INTRODUCTION
Acute pancreatitis (AP) is a relatively prevalent disease that results in localized pancreatic injury, a systemic inflammatory response, and, in severe cases, organ failure.[1⇓-3] Mortality rates for patients with AP vary with disease severity and can range from 2% to 20%.[4,5] Despite improvements in treatment options for AP in recent years, certain patients are at an elevated risk of poor outcomes.[6] Stratifying patients with AP based on their risk level is essential for clinical decision-making and treatments.[7,8] AP is a complex and heterogeneous disease, so it remains difficult to predict patient outcomes.
Several models have been developed for predicting outcomes in patients with AP, but these individual systems exhibit substantial drawbacks. Some systems employ digital interfaces that are user-friendly and accurate, enabling the calculation of nomogram-derived results that can guide treatments. Nomograms are graphical models that provide individualized risk estimation. These models are developed using regression analyses and are commonly used to evaluate prognostic outcomes associated with a range of illnesses, as they are simple, intuitive, and practical.[9⇓-11] These tools can readily yield predictive outcomes without the need for complex calculation procedures.
The present study aimed to design and validate a nomogram capable of estimating the odds of survival in patients with AP.
METHODS
Database
The present study utilized the Medical Information Mart for Intensive Care (MIMIC)-IV Critical Care Database, which is a publicly available resource that compiles data pertaining to patients admitted to the Beth Israel Deaconess Medical Center intensive care unit (ICU) from 2001-2012.[12] The right of this database was approved by the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA) and consent was obtained for the original data collection. Patients’ information in the MIMIC-IV database was anonymized; therefore, informed consent was not required. The present study was conducted as per the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis statement guidelines.[13]
Patient selection
Patients eligible for inclusion in this study were those diagnosed with AP as per the International Classification of Diseases, Ninth Revision code (ICD-9, code=5770) who were >18 years old and had been admitted to the ICU for >24 h. If multiple admissions were recorded for a single patient, only the data pertaining to the first ICU admission were analyzed. Patients were excluded if they suffered from chronic liver disease, malignant tumors, acquired immune deficiency syndrome (AIDS), hemolytic anemia, or end-stage kidney disease or if ≥20% of their data were missing from the database.
Data extraction
Structure query language (SQL) was used to extract raw data with DataGrip (v 2021.2.1) followed by further processing using R (v 4.1.1, R Foundation for Statistical Computing, Austria), which was also used to retrieve patient information from the database. All baseline data within 24 h after admission were collected. Data analyzed for this study included: (1) basic demographic characteristics, such as age, sex, ethnicity, and weight; (2) mean values for vital signs within 24 h following admission including temperature, heart rate, respiratory rate, systolic blood pressure (SBP), and diastolic blood pressure (DBP); (3) laboratory test results, including serum levels of creatinine, albumin, bilirubin, calcium, potassium, blood urea nitrogen (BUN), lactate, hemoglobin level, platelet count, and white blood cell (WBC) count; (4) Glasgow Coma Scale (GCS) scores; and (5) Simplified Acute Physiology Score II (SAPS-II), which was used as a measure of pancreatitis severity.
Study outcome
The primary outcome for this study was 90-day survival. The secondary outcomes were 30- and 60-day survival.
Management of missing data
Missing values are common in the MIMIC-IV database. When a given variable exhibited missing data for <20% of the included patients, multiple imputation was used to fill missing values with predictors to minimize bias.[14]
Statistical analysis
Continuous data are described as the mean±standard deviation (SD) or median (interquartile range [IQR]) as appropriate and were compared with Student’s t-test or rank-sum test. Categorical variables are presented as numbers (percentages) and were compared using the Chi-square test. The Shapiro-Wilk test was used to assess the normality of data distributions. Non-normally distributed data or data exhibiting heterogeneity of variance were compared via Kruskal-Wallis or Mann-Whitney U-tests. Cox regression models were used to identify independent predictors of 30-day, 60-day, and 90-day mortality, and hazard ratios (HRs) and 95% confidence intervals (95% CIs) were calculated. Receiver operating characteristic (ROC) curve analyses were used to evaluate the predictive utility of the developed nomogram. Bootstrap resampling-based internal validation test was utilized to evaluate the accuracy of the nomogram. A P-value <0.05 was the significance threshold, and all statistical analyses were performed using R (v 4.1.1).
