INTRODUCTION
Sepsis is life-threatening organ dysfunction caused by a dysregulated host response to infection.[1] Tissue hypoperfusion is an important pathophysiological process in the occurrence and development of sepsis.[2] Long-lasting microcirculation dysfunction can drive the pathological process of sepsis. The intestinal mucosa is particularly sensitive to microcirculation dysfunction, which can easily lead to the impaired intestinal barrier function and the migration of intestinal bacteria to other organs. The related research on intestinal microcirculation dysfunction in sepsis is an important direction in the medical field, and some research progress has been made. This study aims to provide a summary of the current research.
METHODS
Search strategy
We systematically performed an electronic search of PubMed, Web of Science, and China National Knowledge Infrastructure (CNKI) from inception to August 1, 2021. The search was limited to the English language only. We combined MeSH and title/abstract keywords, such as “sepsis”, “septic shock”, “intestinal”, “gastrointestinal”, “dysfunction”, and “microcirculation” to identify all studies related to intestinal microcirculation dysfunction in sepsis.
Study selection
Two authors independently identified the articles for inclusion based on their titles and abstracts, evaluated the full texts of the papers, and reviewed the bibliographies of each article to identify additional studies.
Exclusion criteria
Studies that were published before 2010 and duplicate articles were excluded.
RESULTS
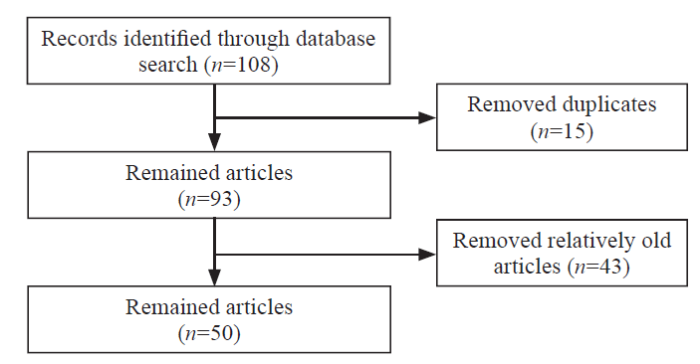
A total of 108 articles were identified. After reviewing the titles and abstracts, which was followed by assessing the full text, 50 articles[3⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-38,40⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-53] were included (Figure 1), and most of them were animal studies. These studies reported pathogenesis, including endothelial dysfunction, leukocyte recruitment and adhesion, microthrombus formation, microcirculation hypoperfusion, and redistribution of intestinal wall blood flow. The monitoring methods of intestinal microcirculation were also diverse. In view of the related pathogenesis of intestinal microcirculation disorder in sepsis, existing studies also have different opinions on its treatment.
Figure 1.
Figure 1.
The flowchart of the study.
DISCUSSION
Pathogenesis
Endothelial dysfunction
In patients with sepsis, endothelial dysfunction is one of the key pathophysiological mechanisms of microcirculation dysfunction,[3] which is also an essential link in leukocyte-endothelial interactions. Studies have shown that nitric oxide (NO) plays an important role in vascular endothelial function. In the vascular endothelium, an imbalance between inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) may play a certain role in sepsis microcirculation dysfunction.[4] The integrity of the vascular endothelial glycocalyx plays a key role in the intestinal microcirculation, which can inhibit intercellular adhesion.[5] Glycocalyx degradation can occur in certain pathologic conditions, such as septic shock.[6] The excessive release of histones may cause changes in the structure and function of the endothelium, which will further lead to microcirculation dysfunction.[7] When the cell is severely damaged, histones can be passively released outside the cell or formed through neutrophil extracellular traps (NETs),[8] which is a special type of programmed cell death (PCD). The increase in leukocyte-endothelial interactions may be related to the increase in E-selectin expression in endothelial cells.[9]
Recruitment and adhesion of leukocytes
Various proinflammatory signals appear in sepsis, including the expression of adhesion molecules. Selectin and integrin are important adhesion molecules in the initial steps of leukocyte recruitment,[10] which can trigger the rolling and adhesion process of leukocytes. When leukocytes adhere to the vascular endothelium, they release a series of inflammatory mediators, cytotoxic substances, elastase, myeloperoxidase, and reactive oxygen species (ROS). These substances can not only damage the function of the vascular endothelium, but also indirectly activate the coagulation process.
