis showed that the 28-day survival rates were 80.40% and 73.90% in the early standardized EN group and conventional EN group, respectively (P=0.406, Figure 2).
Figure 2.
Figure 2.
The 28-day survival rate of the two groups. EN: enteral nutrition.
DISCUSSION
COPD patients may have a long course of the disease, and the repeated inflammatory consumption may lead to malnutrition.[10] The study has not focused on EN standardization in patients with AECOPD during MV,[11] which might lead to insufficient nutrition, immune function injury, infection, or an increase in death. Therefore, it is important to implement early standardized EN for AECOPD patients with MV.
The results showed that the ESMcsa loss and plasma levels of GDF-15 were significantly lower in the early standardized EN group than in the conventional EN group. This indicated that early standardized EN could significantly reduce ICU-AW. The acute reduction in the mass of skeletal muscles is a main reason for the loss of muscle strength in critically ill patients.[12] Since the ESM is an important anti-gravity skeletal muscle in the human body, clinical studies have reported that ESMcsa could objectively reflect the contents and function of skeletal muscle in patients and was related to the prognosis of lung diseases, such as COPD and pulmonary fibrosis.[13] Gosker et al[14] reported that AECOPD patients were prone to acute muscular atrophy due to acute energy consumption, nutritional deficiencies, and passive bed rest, resulting in skeletal muscle atrophy and acute loss of muscle function and strength.
Early standardized EN in critically ill patients could effectively alleviate the acute consumption of muscle tissues. In addition, COPD patients on MV often have a high risk of ICU-AW.[15] In COPD patients with acute respiratory failure, early respiratory muscle weakness might prolong the MV time and increase the risk of complications, such as VAP and ICU-AW,[16] which was consistent with our study results. Our previous study also showed that a decrease in the ESMcsa had a certain clinical diagnostic potential for ICU-AW.[17] In addition, this study also showed that the ESMcsa loss was significantly positively correlated with plasma GDF-15 levels on day 7. This indicated that GDF-15 might reflect the muscle loss. Therefore, GDF-15 levels might synergistically evaluate the degree of sarcopenia in patients. GDF-15 is an important regulator of the protein synthesis/catabolism balance and might be involved in the activation process of these proteolytic pathways.[18] The abnormal activation of GDF-15 in the human body might reduce muscle protein synthesis, thereby causing muscle atrophy. The involvement of GDF-15 in muscle atrophy has also been confirmed in an in vitro study.[19]
Limitations
There were some limitations to this study. First, the lack of information on the EN-related complications and dosage of PN might affect the results. Second, this was a before-and-after study. Therefore, confounding factors could not be fully controlled, especially unmeasured confounding factors. We tried to control confounding factors using multivariable analysis, and the results were consistent with those obtained from the unadjusted analysis. Last, this study was a single-center clinical trial. Therefore, there might be a certain bias. These results need to be further verified in a multicenter study.
CONCLUSIONS
The ESMcsa loss in AECOPD patients with MV was correlated with GDF-15 levels, both of which indicated acute muscular atrophy and skeletal muscle dysfunction. Early standardized EN may prevent acute muscle loss and ICU-AW in AECOPD patients.
Funding: This study was funded by the Social Development Project of Jiangsu Provincial Department of Science and Technology (BE2020670) and the Social Development Project of Lianyungang Science and Technology (SF2117).
Ethical approval: The study protocol was approved by the Ethics Committee of the First People’s Hospital of Lianyungang (approval number: LCYJ2020032001).
Conflicts of interest: The authors do not have a financial interest or relationship to disclose regarding this research.
Contributors: YL and YPX contributed equally to this work. All authors approved the final version.
All the supplementary files are available at http://wjem.com.cn.
Reference
Effects of Maxingloushi decoction on immune inflammation and programmed death markers in mice with chronic obstructive pulmonary disease
DOI:10.5847/wjem.j.1920-8642.2022.023
PMID:35003414
[Cited within: 1]

