INTRODUCTION
Heat stroke (HS) is a common critical disease characterized by elevated core body temperature (over 40.5 °C) and neurological disorders.[1] Environmental heat exposure causes more deaths than all natural disasters taken together.[2⇓-4] It is estimated that between 1979 and 2002, more Americans died from HS than from hurricanes, lightning, earthquakes, floods, and tornadoes combined.[5] In Australia, at least 1,700 people died of HS between 2006 and 2017.[6] On average, heat-related mortality in Chinese metropolises was 32.1 deaths per million people, according to multiple global climate models of 1986-2005. [7] Additionally, critical cases of HS increased significantly in the summer of 2018 in Northern China due to unprecedented high temperatures.
There have been several studies on the risk factors, pathogenesis, treatment, and prevention of HS, such as evidence within sports medicine to support good prognosis with proper management and care,[8⇓-10] but little is known about how to determine the prognosis of HS early. Moreover, the lack of early, noninvasive, predictable biomarkers of organ damage hinders the identification of timely and effective treatments to mitigate it.[11] The purpose of this study was to screen the factors that can aid in the evaluation of HS prognosis, as well as to develop and validate a nomogram to predict survival in HS patients.
METHODS
Study design
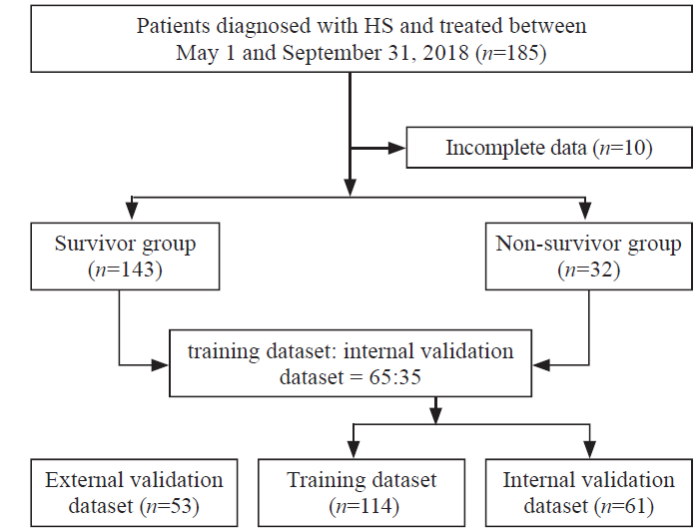
This was a retrospective, observational multi-center study of patients diagnosed with HS and treated between May 1 and September 30, 2018. We collected data on HS-related hospital admissions from 15 tertiary hospitals in 11 cities of 9 provinces in Northern China during a 5-month period.
The eligibility criteria were as follows: patients older than 18 years; meeting the diagnostic criteria of severe HS: classic or exertional HS with a history of exposure to hot and humid weather or strenuous activity, concurrent hyperthermia (core temperature of >40.5 °C), and related neurological dysfunction including confusion, convulsions, or coma on admission.
We excluded patients who had irreversible underlying diseases affecting mortality and pregnancy.
Data collection
For each patient, we collected the following information: demographic data (age, sex, location where HS occurred), condition of hospitalization (initial mean arterial pressure, invasive ventilation, anal temperature, maximum heart rate, Glasgow admission prediction score, hemofiltration, intravascular hypothermia, and mild hypothermia in vitro), and laboratory tests on days 1 and 3, including white blood cell count (WBC), red blood cell count (RBC), hemoglobin count (HBG), platelet count (PLT), alanine aminotransferase (ALT), creatine (CRE), potassium, calcium, myoglobin (MYO), and brain natriuretic peptide (BNP). All the data were collected from the electronic medical records or the hospital database at the time of initial hospital admission.
Outcome analysis
The primary outcome was survival until hospital discharge after HS. The secondary outcomes included 7-d and 14-d survival.
Statistical analysis
We divided the whole dataset into training and internal validation datasets at a ratio of 65:35 using stratified resampling to ensure that both datasets had the approximately same percentage of survivors and non-survivors. An external validation dataset from Beijing Daxing Hospital, including data from 53 patients with HS, was collected concurrently.