RESULTS
Baseline characteristics of the study population
In total, 632 patient records that met our inclusion criteria were identified within the MIMIC-IV database and extracted for analysis. analysis. A total of 75 patients died within 90 days in the hospital. The following baseline characteristics differed significantly between survivors and non-survivors (all P<0.05): age, sex, ethnicity, temperature, WBC, serum levels of albumin, creatinine, BUN and lactate, SBP, DBP, and SAPS-II (supplementary Table 1).
Identification of prognostic factors
Univariate Cox proportional hazards regression model analysis showed that age, WBC, creatinine, BUN, SBP, DBP, lactate, and SAPS-II were potential predictors of 90-day mortality in patients with AP (all P<0.001). Multivariate Cox proportional hazard model analysis showed that age, WBC, SBP, serum lactate, and SAPS-II were independent predictors of 90-day mortality in patients with AP (Table 1).
Table 1. Multivariate analysis of predictors of 90-day mortality in patients with acute pancreatitis
| Variables | Univariate regression | Multivariate Cox regression | ||
|---|---|---|---|---|
| HR, 95% CI | P-value | HR, 95% CI | P-value | |
| Age, years | 1.09, 1.07-1.12 | <0.001 | 1.06, 1.03-1.08 | <0.001 |
| WBC, ×109/L | 1.08, 1.05-1.12 | <0.001 | 1.03, 1.00-1.06 | 0.046 |
| Creatinine, mg/dL | 1.52, 1.32-1.74 | <0.001 | ||
| BUN, mg/dL | 1.03, 1.02-1.04 | <0.001 | ||
| SBP, mmHg | 0.97, 0.96-0.98 | <0.001 | 0.99, 0.97-1.00 | 0.015 |
| DBP, mmHg | 0.95, 0.94-0.97 | <0.001 | ||
| Lactate, mmol/L | 1.24, 1.13-1.36 | <0.001 | 1.10, 1.01-1.20 | 0.023 |
| SAPS-II | 1.09, 1.07-1.11 | <0.001 | 1.04, 1.02-1.06 | <0.001 |
Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were approximated through Cox proportional hazards regression model analyses. Statistical tests were two-sided, and a final predictive model was developed via backward stepwise selection. WBC: white blood cell; BUN: blood urea nitrogen; SBP: systolic blood pressure; DBP: diastolic blood pressure; SAPS-II: Simplified Acute Physiology Score II.
Development of the prognostic nomogram
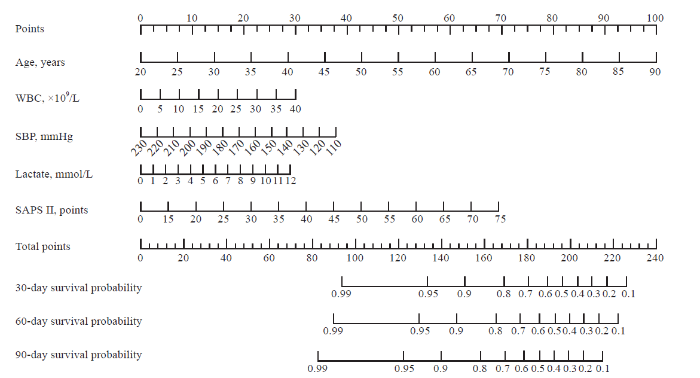
The five prognostic variables identified above were next used to establish a prognostic nomogram for estimating the probability of 30-day, 60-day and 90-day survival in patients with AP. For this nomogram, a weighted score was assigned to each of these predictors, with the highest possible score being 240 points, and the survival probability scale ranged from 0.10 to 0.99. In this model, a higher total score derived by summing the five individual predictor scores was associated with lower odds of surviving for 30 days, 60 days or 90 days (Figure 1).
Figure 1.
Figure 1.
A weighted score assigned to each of the predictors for the prognostic nomogram.
Evaluation of the performance of the prognostic nomogram
The predictive utility of the developed nomogram was assessed using ROC curve analysis. The area under the ROC curve (AUC) values for 30-day, 60-day, and 90-day survival were calculated to be 0.796 (95% CI 0.732-0.859), 0.812 (95% CI 0.755-0.869), and 0.854 (95% CI 0.805-0.903), respectively (supplementary Figures 1 A to C). When the nomogram was subjected to 1,000 bootstrap resampling-based internal validation, it was found to exhibit consistently good accuracy as a tool for predicting 30-day, 60-day, and 90-day survival odds in patients with AP, yielding bootstrap-corrected C-indexes of 0.782, 0.799, and 0.846, respectively (supplementary Figures 1 D to F). The calibration curves suggested that the model was acceptably calibrated, revealing good correlations between the odds of survival derived from this nomogram and those from estimates.