To investigate effects of Maxingloushi decoction on lung inflammation and programmed death markers (programmed death-1 [PD-1], programmed death-ligand 1 [PD-L1]) in the lung tissue, peripheral blood, and bronchoalveolar lavage fluid (BLF) in a mouse model of chronic obstructive pulmonary disease (COPD).Thirty-six mature male BALB/C mice were randomly divided into normal group (group A, =6), COPD model group (group B, =10), Maxingloushi decoction + COPD group (group C, =10), and PD-1 inhibitor + COPD group (group D, =10). The COPD model was established by smoke inhalation combined with lipopolysaccharide (LPS). Levels of PD-1 and PD-L1 in plasma and BLF were measured by enzyme-linked immunosorbent assay (ELISA). Histopathological techniques were used to semi-quantitatively analyze the immuno-fluorescence optical density (IOD) value of the lung tissue.In plasma and BLF, the expression of PD-1 in the group B was higher than that in the group A, and the expression of PD-L1 was lower than that in the group A. The expression of PD-1 and PD-L1 in the lung tissue was normalized in the group C in comparison with the group B (<0.05) and the group D (<0.05), and inflammatory cell infiltration in the lung tissue was also improved.These findings reveal that COPD causes an immune imbalance in the peripheral blood and lung tissue, and that both Maxingloushi decoction and PD-1 inhibitor treatment can mitigate lung inflammation in COPD by reducing PD-1 expression and increasing PD-L1 expression. The treatment effect of Maxingloushi decoction may be superior to that of PD-1 inhibitor.Copyright: © World Journal of Emergency Medicine.
Pulmonary hypertension caused by fibrosing mediastinitis
DOI:10.1016/j.jacasi.2021.11.016
PMID:36338410
[Cited within: 1]

Pulmonary hypertension (PH) is a progressive and severe disorder in pulmonary hemodynamics. PH can be fatal if not well managed. Fibrosing mediastinitis (FM) is a rare and benign fibroproliferative disease in the mediastinum, which may lead to pulmonary vessel compression and PH. PH caused by FM (PH-FM) is a pathologic condition belonging to group 5 in the World Health Organization PH classification. PH-FM has a poor prognosis because of a lack of effective therapeutic modalities and inappropriate diagnosis. With the development of percutaneous pulmonary vascular interventional therapy, the prognosis of PH-FM has been greatly improved in recent years. This article provides a comprehensive review on the epidemiology, pathophysiologic characteristics, clinical manifestations, diagnostic approaches, and treatment modalities of PH-FM based on data from published reports and our medical center with the goal of facilitating the diagnosis and treatment of this fatal disease.© 2022 The Authors.
Chronic obstructive pulmonary disease exacerbation fundamentals: diagnosis, treatment, prevention and disease impact
DOI:10.1111/resp.14041
PMID:33893708
[Cited within: 1]

In chronic obstructive pulmonary disease (COPD), exacerbations (ECOPD), characterized by an acute deterioration in respiratory symptoms, are fundamental events impacting negatively upon disease progression, comorbidities, wellbeing and mortality. ECOPD also represent the largest component of the socioeconomic burden of COPD. ECOPDs are currently defined as acute worsening of respiratory symptoms that require additional therapy. Definitions that require worsening of dyspnoea and sputum volume/purulence assume that acute infections, especially respiratory viral infections, and/or exposure to pollutants are the main cause of ECOPD. But other factors may contribute to ECOPD, such as the exacerbation of other respiratory diseases and non-respiratory diseases (e.g., heart failure, thromboembolism). The complexity of worsening dyspnoea has suggested a need to improve the definition of ECOPD using objective measurements such as blood counts and C-reactive protein to improve accuracy of diagnosis and a personalized approach to management. There are three time points when we can intervene to improve outcomes: acutely, to attenuate the length and severity of an established exacerbation; in the aftermath, to prevent early recurrence and readmission, which are common, and in the long-term, establishing preventative measures that reduce the risk of future events. Acute management includes interventions such as corticosteroids or antibiotics and measures to support the respiratory system, including non-invasive ventilation (NIV). Current therapies are broad and better understanding of clinical phenotypes and biomarkers may help to establish a more tailored approach, for example in relation to antibiotic prescription. Other unmet needs include effective treatment for viruses, which commonly cause exacerbations. Preventing early recurrence and readmission to hospital is important and the benefits of interventions such as antibiotics or anti-inflammatories in this period are not established. Domiciliary NIV in those patients who are persistently hypercapnic following discharge and pulmonary rehabilitation can have a positive impact. For long-term prevention, inhaled therapy is key. Dual bronchodilators reduce exacerbation frequency but in patients with continuing exacerbations, triple therapy should be considered, especially if blood eosinophils are elevated. Other options include phosphodiesterase inhibitors and macrolide antibiotics. ECOPD are a key component of the assessment of COPD severity and future outcomes (quality of life, hospitalisations, health care resource utilization, mortality) and are a central component in pharmacological management decisions. Targeted therapies directed towards specific pathways of inflammation are being explored in exacerbation prevention, and this is a promising avenue for future research.© 2021 Asian Pacific Society of Respirology.
Analysis of clinical effects of early enteral nutrition standardized treatment process management on patients with acute exacerbation of chronic obstructive pulmonary disease on invasive mechanical ventilation
Growth differentiation factor-15 (GDF-15): from biomarker to novel targetable immune checkpoint
DOI:10.3389/fimmu.2020.00951
PMID:32508832
[Cited within: 1]