The categorical variables of survivors and non-survivors were compared using either a Chi-squared test or Fisher’s exact test, as appropriate. Continuous variables are presented as the mean±standard deviation or the median with interquartile range. Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. Univariate Cox regression analyses were performed to identify potentially relevant prognostic factors. Significant factors with P-values less than 0.2 in univariate analysis, combined with the factors from clinicians’ treatment experiences, were included in a forward stepwise multivariable Cox proportional hazards model.
The model discrimination was measured by the area under the receiver operating characteristic (ROC) curve, also known as the concordance index (C-index). A C-index of 1.00 indicates perfect discrimination, whereas a C-index of 0.5 indicates no discrimination. A nomogram based on the results of the Cox proportional hazard regression was established to estimate overall survival using the rms package of R software, whereas the C-index was used to estimate the accuracy of the nomogram. A prognostic index (PI) was established based on the Cox regression model, and patients were categorized into high-risk and low-risk groups according to the optimal cut-off value on the ROC curve. Survival curves were compared between these two groups. The model was also evaluated by a calibration curve that compared the predicted and observed survival probabilities, which facilitated an additional comparison of prognostic accuracy. We evaluated the predictive criterion validity by assessing model discrimination and calibration in the internal and external validation datasets. All statistical analyses were conducted with R software, version 4.0.1.
RESULTS
Figure 1.
Figure 1.
Flowchart diagram illustrating patients included in each group throughout the study. HS: heat stroke.
Table 1. Demographic and clinical characteristics of the study population (n=175)
| Characteristics | Overall (n=175) | Survivor (n=143) | Non-survivor (n=32) | P-value |
|---|---|---|---|---|
| Demographic data | ||||
| Sex, n (%) | ||||
| Female | 61 (34.86) | 49 (34.27) | 12 (37.50) | 0.887 |
| Male | 114 (65.14) | 94 (65.73) | 20 (62.50) | |
| Age, years, median (interquartile range) | 64 (51-75) | 64 (50-75) | 63 (53-71) | 0.960 |
| Condition of hospitalization | ||||
| Initial mean arterial pressure, n (%) | ||||
| 70-105 mmHg | 117 (66.86) | 103 (72.03) | 14 (43.75) | <0.001 |
| <70 mmHg | 29 (16.57) | 14 (9.79) | 15 (46.88) | |
| >105 mmHg | 29 (16.57) | 26 (18.18) | 3 (9.38) | |
| Invasive ventilation, n (%) | ||||
| No | 111 (63.43) | 107 (74.83) | 4 (12.50) | <0.001 |
| Yes | 64 (36.57) | 36 (25.17) | 28 (87.50) | |
| Core temperature, °C, median (interquartile range) | 40.0 (38.1-41.0) | 39.7 (37.5-41.0) | 40.8 (40.0-42.0) | 0.002 |
| Maximum heart rate, n (%) | ||||
| 60-100 beats/min | 52 (29.71) | 49 (34.27) | 3 (9.38) | 0.010 |
| >100 beats/min | 123 (70.29) | 94 (65.73) | 29 (90.62) | |
| Glasgow admission prediction score, median (interquartile range) | 8 (5-12) | 8 (6-14) | 5 (3-7) | <0.001 |
| Hemofiltration, n (%) | ||||
| No | 147 (84.00) | 129 (90.21) | 18 (56.25) | <0.001 |
| Yes | 28 (16.00) | 14 (9.79) | 14 (43.75) | |
| Intravascular hypothermia, n (%) | ||||