DISCUSSION
The present analysis leveraged clinical and survival-related data from 632 patients with AP included within the MIMIC-IV database to develop a model capable of predicting survival in patients with AP. Multivariate analysis identified five predictors of 90-day mortality among these patients with AP, namely, age, WBC, SBP, lactate, and SAPS-II. A predictive nomogram incorporating these five variables was then established and found to exhibit satisfactory performance in the prediction of 30-day, 60-day and 90-day survival according to AUC and calibration curve analyses. It is suggested that the nomogram might be useful in the clinical setting.
As a severe inflammatory condition with a rapid and heterogeneous clinical course, AP remains a major threat to the patients and imposes significant economic and health burdens on affected individuals.[1,2] A majority of patients with AP exhibit mild disease that resolves without any long-term complications, but an estimated 20% of patients will develop moderate or severe disease characterized by peripancreatic or pancreatic necrosis, organ failure, and mortality rates ranging from 20% to 40%.[3,4] These high-risk patients may be more likely to benefit from intensive monitoring for organ failure, aggressive fluid resuscitation efforts, appropriate antibiotic treatment, and other therapeutic interventions, including radiologic treatment or endoscopic sphincterotomy.[5] The ability to accurately evaluate AP severity during the early stage of the disease is thus essential as a means of facilitating timely intervention aimed at improving patient prognosis. Several scoring systems have been used to assess AP severity, including the Ranson score, Acute Physiology and Chronic Health Evaluation (APACHE)-Ⅱ score, Balthazar computed tomography severity index (CTSI), and Bedside Index for Severity in Acute Pancreatitis (BISAP) score.[15⇓-17] However, these individual systems exhibit specific drawbacks and limitations, and no scoring system can currently achieve maximal sensitivity and specificity. The development of additional novel prognostic models is essential to further improve predictive accuracy for various clinical populations.
Nomograms can be readily employed to evaluate the odds of a given clinical outcome for an individual patient, leading to their increasingly frequent use as prognostic tools in oncological contexts.[9⇓-11] In this study, we sought to develop a nomogram capable of quickly identifying high-risk patients with AP in an ICU setting. The developed nomogram consisted of five variables that can all be readily measured within 24 h following admission, and it was able to predict the odds of short-term survival for this patient population. This model exhibited moderate predictive utility, and the model-predicted odds of 30-day, 60-day and 90-day mortality were similar to the observed survival rates in this patient cohort.
Our final prognostic nomogram incorporated five predictors of AP patient survival. Age is well known as a primary predictor of risk among patients with AP.[3,18] An increase in WBCs may occur due to the inflammation associated with AP or due to pancreatic infection (a complication of AP). WBC count is significantly higher in patients with severe pancreatitis than in those with mild pancreatitis,[19] and WBC count is a predictor of mortality in patients with AP.[20] A low SBP is an indicator of organ failure secondary to systemic inflammation (i.e., more severe pancreatitis), and hypotension during the first week of AP is associated with an increased risk of infected pancreatic necrosis.[21] An elevated level of serum lactate is indicative of inadequate organ perfusion, and lactate level was reported to be a predictor of mortality in patients with AP,[22] including those with intraabdominal hypertension.[23] SAPS-II is an alternative version of the APACHE scale that is frequently used in the ICU setting.[24] Notably, SAPS-II is a predictor of mortality in patients with AP.[25] As such, all five of the predictors incorporated into our developed nomogram represent credible mortality-related risk factors worthy of consideration in clinical research.
There are several limitations to this study. First, several mortality risk factors that have been previously reported, including serum lipid level and serum calcium level, were not included as a means of reducing potential bias stemming from missing data. Second, we were unable to assess imaging findings, which potentially reduced the overall nomogram efficacy. Third, the nomogram model was established using a single dataset without a validation set, which will necessitate further large-scale studies aimed at validating its clinical utility. While internal bootstrap resampling validation was performed in this study, larger external cohorts will be critical to bolstering the overall efficacy of this predictive tool in the future. Fourth, given that these data came from a US population, whether these results can be generalized to non-US populations remains to be validated. In addition, other potentially relevant information, such as the ICU admission criteria, was not available from the database and hence could not be considered during the analysis.