Growth/differentiation factor-15 (GDF-15), also named macrophage inhibitory cytokine-1, is a divergent member of the transforming growth factor β superfamily. While physiological expression is barely detectable in most somatic tissues in humans, GDF-15 is abundant in placenta. Elsewhere, GDF-15 is often induced under stress conditions, seemingly to maintain cell and tissue homeostasis; however, a moderate increase in GDF-15 blood levels is observed with age. Highly elevated GDF-15 levels are mostly linked to pathological conditions including inflammation, myocardial ischemia, and notably cancer. GDF-15 has thus been widely explored as a biomarker for disease prognosis. Mechanistically, induction of anorexia via the brainstem-restricted GDF-15 receptor GFRAL (glial cell-derived neurotrophic factor [GDNF] family receptor α-like) is well-documented. GDF-15 and GFRAL have thus become attractive targets for metabolic intervention. Still, several GDF-15 mediated effects (including its physiological role in pregnancy) are difficult to explain via the described pathway. Hence, there is a clear need to better understand non-metabolic effects of GDF-15. With particular emphasis on its immunomodulatory potential this review discusses the roles of GDF-15 in pregnancy and in pathological conditions including myocardial infarction, autoimmune disease, and specifically cancer. Importantly, the strong predictive value of GDF-15 as biomarker may plausibly be linked to its immune-regulatory function. The described associations and mechanistic data support the hypothesis that GDF-15 acts as immune checkpoint and is thus an emerging target for cancer immunotherapy.Copyright © 2020 Wischhusen, Melero and Fridman.
Guidelines for reporting medical research: a critical appraisal
Revision to the guidelines for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021): process and perspective
American Society for Parenteral and Enteral Nutrition clinical guidelines: the validity of body composition assessment in clinical populations
DOI:10.1002/jpen.1669
PMID:31216070
[Cited within: 1]

On behalf of the American Society for Parenteral and Enteral Nutrition (ASPEN), a systematic review was conducted to evaluate the best available evidence regarding the validity of relevant body composition methods (eg, dual energy X-ray absorptiometry [DXA], ultrasound [US], and bioelectrical impedance analysis [BIA]) in clinical populations. The guidelines targeted adults >18 years of age with a potentially inflammatory condition or pathological end point associated with a specific disease or clinical condition. In total, 7375 studies were retrieved, and 15 DXA, 7 US, and 23 BIA studies provided applicable data. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses was used to assess the diagnostic accuracy of the test method against a "gold standard" reference. The Grading of Recommendations, Assessment, Development and Evaluation criteria were used to separate the evaluation of the body of evidence from the recommendations. Based on a limited number of studies and expert opinion, DXA is recommended for the assessment of fat mass in patients with a variety of disease states; however, the validity of DXA for lean mass assessment in any clinical population remains unknown. No recommendations can be made at this time to support the use of US or BIA in the clinical setting, as data to support its validity in any specific patient population are limited in scope or by the proprietary nature of manufacture-specific BIA regression models to procure body composition data, respectively. Directions for future research are provided. These clinical guidelines were approved by the ASPEN Board of Directors.© 2019 American Society for Parenteral and Enteral Nutrition.
Effectiveness of enteral feeding protocol on clinical outcomes in critically ill patients: a before and after study
Effects of Maxingloushi decoction on immune inflammation and programmed death markers in mice with chronic obstructive pulmonary disease
DOI:10.5847/wjem.j.1920-8642.2022.023
PMID:35003414
[Cited within: 1]