| No | 164 (93.71) | 133 (93.01) | 31 (96.88) | 0.692 |
| Yes | 11 (6.29) | 10 (6.99) | 1 (3.12) | |
| Mild hypothermia in vitro, n (%) | ||||
| No | 106 (60.57) | 90 (62.94) | 16 (50.00) | 0.249 |
| Yes | 69 (39.43) | 53 (37.06) | 16 (50.00) | |
| Laboratory tests on day 1 | ||||
| White blood cell count (WBC), n (%) | ||||
| (3.5-9.5)×109/L | 60 (34.29) | 53 (37.06) | 7 (21.88) | 0.153 |
| >9.5×109/L | 115 (65.71) | 90 (62.94) | 25 (78.12) | |
| Red blood cell count (RBC), n (%) | ||||
| (3.8-5.1)×109/L | 129 (73.71) | 103 (72.03) | 26 (81.25) | 0.704 |
| <3.8×109/L | 38 (21.71) | 33 (23.08) | 5 (15.62) | |
| >5.1×109/L | 8 (4.57) | 7 (4.90) | 1 (3.12) | |
| Hemoglobin count (HBG), n (%) | ||||
| 115-150 g/L | 122 (69.71) | 100 (69.93) | 22 (68.75) | 0.211 |
| <115 g/L | 34 (19.43) | 30 (20.98) | 4 (12.50) | |
| >150 g/L | 19 (10.86) | 13 (9.09) | 6 (18.75) | |
| Platelet count (PLT), n (%) | ||||
| (125-350)×109/L | 92 (52.57) | 80 (55.94) | 12 (37.50) | 0.086 |
| <125×109/L | 81 (46.29) | 61 (42.66) | 20 (62.50) | |
| >350×109/L | 2 (1.14) | 2 (1.40) | 0 | |
| Alanine aminotransferase (ALT), n (%) | ||||
| 7-40 U/L | 72 (41.14) | 67 (46.85) | 5 (15.62) | 0.002 |
| >40 U/L | 103 (58.86) | 76 (53.15) | 27 (84.38) | |
| Creatine (CRE), n (%) | ||||
| 41.0-81.0 U/L | 59 (33.71) | 56 (39.16) | 3 (9.38) | 0.003 |
| >81.0 U/L | 116 (66.29) | 87 (60.84) | 29 (90.62) | |
| Potassium, n (%) | ||||
| 3.5-5.3 m/L | 92 (52.57) | 71 (49.65) | 21 (65.62) | 0.053 |
| <3.5 m/L | 78 (44.57) | 69 (48.25) | 9 (28.12) | |
| >5.3 m/L | 5 (2.86) | 3 (2.10) | 2 (6.25) | |
| Calcium, n (%) | ||||
| 2.11-2.52 m/L | 57 (32.57) | 46 (32.17) | 11 (34.38) | 0.440 |
| <2.11 m/L | 116 (66.29) | 96 (67.13) | 20 (62.50) | |
| >2.52 m/L | 2 (1.14) | 1 (0.70) | 1 (3.12) | |
| Myoglobin (MYO), n (%) | ||||
| 0-107 μg/L | 16 (9.14) | 15 (10.49) | 1 (3.12) | 0.311 |
| >107 μg/L | 159 (90.86) | 128 (89.51) | 31 (96.88) | |
| Brain natriuretic peptide (BNP), n (%) | ||||
| 0-100 pg/mL | 39 (22.29) | 33 (23.08) | 6 (18.75) | 0.767 |
| >100 pg/mL | 136 (77.71) | 110 (76.92) | 26 (81.25) | |
| Laboratory tests on day 3 | ||||
| WBC, n (%) | ||||
| (3.5-9.5)×109/L | 50 (28.57) | 47 (32.87) | 3 (9.38) | 0.007 |
| <3.5×109/L | 2 (1.14) | 1 (0.70) | 1 (3.12) | |
| >9.5×109/L | 123 (70.29) | 95 (66.43) | 28 (87.50) | |
| HBG, n (%) | ||||
| (115-150)×109/L | 136 (77.71) | 112 (78.32) | 24 (75.00) | 0.567 |
| <115×109/L | 36 (20.57) | 29 (20.28) | 7 (21.88) | |
| >150×109/L | 3 (1.71) | 2 (1.40) | 1 (3.12) | |
| PLT, n (%) | ||||
| (125-350)×109/L | 33 (18.86) | 31 (21.68) | 2 (6.25) | 0.077 |
| <125×109/L | 142 (81.14) | 112 (78.32) | 30 (93.75) | |
| ALT, n (%) | ||||
| 7-40 U/L | 21 (12.00) | 21 (14.69) | 0 | 0.015 |
| >40 U/L | 154 (88.00) | 122 (85.31) | 32 (100.00) | |
| AST, n (%) | ||||
| 13-35 U/L | 20 (11.43) | 19 (13.29) | 1 (3.12) | 0.295 |
| <13 U/L | 1 (0.57) | 1 (0.70) | 0 | |
| >35 U/L | 154 (88.00) | 123 (86.01) | 31 (96.88) | |
| International normalized ratio (INR), n (%) | ||||
| 0.8-1.2 | 115 (65.71) | 93 (65.03) | 22 (68.75) | 0.846 |
| >1.2 | 60 (34.29) | 50 (34.97) | 10 (31.25) | |
| CRE, n (%) | ||||
| 41.0-81.0 U/L | 117 (66.86) | 95 (66.43) | 22 (68.75) | 1 |
| <41.0 U/L | 4 (2.29) | 4 (2.80) | 0 | |
| >81.0 U/L | 54 (30.86) | 44 (30.77) | 10 (31.25) | |
| Potassium, n (%) | ||||
| 3.5-5.3 m/L | 146 (83.43) | 119 (83.22) | 27 (84.38) | 0.392 |