CONCLUSIONS
We herein developed a bootstrap-resampling validated nomogram capable of predicting 30-day, 60-day and 90-day mortality rates in patients with AP who are critically ill and have been admitted to the ICU. This prognostic nomogram might be able to predict patient outcomes and thus may be used as a tool for risk stratification and clinical decision-making for patients with AP. However, external validation by an independent, prospective study with a large sample size will be vital to improve the reliability of this model.
Funding: This study was supported by the Clinical Research Funds of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (ynhg202125).
Ethical approval: The right of this database was approved by the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA) and consent was obtained for the original data collection. Patients’ information in the MIMIC-III database was anonymized; therefore, informed consent was not required.
Conflicts of interest: The authors report that there are no competing interests to declare.
Contributors: XGZ: conceptualization, data curation, funding acquisition, visualization, writing - original draft; JMJ: visualization, writing - original draft; YXL: methodology; JG: methodology; WW: writing - review & editing; QMF: conceptualization, funding acquisition, supervision, writing - review & editing.
All the supplementary files in this paper are available at http://wjem.com.cn.
Reference
Acute pancreatitis
DOI:S0140-6736(20)31310-6
PMID:32891214
[Cited within: 2]

Acute pancreatitis is an unpredictable and potentially lethal disease. The prognosis mainly depends on the development of organ failure and secondary infection of pancreatic or peripancreatic necrosis. In the past 10 years, treatment of acute pancreatitis has moved towards a multidisciplinary, tailored, and minimally invasive approach. Despite improvements in treatment and critical care, severe acute pancreatitis is still associated with high mortality rates. In this Seminar, we outline the latest evidence on diagnostic and therapeutic strategies for acute pancreatitis.Copyright © 2020 Elsevier Ltd. All rights reserved.
Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies
DOI:10.1016/S2468-1253(16)30004-8 URL [Cited within: 2]
One-step laparoscopic pancreatic necrosectomy verse surgical step-up approach for infected pancreatic necrosis: a case-control study
DOI:10.5847/wjem.j.1920-8642.2022.058 URL [Cited within: 3]
Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis
DOI:10.1136/gutjnl-2017-314657
PMID:29950344
[Cited within: 2]

In patients with pancreatitis, early persisting organ failure is believed to be the most important cause of mortality. This study investigates the relation between the timing (onset and duration) of organ failure and mortality and its association with infected pancreatic necrosis in patients with necrotising pancreatitis.We performed a post hoc analysis of a prospective database of 639 patients with necrotising pancreatitis from 21 hospitals. We evaluated the onset, duration and type of organ failure (ie, respiratory, cardiovascular and renal failure) and its association with mortality and infected pancreatic necrosis.In total, 240 of 639 (38%) patients with necrotising pancreatitis developed organ failure. Persistent organ failure (ie, any type or combination) started in the first week in 51% of patients with 42% mortality, in 13% during the second week with 46% mortality and in 36% after the second week with 29% mortality. Mortality in patients with persistent multiple organ failure lasting <1 week, 1-2 weeks, 2-3 weeks or longer than 3 weeks was 43%, 38%, 46% and 52%, respectively (p=0.68). Mortality was higher in patients with organ failure alone than in patients with organ failure and infected pancreatic necrosis (44% vs 29%, p=0.04). However, when excluding patients with very early mortality (within 10 days of admission), patients with organ failure with or without infected pancreatic necrosis had similar mortality rates (28% vs 34%, p=0.33).In patients with necrotising pancreatitis, early persistent organ failure is not associated with increased mortality when compared with persistent organ failure which develops further on during the disease course. Furthermore, no association was found between the duration of organ failure and mortality.© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2019. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
The incidence and aetiology of acute pancreatitis across Europe
DOI:S1424-3903(17)30017-0
PMID:28159463
[Cited within: 2]

Acute pancreatitis is increasingly one of the most important acute gastrointestinal conditions throughout much of the world, although incidence and aetiology varies across countries and regions. This study investigated regional and national patterns in the incidence and aetiology of acute pancreatitis, demographic patterns in incidence and trends over time in incidence across Europe.A structured review of acute pancreatitis incidence and aetiology from studies of hospitalised patient case series, cohort studies or other population based studies from 1989 to 2015 and a review of trends in incidence from 1970 to 2015 across all 51 European states.The incidence of acute pancreatitis was reported from 17 countries across Europe and ranged from 4.6 to 100 per 100 000 population. Incidence was usually highest in eastern or northern Europe, although reported rates often varied according to case ascertainment criteria. Of 20 studies that reported on trends in incidence, all but three show percentage increases over time (overall median increase = 3.4% per annum; range = -0.4%-73%). The highest ratios of gallstone to alcohol aetiologies were identified in southern Europe (Greece, Turkey, Italy and Croatia) with lowest ratios mainly in eastern Europe (Latvia, Finland, Romania, Hungary, Russia and Lithuania).The incidence of acute pancreatitis varies across Europe. Gallstone is the dominant aetiology in southern Europe and alcohol in eastern Europe with intermediate ratios in northern and western Europe. Acute pancreatitis continues to increase throughout most of Europe.Copyright © 2017. Published by Elsevier B.V.