To investigate effects of Maxingloushi decoction on lung inflammation and programmed death markers (programmed death-1 [PD-1], programmed death-ligand 1 [PD-L1]) in the lung tissue, peripheral blood, and bronchoalveolar lavage fluid (BLF) in a mouse model of chronic obstructive pulmonary disease (COPD).Thirty-six mature male BALB/C mice were randomly divided into normal group (group A, =6), COPD model group (group B, =10), Maxingloushi decoction + COPD group (group C, =10), and PD-1 inhibitor + COPD group (group D, =10). The COPD model was established by smoke inhalation combined with lipopolysaccharide (LPS). Levels of PD-1 and PD-L1 in plasma and BLF were measured by enzyme-linked immunosorbent assay (ELISA). Histopathological techniques were used to semi-quantitatively analyze the immuno-fluorescence optical density (IOD) value of the lung tissue.In plasma and BLF, the expression of PD-1 in the group B was higher than that in the group A, and the expression of PD-L1 was lower than that in the group A. The expression of PD-1 and PD-L1 in the lung tissue was normalized in the group C in comparison with the group B (<0.05) and the group D (<0.05), and inflammatory cell infiltration in the lung tissue was also improved.These findings reveal that COPD causes an immune imbalance in the peripheral blood and lung tissue, and that both Maxingloushi decoction and PD-1 inhibitor treatment can mitigate lung inflammation in COPD by reducing PD-1 expression and increasing PD-L1 expression. The treatment effect of Maxingloushi decoction may be superior to that of PD-1 inhibitor.Copyright: © World Journal of Emergency Medicine.
Our great forgotten, chronic respiratory sufferers
DOI:10.20960/nh.1238
PMID:28585855
[Cited within: 1]

Lung’s own properties make that nutritional support, besides covering the requirements can modulate its infl ammatory response.Lung tissue has a low glucose stock. Fatty acids are the main energy producer of type II pneumocytes, which use them in order to form phospholipids, essential for surfactant whose creation and release decrease in acute lung injury (ALI). Glutamine is a good substratum for endocrine cells and type II pneumocytes. Due to high nutritional risk, it is important its assessments in disorders as COPD and acute respiratory distress syndrome (ADRS). Indirect calorimetry values the effect of ventilation and nutritional support, avoiding overfeeding. Hypophosphatemia and refeeding syndrome are frequent and need to be avoided because of their morbidity.In critically ill patients, malnutrition can lead to respiratory failure and increasing mechanical ventilation time. To avoid hypercapnia in weaning, glucose levels should be controlled.High lipids/carbohydrates ratio do not show usefulness in COPD neither mechanical ventilation removal. ALI patients beneficiate from an early start and the volume administered. Enteral nutrition with high fatty acids ratio (EPA, DHA and γ-linolenic acid) and antioxidants do not show any superiority. Omega-3 fatty acid in parenteral nutrition could modulate infl ammation and immunosuppression in a positive manner. The use of glutamine, vitamins or antioxidants in these patients could be justified.
Causes of mortality in ICU-acquired weakness
DOI:10.1177/0885066617745818
PMID:29241382
[Cited within: 1]

Intensive care unit-acquired weakness (ICU-AW) is a common complication of critical illness and is associated with increased mortality, longer mechanical ventilation and longer hospital stay. Little is known about the causes of mortality in patients with ICU-AW. In this study, we aimed to give an overview of the causes of death in a population diagnosed with ICU-AW during hospital admission.Data from a prospective cohort study in the mixed medical-surgical ICU of the Academic Medical Center in Amsterdam were used. Patients were included when mechanically ventilated for more than 48 hours. Intensive care unit-acquired weakness was defined as a mean medical research council score <4. Baseline data and data on the time of death were collected.Fifty-three patients were included. Irreversible shock with multiple organ failure (MOF) was the most common cause of death (28/53 of patients; 26 patients with septic shock and 2 patients with hypovolemic shock). Most common site of sepsis was abdominal (38.5%) and pulmonary (19.2%). On admission to the ICU, 53% had a do-not-resuscitate code. In 74% of the patients, further treatment limitations were implemented during their ICU stay.In this cohort of patients with ICU-AW, most patients died of irreversible shock with MOF, caused by sepsis.
Accelerated loss of antigravity muscles is associated with mortality in patients with COPD
DOI:10.1159/000506520
PMID:32235124
[Cited within: 1]