Formation of microthrombi
In sepsis, inflammation and clotting interact with each other.[11] This pathological condition leads to the formation of microthrombi. Pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) in the early stages of sepsis are thought to be involved in the formation of immune thrombosis. At the same time, the von Willebrand factor (vWF) is released to the outside of the cell in large quantities. It can be used as a carrier protein for factor VIII and mediate platelet aggregation and adhesion at the site of endothelial cell injury.[12] With the activation of the coagulation cascade, thrombin activates endothelial cells and platelets, which will lead to excessive release of P-selectin. This process promotes the granulocyte-platelet interaction, which in turn leads to platelet-neutrophil aggregation.[13]
Hypoperfusion of microcirculation
Small blood vessels need sufficient arterial pressure to maintain perfusion. The increased permeability of blood vessels leads to continuous leakage of fluid and protein to the outside of the vessels, and the effective blood volume is reduced. These pathologic conditions lead to hypoperfusion of the microcirculation. Microcirculation changes are not related to systemic hemodynamics and the microcirculation response is not synchronized with systemic hemodynamics during treatment.[14]
Redistribution of blood flow in the intestinal wall
Physiologically, the blood flow distribution in the intestinal wall is uneven, and the mucosal layer receives 70%-80% of the blood supply.[15] In sepsis, the reduction in mucosal blood flow is greater than that in serosal/muscular blood flow.[16] Intestinal villous vessels have physiological defects, and their unique “hairpin capillary structure” causes the blood supply to the villous tip to be uneven.[17] As a result, intestinal villous microcirculation is more prone to disturbance than other parts of the intestinal wall. In addition, similarities between intestinal and sublingual microcirculation also exist.[18]
Other pathogeneses
During sepsis, excess oxygen free radicals are produced, which results in depletion of endogenous antioxidants and loss of protection against potential oxygen free radical damage. Some studies have also confirmed mitochondrial dysfunction.[19,20] Dysfunction of the mitochondrial electron transport system (ETS) makes aerobic metabolism disorders more serious. A study found that in septic rats, the increase in GPR55 expression mediates the interaction between leukocytes and endothelium in the microcirculation.[21] Intestinal microcirculation disorders can be reversed by Toll-like receptor 4 (TLR4) antagonists.[22]
Monitoring methods
Handheld microscope
Currently, there are three different handheld microscopes that can be used to directly observe the intestinal microcirculation, including the orthogonal polarization spectral (OPS)[23] imaging technique, sidestream dark-field (SDF) [24] imaging, and incident dark field (IDF)[25] imaging. The microvascular flow index (MFI), total vascular density (TVD), perfusion vascular density (PVD), and perfusion vascular vessels (PPV) were commonly used for observation.
Intravital microscopy
Intravital microscopy (IVM) is often used to observe the intestinal microcirculation in sepsis. Functional capillary density (FCD) was used to represent the microcirculatory perfusion of the tissue. The number of adherent leukocytes (cells/mm2) and the number of rolling leukocytes (cells/min) were recorded.[26] This technology can be used not only to observe the perfusion of the microcirculation but also to observe the adhesion and rolling of the microcirculation leukocytes, which reflects the leucocyte-endothelial interaction and the inflammation of the microcirculation.
Laser Doppler blood flow instruments
The principle is based on the emitted laser beam being scattered by the tissue and part of the light being absorbed. In applications involving intestinal microcirculation, the main observation index is the blood flow of the microcirculation, which is expressed as the percentage of the blood flow to the baseline after intervention and reflects the blood flow velocity and blood flow of the microcirculation.
Laser speckle contrast imaging
This technique is currently commonly used to monitor blood flow in brain tissue, and it is also used in septic intestinal microcirculation monitoring. The main observation index is the blood flow intensity of the microcirculation, which is expressed in perfusion units (PU) of flow and is related to the product of the moving speed and the concentration of the moving red blood cells in the tissue sample.[27]
Tissue reflectance spectrophotometry
In applications involving intestinal microcirculation, through tissue reflectance spectrophotometry, the monitoring of intestinal microcirculation will not be limited to the perfusion of microcirculation but can also provide a timely understanding of the utilization of oxygen.