American Gastroenterological Association Institute guideline on initial management of acute pancreatitis
DOI:S0016-5085(18)30076-3 PMID:29409760 [Cited within: 1]
Comparison of different scoring systems in predicting the severity of acute pancreatitis: a prospective observational study
Prediction models of mortality in acute pancreatitis in adults: a systematic review
Nomograms in oncology: more than meets the eye
DOI:10.1016/S1470-2045(14)71116-7 URL [Cited within: 2]
Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis
DOI:10.1016/S1470-2045(16)00010-3
PMID:27068860
[Cited within: 2]

The current American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging system does not have sufficient details to encompass the variety of soft-tissue sarcomas, and available prognostic methods need refinement. We aimed to develop and externally validate two prediction nomograms for overall survival and distant metastases in patients with soft-tissue sarcoma in their extremities.Consecutive patients who had had an operation at the Istituto Nazionale Tumori (Milan, Italy), from Jan 1, 1994, to Dec 31, 2013, formed the development cohort. Three cohorts of patient data from the Institut Gustave Roussy (Villejuif, France; from Jan 1, 1996, to May 15, 2012), Mount Sinai Hospital (Toronto, ON, Canada; from Jan 1, 1994, to Dec 31, 2013), and the Royal Marsden Hospital (London, UK; from Jan 1, 2006, to Dec 31, 2013) formed the external validation cohorts. We developed the nomogram for overall survival using a Cox multivariable model, and a Fine and Gray multivariable model for the distant metastases nomogram. We applied a backward procedure for variables selection for both nomograms. We assessed nomogram model performance by examining overall accuracy (Brier score), calibration (calibration plots and Hosmer-Lemeshow calibration test), and discrimination (Harrell C index). We plotted decision curves to evaluate the clinical usefulness of the two nomograms.1452 patients were included in the development cohort, with 420 patients included in the French validation cohort, 1436 patients in the Canadian validation cohort, and 444 patients in the UK validation cohort. In the development cohort, 10-year overall survival was 72·9% (95% CI 70·2-75·7) and 10-year crude cumulative incidence of distant metastases was 25·0% (95% CI 22·7-27·5). For the overall survival nomogram, the variables selected applying a backward procedure in the multivariable Cox model (patient's age, tumour size, Fédération Française des Centres de Lutte Contre le Cancer [FNCLCC] grade, and histological subtype) had a significant effect on overall survival. The same variables, except for patient age, were selected for the distant metastases nomogram. In the development cohort, the Harrell C index for overall survival was 0·767 (95% CI 0·743-0·789) and for distant metastases was 0·759 (0·736-0·781). In the validation cohorts, the Harrell C index for overall survival and distant metastases were 0·698 (0·638-0·754) and 0·652 (0·605-0·699; French), 0·775 (0·754-0·796) and 0·744 (0·720-0·768; Canadian), and 0·762 (0·720-0·806) and 0·749 (0·707-0·791; UK). The two nomograms both performed well in terms of discrimination (ability to distinguish between patients who have had an event from those who have not) and calibration (accuracy of nomogram prediction) when applied to the validation cohorts.Our nomograms are reliable prognostic methods that can be used to predict overall survival and distant metastases in patients after surgical resection of soft-tissue sarcoma of the extremities. These nomograms can be offered to clinicians to improve their abilities to assess patient prognosis, strengthen the prognosis-based decision making, enhance patient stratification, and inform patients in the clinic.None.Copyright © 2016 Elsevier Ltd. All rights reserved.