| <3.5 m/L | 27 (15.43) | 23 (16.08) | 4 (12.50) | |
| >5.3 m/L | 2 (1.14) | 1 (0.70) | 1 (3.12) | |
| MYO, n (%) | ||||
| 0-107 μg/L | 19 (10.86) | 19 (13.29) | 0 | |
| >107 μg/L | 156 (89.14) | 124 (86.71) | 32 (100.00) | 0.026 |
| BNP, n (%) | ||||
| 0-100 pg/mL | 4 (2.29) | 4 (2.80) | 0 | 1 |
| >100 pg/mL | 171 (97.71) | 139 (97.20) | 32 (100.00) | |
The patients’ average age was 64 years (51-75 years), nearly two-thirds (65.14%) were male, and 32 (18.29%) died before hospital discharge (Table 1). Sex, age, intravascular hypothermia, mild hypothermia in vitro, 8 laboratory tests on day 1 (PLT, BNP, WBC, HBG, calcium, MYO, potassium, and RBC), and 7 laboratory tests on day 3 (HBG, AST, international normalized ratio [INR], CRE, potassium, PLT, and BNP) did not differ significantly between survivors and non-survivors. Survivors’ initial mean arterial pressure was significantly higher than that of the non-survivors. The median temperature of non-survivors was significantly higher than that of survivors (40.8 °C vs. 39.7 °C, P=0.002). The mean of the Glasgow admission prediction score was 3 points higher in the survivor group than in the non-survivor group (P<0.001). A significantly higher proportion of non-survivors had invasive ventilation (87.50% vs. 25.17%, P<0.001), maximum heart rate >100 beats/min (90.62% vs. 65.73%, P=0.010), hemofiltration (43.75% vs. 9.79%, P<0.001), and MYO values >107 μg/L on day 3 (100% vs. 86.71%, P=0.026).
After the univariate analysis, invasive ventilation, initial mean arterial pressure <70 mmHg, core temperature, maximum heart rate, hemofiltration, and Glasgow admission prediction score were included (P<0.2). In addition, WBC >9.5×109/L, ALT >40 U/L, CRE >81.0 U/L, HBG > 150 g/L, and PLT <125×109/L on day 1, as well as WBC <3.5×109/L or >9.5×109/L, PLT < 125×109/L and AST >35 U/L on day 3, were found to be significant. Based on our univariate analysis and the clinical experience of our clinicians, we included 8 predictors in our multivariate analysis: invasive ventilation, initial mean arterial pressure (<70 mmHg [1 mmHg=0.133 kPa] or >105 mmHg), maximum heart rate, lab results on day 1 (WBC, ALT, and CRE), and Glasgow admission prediction score (supplementary Table 1).
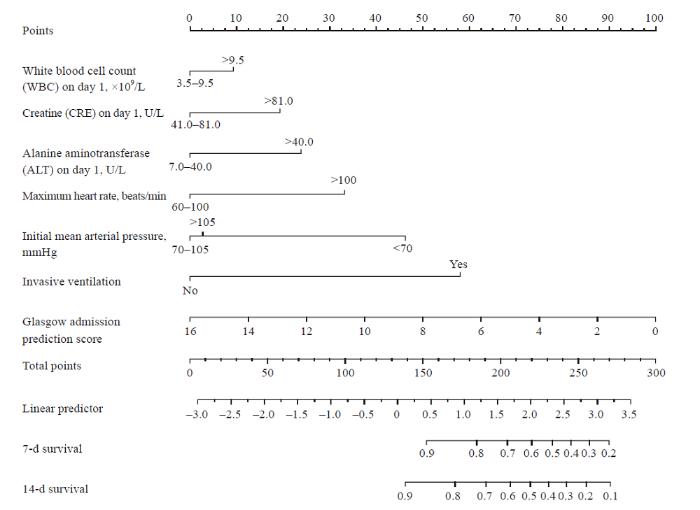
Multivariate Cox regression indicated that invasive ventilation, initial mean arterial pressure <70 mmHg, and Glasgow admission prediction score were independent risk factors for the prognosis of HS patients (supplementary Table 1). To further interpret our Cox regression model and obtain individualized estimates of outcomes, a nomogram for predicting 7-d and 14-d survival in the training dataset was established (Figure 2). The nomogram had a C-index of 0.880 (95% confidence interval [95% CI] 0.831-0.930) by bootstrapping validation (B=1000). As indicated in Figure 2, higher values for lab results on day 1 (WBC, CRE, ALT) or maximum heart rate resulted in a higher total score and lower chance of survival on day 7 and day 14. Meanwhile, a higher value of the initial mean arterial pressure or Glasgow admission prediction score contributed to a lower total score and a higher chance of survival on day 7 and day 14. In addition, patients with no invasive ventilation are more likely to survive until day 7 and day 14.