Serological markers
The intestinal fatty acid-binding protein (I-FABP) is a very sensitive indicator of intestinal ischemic disease, which can increase in the early stage of ischemia.[28] D-lactic acid (D-Lac) is the fermentation product of intestinal bacteria. When intestinal barrier function is impaired, a large amount of D-Lac in the intestinal tract enters the blood, which will result in the elevation of plasma D-Lac. Günel et al[29] believe that this marker also has a certain role in the diagnosis of intestinal ischemic diseases. Sessa et al[30] found that intestinal serum diamine oxidase (DAO) activity decreased during intestinal ischemia, while serum DAO levels significantly increased.
Pathological examination
Through transmission electron microscopy, the glycocalyx of the intestinal capillary endothelium and the concentration of vWF and soluble thrombomodulin (sTM) can be observed.[7] HE-stained pathological sections of small intestine tissue are scored to understand the destruction of intestinal tissue in sepsis microcirculation disorder.
Treatment
Antibiotics
Infection is the initiating factor of sepsis, and antibiotics have become the central link in the treatment of sepsis. Polymyxin B can reduce the damage from endotoxin to the intestinal microcirculation by reducing the level of endotoxin. Daptomycin, tigecycline, erythromycin, and linezolid can improve intestinal microcirculation perfusion or reduce leukocyte adhesion. Vancomycin and tobramycin may increase the adhesion of leukocytes in the intestinal microcirculation.[31] But another study[26] showed that vancomycin and tobramycin had microcirculation vasodilator effects and increased FCD, which may be related to the promotion of histamine release from mast cells.
Anticoagulants
The use of anticoagulants is a direction that can be explored. Excessive release of histones is a cause of intestinal microcirculation disorder in sepsis, which can be ameliorated by unfractioned heparin. The mechanism may be to reduce the inflammation induced by histones,[7] enhance the affinity between antithrombin III and thrombin, and accelerate the inactivation of thrombin. Heparin itself is also thought to have anti-inflammatory effects. Argatroban can reduce not only the formation of microthrombi but also the activation of leukocytes.[32]
Fluid resuscitation
Currently, the Surviving Sepsis Campaign (SSC) recommends that patients with sepsis hypoperfusion should receive an intravenous infusion of 30 mL/kg crystalloid solution within 3 h after diagnosis. Which type of fluid resuscitation can best alleviate sepsis intestinal microcirculation dysfunction? Langanke et al[33] found that compared with balanced crystalloids, the third-generation hydroxyethyl starch (HES) solution was more effective in improving the intestinal microcirculation. However, Schick et al[34] believed that compared with colloidal liquid, balanced crystalloids had a more positive effect on intestinal microcirculation.
Vasoactive drugs
As sepsis can lead to hypotension, vasoactive drugs are needed to maintain blood pressure and ensure the perfusion of vital organs. Current guidelines recommend the use of norepinephrine (NE) as a first-line vasoactive agent, but its effect on intestinal microcirculation remains controversial. Nantais et al[35] reported that NE could improve intestinal microcirculation dysfunction; while Nacul et al[36] found that NE could aggravate or have no significant effect on intestinal microcirculation dysfunction. Dopexamine, dopamine, and dobutamine are used alone, and the combination of dobutamine and NE is thought to improve intestinal microcirculation dysfunction.[36]
Lipid-lowering drugs
In a study[37] of brain dysfunction in sepsis, statins were believed to inhibit leukocyte-endothelial interactions and improve microcirculation dysfunction. It has also been reported that bezafibrate can improve intestinal microcirculation dysfunction in sepsis by inhibiting the expression of iNOS; however, the adhesion and rolling of leukocytes in the microcirculation increases when high-density lipoprotein (HDL) is given.[38]
Traditional Chinese medicine treatment
Based on “Expert consensus on diagnosis and treatment of septic shock with integrated traditional Chinese and Western medicine”,[39] intestinal microcirculation disorders in sepsis are classified as syndromes of intestinal dysfunction and obstruction of “Fu-qi” and are often treated by purgation. Therefore, it is recommended that the heat-clearing and purgation prescriptions Dachengqi decoction be used, to promote blood circulation.