Nomograms predicting progression-free survival, overall survival, and pelvic recurrence in locally advanced cervical cancer developed from an analysis of identifiable prognostic factors in patients from NRG oncology/gynecologic oncology group randomized trials of chemoradiotherapy
DOI:10.1200/JCO.2014.57.7122
PMID:25732170
[Cited within: 2]

To evaluate the prognostic factors in locally advanced cervical cancer limited to the pelvis and develop nomograms for 2-year progression-free survival (PFS), 5-year overall survival (OS), and pelvic recurrence.We retrospectively reviewed 2,042 patients with locally advanced cervical carcinoma enrolled onto Gynecologic Oncology Group clinical trials of concurrent cisplatin-based chemotherapy and radiotherapy. Nomograms for 2-year PFS, five-year OS, and pelvic recurrence were created as visualizations of Cox proportional hazards regression models. The models were validated by bootstrap-corrected, relatively unbiased estimates of discrimination and calibration.Multivariable analysis identified prognostic factors including histology, race/ethnicity, performance status, tumor size, International Federation of Gynecology and Obstetrics stage, tumor grade, pelvic node status, and treatment with concurrent cisplatin-based chemotherapy. PFS, OS, and pelvic recurrence nomograms had bootstrap-corrected concordance indices of 0.62, 0.64, and 0.73, respectively, and were well calibrated.Prognostic factors were used to develop nomograms for 2-year PFS, 5-year OS, and pelvic recurrence for locally advanced cervical cancer clinically limited to the pelvis treated with concurrent cisplatin-based chemotherapy and radiotherapy. These nomograms can be used to better estimate individual and collective outcomes.© 2015 by American Society of Clinical Oncology.
Atrial fibrillation is not an independent determinant of mortality among critically ill acute ischemic stroke patients: a propensity score-matched analysis from the MIMIC-IV database
Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement
Multiple imputation with multivariate imputation by chained equation (MICE) package
DOI:10.3978/j.issn.2305-5839.2015.12.63
PMID:26889483
[Cited within: 1]

Multiple imputation (MI) is an advanced technique for handing missing values. It is superior to single imputation in that it takes into account uncertainty in missing value imputation. However, MI is underutilized in medical literature due to lack of familiarity and computational challenges. The article provides a step-by-step approach to perform MI by using R multivariate imputation by chained equation (MICE) package. The procedure firstly imputed m sets of complete dataset by calling mice() function. Then statistical analysis such as univariate analysis and regression model can be performed within each dataset by calling with() function. This function sets the environment for statistical analysis. Lastly, the results obtained from each analysis are combined by using pool() function.
Comparison of scoring systems used in acute pancreatitis for predicting major adverse events
A comparison of APACHE II, BISAP, Ranson’s score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta Classification
Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis
DOI:10.1053/j.gastro.2012.03.005
PMID:22425589
[Cited within: 1]

It is important to identify patients with acute pancreatitis who are at risk for developing persistent organ failure early in the course of disease. Several scoring systems have been developed to predict which patients are most likely to develop persistent organ failure. We head-to-head compared the accuracy of these systems in predicting persistent organ failure, developed rules that combined these scores to optimize predictive accuracy, and validated our findings in an independent cohort.Clinical data from 2 prospective cohorts were used for training (n = 256) and validation (n = 397). Persistent organ failure was defined as cardiovascular, pulmonary, and/or renal failure that lasted for 48 hours or more. Nine clinical scores were calculated when patients were admitted and 48 hours later. We developed 12 predictive rules that combined these scores, in order of increasing complexity.Existing scoring systems showed modest accuracy (areas under the curve at admission of 0.62-0.84 in the training cohort and 0.57-0.74 in the validation cohort). The Glasgow score was the best classifier at admission in both cohorts. Serum levels of creatinine and blood urea nitrogen provided similar levels of discrimination in each set of patients. Our 12 predictive rules increased accuracy to 0.92 in the training cohort and 0.84 in the validation cohort.The existing scoring systems seem to have reached their maximal efficacy in predicting persistent organ failure in acute pancreatitis. Sophisticated combinations of predictive rules are more accurate but cumbersome to use, and therefore of limited clinical use. Our ability to predict the severity of acute pancreatitis cannot be expected to improve unless we develop new approaches.Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
A 5-year retrospective cohort study: epidemiology, etiology, severity, and outcomes of acute pancreatitis
DOI:10.1097/MPA.0000000000001637 URL [Cited within: 1]
Can Hematological parameters predict the severity of acute pancreatitis?