Figure 2.
Figure 2.
Nomograms for predicting 7-d survival and 14-d survival in the training set.
The PI cut-off value was set at 2.085 according to the ROC curve for overall survival prediction. Patients were divided into high-risk (PI ≥2.085) and low-risk groups (PI <2.085). Supplementary Figure 1 shows significant survival heterogeneity between the two groups (P<0.001), with a larger gap for 14-d survival than for 7-d survival.
Supplementary Figure 2 shows good model discrimination in both the internal and external validation datasets. The C-indexes in the training datasets were lower than those in the external validation datasets but were higher than those in the internal validation datasets. Moreover, in the comparison of the calibration curves for predicting 7-d and 14-d survival in the internal and external validation datasets, the model showed good calibration ability in both datasets. Overall, the calibration ability for 14-d survival was superior to that for 7-d survival. With regard to the difference between the validation datasets, the calibration ability in the external validation dataset was much better than that in the internal validation dataset for 7-d survival but slightly worse for 14-d survival.
DISCUSSION
Shock, acute renal failure, rhabdomyolysis, acute respiratory distress syndrome, disseminated intravascular coagulation, and acid-base or electrolyte disorders are common potential or direct complications of severe HS.[12] In this study, we observed an elevation of cardiac troponins (cTn) I in nearly half of the patients; however, there was no significant difference when compared with the mortality rate. Finally, we included the maximum heart rate to establish the nomogram. Routinely, people are exposed to high temperatures and cool down through an increase in heart rate, which in turn increases blood flow to the skin.[13] However, this protective mechanism increases myocardial oxygen consumption and may lead to myocardial infarction.[14] Patients with HS combined with acute myocardial infarction often have poor prognosis and high treatment costs.[14] Furthermore, numerical changes in cTn I can be used to predict the prognosis of HS. [15,16]
Rhabdomyolysis, which usually occurs in the acute phase of HS, was observed in over half of this study’s participants. Heat stress can induce kidney disease through acute tubular injury from heat- and exercise-induced rhabdomyolysis, acute elevation in serum and urine uric acid with crystalluria, extracellular volume depletion, and resultant renal hypoperfusion.[17] Furthermore, simple elevation of core body temperature leads to severe kidney injury, which is associated with tubular death.[18] Increased plasma creatinine concentration, reduced urine volume and urine flow rate, reduced fractional excretion of sodium, and mildly increased urinary specific gravity occur after impaired renal function in patients with HS.[19] In this study, we included CRE in the development of the nomogram.
Liver injury has been reported among patients secondary to the IV infusions of amiodarone[20] or among patients with HS because of an inflammatory response rather than as an acute response to hyperthermia. Some studies have reported liver injury with an early increase in AST and lactate dehydrogenase (LDH), which peak after 3 to 4 d, and an increase in bilirubin by the second or third day.[21]
In our study, the mortality rate was 18.29%, which was lower than that in Western countries.[22] The increase in HS cases (especially exertional HS) in the summer of 2018 in Northern China has been associated with high temperatures and humidity conditions lasting over two months, which is rare for Northern China. After several outbreaks, media reports on HS gained public attention, which in turn caused related regulations to be introduced. Hospitals and prehospital rescue institutions implemented an emergency plan for treating patients with HS in most cities in Northern China. The prevention of HS is as crucial as proper and timely treatment.