Other treatments
In view of the microcirculation dysfunction caused by the vasoconstrictor effect of endothelin (ET) in sepsis, selective endothelin type B receptor (ETB-R) agonists combined with endothelin type A receptors (ETA-R) antagonists could maintain systemic circulation blood pressure and improve microcirculation dysfunction.[23] Dexmedetomidine is an α2 adrenergic receptor agonist. A study[24] reported that this drug could improve intestinal microcirculation dysfunction in sepsis and reduce vascular endothelial damage. Insulin administration improves intestinal microcirculation and reduces leukocyte activation in septic rats through immune regulation, vascular regulation and regulation of the prostaglandin A2 (PGA2)/thromboxane A2 (TxA2) balance of insulin.[4] The new iron chelator DIBI can inhibit iron-catalyzed ROS production, thereby improving microcirculation.[40] Deletion of cIAP2 showed potential therapeutic benefit in endotoxemia.[41] Selective inhibition of adenosine triphosphate (ATP)-sensitive potassium (KATP) channels in vasodilatory shock by continuous infusion of glipizide could partially restore vasomotor tone in endotoxemic rats and improve systemic hemodynamics in septic rats.[42] Tetrahydrobiopterinl,[22] toll-like receptor 4 (TLR4) antagonist,[43] and desmopressin[44] have an effect on improving the intestinal microcirculation. There are individual reports of vasopressin V1A receptors,[45,46] physostigmine,[47] and dehydroepiandrosterone combined with orthovanadate,[48] showing that they could improve microcirculation. Activation of cannabinoid 2 receptors[49] and estradiol receptors,[50] inhibition of cannabinoid receptor,[51] endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH),[52] and lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1)[53] are possible therapeutic targets to consider.
CONCLUSIONS
Intestinal microcirculation dysfunction in sepsis needs more attention, and its pathogenesis is complex, which is an important factor affecting the prognosis of patients with sepsis. However, due to the limitation of detection methods, most of the studies on its pathogenesis and treatment are animal studies, without the support of clinical research data. It is expected that future studies will find more convenient indicators to monitor the changes in intestinal microcirculation and supplement the lack of clinical data.
Funding: None.
Ethical approval: Not needed.
Conflicts of interest: The authors declare that they have no competing interests.
Contributors: ALT proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
Reference
Effect of neutrophil CD64 for diagnosing sepsis in emergency department
DOI:10.5847/wjem.j.1920-8642.2020.02.003 URL [Cited within: 1]
Severe sepsis and septic shock
DOI:10.1056/NEJMra1208623 URL [Cited within: 1]
Poor microcirculatory flow dynamics are associated with endothelial cell damage and glycocalyx shedding after traumatic hemorrhagic shock
DOI:10.1097/TA.0000000000001695
PMID:28885470
[Cited within: 2]

Endothelial cell damage and glycocalyx shedding after trauma can increase the risk of inflammation, coagulopathy, vascular permeability, and death. Bedside sublingual video-microscopy may detect worse flow and perfusion associated with this endotheliopathy. We compared markers of endotheliopathy with physical flow dynamics after traumatic hemorrhagic shock.Sublingual incident dark field video-microscopy was performed at three time points after injury (<10 hours, 10-30 hours, and 30-50 hours). Values for microcirculatory flow index (MFI), Point Of carE Microcirculation assessment (POEM) score, proportion of perfused vessels (PPV), microcirculatory heterogeneity index (MHI), perfused vessel density (PVD), and total vessel density (TVD) were obtained. ELISAs were performed to measure concentrations of thrombomodulin and syndecan-1 as biomarkers of endothelial cell damage and glycocalyx shedding respectively. Flow parameters were dichotomized to above and below average, and biomarkers compared between groups; below average MFI, POEM, PPV, PVD, and TVD, and above average MHI were considered poor microcirculatory flow dynamics.A total of 155 sublingual video-microscopy clips corresponding to 39 time points from 17 trauma patients were analyzed. Median age was 35 (IQR 25-52); 16/17 were men. Within 10 hours of injury, syndecan-1 concentrations were significantly higher compared to 17 age- and sex-matched healthy controls (30 [IQR 20-44] ng/mL) for worse TVD (78 [IQR 63-417] ng/mL), PVD (156 [IQR 63-590] ng/mL), PPV (249 [IQR 64-578] ng/mL), MFI (249 [IQR 64-578] ng/mL), MHI (45 [IQR] 38-68) ng/mL), and POEM scores (108 [IQR 44-462] ng/mL) (all p < 0.01). Thrombomodulin was also raised within 10 hours of injury when compared to healthy controls (2.9 [IQR 2.2-3.4] ng/mL) for worse PPV (4.1 [IQR 3.4-6.2] ng/mL) and MFI (4.1 [IQR 3.4-6.2] ng/mL) (both p < 0.05).Endothelial cell damage and glycocalyx shedding are associated with worse flow, density, and heterogeneity within microvessels after traumatic hemorrhagic shock. The clinical utility of these biomarkers and flow parameters at the bedside are yet to be elucidated.Prognostic study, level III.