Low antigravity muscle mass is strongly associated with poor prognosis in patients with chronic obstructive pulmonary disease (COPD). However, the significance of longitudinal changes in antigravity muscle mass remains unclear in patients with COPD.The aims of this study were to investigate the factors associated with the longitudinal loss of antigravity muscles and whether the accelerated loss of these muscles has a negative impact on prognosis.This study was part of a prospective observational study at Kyoto University. We enrolled stable male patients with COPD who underwent longitudinal quantitative CT analysis of the cross-sectional area of the erector spinae muscles (ESMCSA) at an interval of 3 years. The associations between the rate of change in ESMCSA (%ΔESM) and clinical parameters, such as anthropometry, symptoms, lung function, exacerbation frequency, and all-cause mortality, were investigated.In total, 102 stable male COPD patients were successfully evaluated in this study (71.3 ± 8.3 years, GOLD stage I/II/III/IV = 20/47/28/7 patients). ESMCSA significantly decreased from 30.53 to 28.98 cm2 (p < 0.0001) in 3 years, and the mean %ΔESM was 5.21 ± 7.24%. The rate of survival during the observation period was 85.3% (87/102). Patients with an accelerated decline in ESMCSA (n = 31; more than double the mean rate of decline) had a significantly higher frequency of moderate-to-severe exacerbations during the interval (p = 0.015). They also had significantly worse survival (p = 0.035 by log-rank test). A multivariate Cox proportional hazard model showed that lower ESMCSA and greater %ΔESM decline were independently and significantly associated with mortality.Frequent exacerbations were related to the loss of antigravity muscles in COPD patients. The accelerated loss of antigravity muscles was associated with a poor prognosis.© 2020 S. Karger AG, Basel.
Role of acute exacerbations in skeletal muscle impairment in COPD
DOI:10.1080/17476348.2021.1843429 URL [Cited within: 1]
Deterioration of limb muscle function during acute exacerbation of chronic obstructive pulmonary disease
DOI:10.1164/rccm.201703-0615CI URL [Cited within: 1]
Effects of inspiratory muscle training and calisthenics-and-breathing exercises in COPD with and without respiratory muscle weakness
DOI:10.4187/respcare.03947
PMID:26556894
[Cited within: 1]

Patients with COPD may experience respiratory muscle weakness. Two therapeutic approaches to the respiratory muscles are inspiratory muscle training and calisthenics-and-breathing exercises. The aims of the study are to compare the effects of inspiratory muscle training and calisthenics-and-breathing exercises associated with physical training in subjects with COPD as an additional benefit of strength and endurance of the inspiratory muscles, thoracoabdominal mobility, physical exercise capacity, and reduction in dyspnea on exertion. In addition, these gains were compared between subjects with and without respiratory muscle weakness.25 subjects completed the study: 13 composed the inspiratory muscle training group, and 12 composed the calisthenics-and-breathing exercises group. Subjects were assessed before and after training by spirometry, measurements of respiratory muscle strength and test of inspiratory muscle endurance, thoracoabdominal excursion measurements, and the 6-min walk test. Moreover, scores for the Modified Medical Research Council dyspnea scale were reported.After intervention, there was a significant improvement in both groups of respiratory muscle strength and endurance, thoracoabdominal mobility, and walking distance in the 6-min walk test. Additionally, there was a decrease of dyspnea in the 6-min walk test peak. A difference was found between groups, with higher values of respiratory muscle strength and thoracoabdominal mobility and lower values of dyspnea in the 6-min walk test peak and the Modified Medical Research Council dyspnea scale in the inspiratory muscle training group. In the inspiratory muscle training group, subjects with respiratory muscle weakness had greater gains in inspiratory muscle strength and endurance.Both interventions increased exercise capacity and decreased dyspnea during physical effort. However, inspiratory muscle training was more effective in increasing inspiratory muscle strength and endurance, which could result in a decreased sensation of dyspnea. In addition, subjects with respiratory muscle weakness that performed inspiratory muscle training had higher gains in inspiratory muscle strength and endurance but not of dyspnea and submaximal exercise capacity. (ClinicalTrials.gov registration NCT01510041.).Copyright © 2016 by Daedalus Enterprises.
Acute reduction of erector spine muscle cross-sectional area is associated with ICU-AW and worse prognosis in patients with mechanical ventilation in the ICU: a prospective observational study
DOI:10.1097/MD.0000000000027806 URL [Cited within: 1]
Growth/differentiation factor 15 causes TGFβ-activated kinase 1-dependent muscle atrophy in pulmonary arterial hypertension
DOI:10.1136/thoraxjnl-2017-211440
PMID:30554141
[Cited within: 1]