This study had certain limitations. First, this study was a retrospective study with a small sample size; thus, a prospective study with a larger population is required to further validate our findings. Second, our data lacked the time from illness onset to hospitalization and coagulation values that were primary indicators of the prognosis of patients with HS, which may have influenced the model we developed. Finally, we did not consider the influence of climatic factors, which may affect HS and the related outcomes of patients.
CONCLUSIONS
A nomogram for predicting survival in HS patients is developed and internally and externally valid. The inclusive nomogram might represent an efficient and valuable tool for prediction and treatment decision-making of HS patients.
ACKNOWLEDGMENTS
We thank all colleagues who collected the medical information of the patients included in this study: Tao Wang, Yan-fen Chai, Qing-bian Ma, Hong-hong Pei, Feng Xu, Chun-ling Zhao, Tian-peng Zhang, Min Zhang, Zhao Tao, and Zhi Liu. We thank the patients and their families for making this study possible. We would like to express our very great appreciation to other colleagues: Qiang Wang and Zhe-ping Yuan from Goodwill Hessian Health Technology Co., Ltd., who contributed greatly throughout the process of data cleaning and analyses.
Funding: None
Ethics approval: The Ethics Committee Board of Beijing Chaoyang Hospital, Capital Medical University, approved this study and waived the requirement for written informed consent.
Conflicts of interest: The authors state that there are no conflicts of interest related to this manuscript.
Contributors: FS and XS contributed equally to this article. ZRT, SZ, and FS contributed conception and design of the study; SHH, QYL, JXS, JK, PG, GXW, LJQ, FW, KF, FYC, YJY, TM, and YL collected the data; YW, HC, and CXY organized the database; SY, WG, SW, LD, and MCZ performed the statistical analysis; FS and XS wrote the first draft of the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
All the supplementary files in this paper are available at http://wjem.com.cn.
Reference
Heatstroke
DOI:10.1056/NEJMra1810762 URL [Cited within: 1]
Heat-related deaths—United States, 1999-2003
Climate change and extreme heat events
DOI:10.1016/j.amepre.2008.08.021 URL [Cited within: 1]
The potential impacts of climate variability and change on temperature-related morbidity and mortality in the United States
Heat-related mortality—Arizona, 1993-2002, and United States, 1979-2002
Heat-related deaths after an extreme heat event—four states, 2012, and United States, 1999-2009
Tens of thousands additional deaths annually in cities of China between 1.5 ℃ and 2.0 ℃ warming
DOI:10.1038/s41467-019-11283-w URL [Cited within: 1]
Athletic training services in public secondary schools: a benchmark study
DOI:10.4085/1062-6050-50.2.03 URL [Cited within: 1]
High schools’ adoption of evidence-based practices for the management of exertional heat stroke
DOI:10.4085/1062-6050-361-20 URL [Cited within: 1]
Roundtable on preseason heat safety in secondary school athletics: prehospital care of patients with exertional heat stroke
DOI:10.4085/1062-6050-0173.20 URL [Cited within: 1]
Heat stroke
DOI:10.1056/NEJMra011089 URL [Cited within: 1]
Heat illnesses: a hot topic in the setting of global climate change
Acute myocardial infarction among hospitalizations for heat stroke in the United States
DOI:10.3390/jcm9051357 URL [Cited within: 2]
Point-of-care cardiac troponin test accurately predicts heat stroke severity in rats
DOI:10.1152/ajpregu.00286.2015 URL [Cited within: 1]
Cardiovascular and thermoregulatory biomarkers of heat stroke severity in a conscious rat model
Chronic kidney disease of unknown cause in agricultural communities
Increase of core temperature affected the progression of kidney injury by repeated heat stress exposure
DOI:10.1152/ajprenal.00259.2019 URL [Cited within: 1]
Exercising in a hot environment with muscle damage: effects on acute kidney injury biomarkers and kidney function
DOI:10.1152/ajprenal.00091.2013 URL [Cited within: 1]
Immediate oral amiodarone re-challenge following the development of parenteral-induced acute liver toxicity
DOI:10.5847/wjem.j.1920-8642.2021.04.012 PMID:34512831 [Cited within: 1]
Biochemical recovery from exertional heat stroke follows a 16-day time course
DOI:10.1371/journal.pone.0229616 URL [Cited within: 1]
Environmental conditions and the occurrence of exertional heat illnesses and exertional heat stroke at the Falmouth Road Race
DOI:10.4085/1062-6050-49.3.26 URL [Cited within: 1]