Mortality prognostic factors in acute pancreatitis
PMID:27928447
[Cited within: 1]

The aim of the study was to present the biological prognostic factors of mortality in patients with acute pancreatitis. Several usual laboratory values were monitored: glucose, urea, partial pressure of oxygen, WBC count, hemoglobin, total bilirubin, and cholesterol. A statistical analysis was performed by using ROC curves and AUC interpretation. The overall mortality rate was 21.1% and was different depending on the severity of the disease. Only 2.22% of the patients with a mild disease died, as opposed to 45.63% of the patients with a severe form. All the analyses studied were significantly elevated in the deceased patients. A close correlation between blood glucose, urea, partial pressure of oxygen, WBC, hemoglobin, total bilirubin, and cholesterol and mortality was objectified by measuring the AUC, which was of 97.1%, 95.5%, 93.4%, 92.7%, 87.4%, 82.2%, and 79.0%. The usual, easy to use, fast, and cheap tests were useful in predicting mortality in patients with acute pancreatitis. Our study confirmed that the combination of several factors led to an accurate mortality prediction.
Hypotension in the first week of acute pancreatitis and APACHE II score predict development of infected pancreatic necrosis
DOI:10.1007/s10620-014-3081-y URL [Cited within: 1]
Initially, elevated arterial lactate as an independent predictor of poor outcomes in severe acute pancreatitis
DOI:10.1186/s12876-020-01268-1 URL [Cited within: 1]
Mortality predictors of patients suffering of acute pancreatitis and development of intraabdominal hypertension
DOI:10.3906/sag-1809-15
PMID:30997789
[Cited within: 1]

Intraabdominal hypertension (IAH) occurs frequently in patients with acute pancreatitis and adds to their morbidity and mortality. The main aim of the study was to identify the determination of the predictive factors connected to IAH that influence the evolution of acute pancreatitis.The prospective cohort study was conducted on 100 patients who had acute pancreatitis. According to obtained intraabdominal pressure (IAP) values, the patients were divided into two groups: one group (n = 40) with normal IAP values and the other (IAH group, n = 60) with increased IAP values. Deceased patients were specially analyzed within the IAH group in order to determine mortality predictors.Statistical significance of IAP (P = 0.048), lactates (P = 0.048), peak pressure (P = 0.043), abdominal perfusion pressure (P = 0.05), and mean arterial pressure (P = 0.041) was greater for deceased than for surviving patients in the IAH group. High mortality appears for patients younger than 65 years old, with lactate level higher than 3.22 mmol/L and filtration gradient (GF) lower than 67 mmHg.Age, lactates, GF, and APACHE II score are determined as mortality predictors for patients suffering from acute pancreatitis who developed IAH. The mortality rate is higher when the level of GF is decreasing and the level of lactate increasing.
Assessment of severity of acute pancreatitis over time
Acute pancreatitis gravity predictive factors: which and when to use them?
DOI:10.1590/S0102-67202015000300016
PMID:26537149
[Cited within: 1]

Acute pancreatitis has as its main causes lithiasic biliary disease and alcohol abuse. Most of the time, the disease shows a self-limiting course, with a rapid recovery, only with supportive treatment. However, in a significant percentage of cases, it runs with important local and systemic complications associated with high mortality rates.To present the current state of the use of these prognostic factors (predictive scores) of gravity, as the time of application, complexity and specificity.A non-systematic literature review through 28 papers, with emphasis on 13 articles published in indexed journals between 2008 and 2013 using Lilacs, Medline, Pubmed.Several clinical, laboratory analysis, molecular and image variables can predict the development of severe acute pancreatitis. Some of them by themselves can be determinant to the progression of the disease to a more severe form, such as obesity, hematocrit, age and smoking. Hematocrit with a value lower than 44% and serum urea lower than 20 mg/dl, both at admission, appear as risk factors for pancreatic necrosis. But the PCR differentiates mild cases of serious ones in the first 24 h. Multifactorial scores measured on admission and during the first 48 h of hospitalization have been used in intensive care units, being the most ones used: Ranson, Apache II, Glasgow, Iget and Saps II.Acute pancreatitis is a disease in which several prognostic factors are employed being useful in predicting mortality and on the development of the severe form. It is suggested that the association of a multifactorial score, especially the Saps II associated with Iget, may increase the prognosis accuracy. However, the professional's preferences, the experience on the service as well as the available tools, are factors that have determined the choice of the most suitable predictive score.