Skeletal muscle dysfunction is a clinically important complication of pulmonary arterial hypertension (PAH). Growth/differentiation factor 15 (GDF-15), a prognostic marker in PAH, has been associated with muscle loss in other conditions. We aimed to define the associations of GDF-15 and muscle wasting in PAH, to assess its utility as a biomarker of muscle loss and to investigate its downstream signalling pathway as a therapeutic target.GDF-15 levels and measures of muscle size and strength were analysed in the monocrotaline (MCT) rat, Sugen/hypoxia mouse and in 30 patients with PAH. In C2C12 myotubes the downstream targets of GDF-15 were identified. The pathway elucidated was then antagonised in vivo.Circulating GDF-15 levels correlated with tibialis anterior (TA) muscle fibre diameter in the MCT rat (Pearson r=-0.61, p=0.003). In patients with PAH, plasma GDF-15 levels of <564 pg/L predicted those with preserved muscle strength with a sensitivity and specificity of ≥80%. In vitro GDF-15 stimulated an increase in phosphorylation of TGFβ-activated kinase 1 (TAK1). Antagonising TAK1, with 5(Z)-7-oxozeaenol, in vitro and in vivo led to an increase in fibre diameter and a reduction in mRNA expression of atrogin-1 in both C2C12 cells and in the TA of animals who continued to grow. Circulating GDF-15 levels were also reduced in those animals which responded to treatment.Circulating GDF-15 is a biomarker of muscle loss in PAH that is responsive to treatment. TAK1 inhibition shows promise as a method by which muscle atrophy may be directly prevented in PAH.NCT01847716; Results.© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
GDF-15 in tumor-derived exosomes promotes muscle atrophy via Bcl-2/caspase-3 pathway
DOI:10.1038/s41420-022-00972-z
PMID:35379793
[Cited within: 1]

Tumor-derived exosomes are emerging mediators of cancer cachexia, a kind of multifactorial syndrome characterized by serious loss of skeletal muscle mass and function. Our previous study had showed that microRNAs in exosomes of C26 colon tumor cells were involved in induction of muscle atrophy. Here, we focus on studying proteins in tumor-derived exosomes which might also contribute to the development of cancer cachexia. Results of comparing the protein profiles of cachexic C26 exosomes and non-cachexic MC38 exosomes suggested that growth differentiation factor 15 (GDF-15) was rich in C26 exosomes. Western blotting analysis confirmed the higher levels of GDF-15 in C26 cells and C26 exosomes, compared with that of MC38 cells. Results of animal study also showed that GDF-15 was rich in tumor tissues, serum exosomes, and gastrocnemius (GA) muscle tissues of C26 tumor-bearing mice. GDF-15 protein could directly induce muscle atrophy of cultured C2C12 myotubes via regulating Bcl-2/caspase-3 pathways. What's more, overexpression of GDF-15 in MC38 cells could increase the potency of MC38 conditioned medium or exosomes in inducing muscle atrophy. Knockdown of GDF-15 in C26 cells decreased the potency of C26 conditioned medium or exosomes in inducing muscle atrophy. These results suggested that GDF-15 in tumor-derived exosomes could contribute to induction of muscle atrophy and also supported the possibility of targeting GDF-15 in treatment of cancer cachexia.© 2022. The Author(s).