Impact of insulin on the intestinal microcirculation in a model of sepsis-related hyperglycemia
DOI:S0026-2862(17)30219-4
PMID:29778648
[Cited within: 3]

Sepsis involves dysfunctional glucose metabolism. Among patients with sepsis, hyperglycemia is frequent and insulin administration has been evaluated for glycemic control to improve patient outcomes. Only few studies have examined the hyperglycemic microcirculation and the impact of insulin on the microvasculature in sepsis.To study the functional capillary density (FCD) and leukocyte activation within the intestinal microcirculation in endotoxin-induced experimental sepsis.In 50 male Lewis rats, endotoxemia was induced with lipopolysaccharide (LPS; 5 mg/kg). Low dose (LD) glucose was administered to avoid insulin-induced hypoglycemia. High dose (HD) glucose was administered to model sepsis-related hyperglycemia. Animals in LD and HD glucose groups received an insulin bolus (1.4 IU/kg). Two hours after LPS administration, intravital microscopy (IVM) of the terminal ileum was performed, and FCD and leukocyte adherence were measured in a blinded fashion. Blood glucose levels were measured every 30 min following the onset of endotoxemia. Plasma samples were collected 3 h after the onset of endotoxemia to measure IFN-γ, TNF-α, IL-1α, IL-4, GM-CSF and MCP-1 levels using multiplex bead immunoassay.Endotoxemia significantly reduced FCD and increased leukocyte adherence within the intestinal microvasculature. LD and HD glucose administration combined with insulin improved the FCD and decreased the adherence of leukocytes in endotoxemic animals as did HD glucose administration alone. Consistent with these results, IL-4, IL-1α, GM-CSF and IFN-γ levels were decreased following combined HD glucose and insulin administration in endotoxemic animals.Insulin administration, as well as an endogenous insulin response triggered by HD glucose administration, improved the FCD and decreased leukocyte activation in endotoxemic rats. The results of this study give insight into the immune and vaso-modulatory role of insulin administration during experimental endotoxemia, and may be extrapolated for clinical sepsis and other critical illnesses with marked microcirculatory dysfunction.Copyright © 2018 Elsevier Inc. All rights reserved.
Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion
DOI:10.1097/SHK.0b013e3181cdc363
PMID:20634656
[Cited within: 2]

Adhesion of polymorphonuclear neutrophils (PMN) to coronary endothelium is a key event for cardiac ischemia/reperfusion injury. Adhesion molecules are normally harbored within the glycocalyx, clothing every healthy vascular endothelium, but shed by ischemia/reperfusion. Our aim was to show whether protection of the glycocalyx with either hydrocortisone or antithrombin can reduce postischemic leukocyte adhesion. Isolated guinea pig hearts, perfused with Krebs-Henseleit buffer, were subjected to 20 min of warm (37 degrees C) no-flow ischemia and consecutive 10 min of reperfusion, either in the absence or presence of hydrocortisone (10 microg/mL) or antithrombin (1 U/mL). An intracoronary bolus of 3 x 10 PMN was applied at the end of reperfusion but without prior contact to the drugs. The sequestration of PMN was calculated from the difference between coronary input and output of cells. Expression of the integrin CD11b on PMN was measured before and after coronary passage. Ischemia/reperfusion induced severe degradation of the glycocalyx (coronary venous syndecan-1 release, 171 +/- 15 ng/g heart vs. basal, 19 +/- 2 ng/g; heparan sulfate, 5.27 +/- 0.28 microg/g vs. basal, 0.26 +/- 0.06 microg/g) and increased PMN adhesion (38.1% +/- 3.5% vs. basal, 11.7% +/- 3.1%). Hydrocortisone and antithrombin both not only reduced glycocalyx shedding (syndecan-1 release, 34 +/- 6 ng/g and 26 +/- 5 ng/g; heparan sulfate, 1.96 +/- 0.24 microg/g and 1.28 +/- 0.2 microg/g, respectively), but also PMN adhesion (17.3% +/- 2.2% and 25.4% +/- 3.3%, respectively) after ischemia/reperfusion. Electron microscopy revealed a mostly intact coronary glycocalyx after pretreatment with either drug. Activation of PMN upon coronary passage was not influenced. Preservation of the glycocalyx mitigates postischemic PMN adhesion. Preconditioning with either hydrocortisone or antithrombin should, thus, alleviate vascular leakage, tissue edema, and inflammation.
TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin
DOI:10.1007/s00395-008-0749-5
PMID:18836678
[Cited within: 2]

Healthy vascular endothelium is clothed by the endothelial glycocalyx. This structure plays a key role in the regulation of inflammation and vascular permeability and is known to be degraded by ischemic and inflammatory stress. Our aim was to show whether hydrocortisone and antithrombin stabilize the glycocalyx and, therefore, the vascular barrier, against damage induced by the inflammatory stimulus TNF-alpha, thus improving the cardio-vascular situation.Isolated guinea pig hearts were perfused with Krebs-Henseleit buffer for 20 min at constant flow (baseline perfusion pressure 70 cmH(2)O). Hydrocortisone in a stress dose (10 microg/ml) or antithrombin in a physiological dose (1 U/ml) were then applied for 15 min before infusion of TNF-alpha (4 ng/ml, 10 min). Coronary net fluid filtration was assessed directly by measuring transudate formation on the epicardial surface. Hearts were perfusion-fixed to visualize the glycocalyx.TNF-alpha induced severe degradation of the glycocalyx, increased coronary resistance, heightened vascular leak and permeability to hydroxyethyl starch and caused mast-cell degranulation. Hydrocortisone and antithrombin both reduced all of these effects. Electron microscopy revealed a mostly intact glycocalyx after treatment with either drug.Both hydrocortisone and antithrombin clearly preserve the endothelial glycocalyx in the face of inflammatory degradation initiated by TNF-alpha, however, with different mechanisms. This is an important new facet in the pathophysiology and therapy of sepsis, since preservation of the glycocalyx should help prevent vasoconstriction, tissue edema as well as leukocyte and platelet adhesion, thus mitigating inflammation and tissue hypoxia.
Unfractionated heparin attenuates histone-mediated cytotoxicity in vitro and prevents intestinal microcirculatory dysfunction in histone-infused rats
DOI:10.1097/TA.0000000000002387 URL [Cited within: 4]
Extracellular histones in tissue injury and inflammation
DOI:10.1007/s00109-014-1148-z URL [Cited within: 2]
Acute fluoxetine treatment induces slow rolling of leukocytes on endothelium in mice
DOI:10.1371/journal.pone.0088316 URL [Cited within: 2]
The microcirculation in adipose tissue inflammation
DOI:10.1007/s11154-013-9236-x URL [Cited within: 2]
Endothelial barrier dysfunction in septic shock
DOI:10.1111/joim.12331
PMID:25418337
[Cited within: 2]

The endothelium provides an essential and selective membrane barrier that regulates the movement of water, solutes, gases, macromolecules and the cellular elements of the blood from the tissue compartment in health and disease. Its structure and continuous function is essential for life for all vertebrate organisms. Recent evidence indicates that the endothelial surface does not have a passive role in systemic inflammatory states such as septic shock. In fact, endothelial cells are in dynamic equilibrium with a myriad of inflammatory mediators and elements of the innate immune and coagulation systems to orchestrate the host response in sepsis. The barrier function of the endothelial surface is almost uniformly impaired in septic shock, and it is likely that this contributes to adverse outcomes. In this review, we will highlight recent advances in the understanding of the signalling events that regulate endothelial function and molecular events that induce endothelial dysfunction in sepsis. Endothelial barrier repair strategies as a treatment for sepsis include modulation of C5a, high-mobility group box 1 and VEGF receptor 2; stimulation of angiopoietin-1, sphingosine 1 phosphate receptor 1 and Slit; and a number of other innovative approaches. © 2014 The Association for the Publication of the Journal of Internal Medicine.
Sepsis and disseminated intravascular coagulation
DOI:10.1186/s40560-016-0149-0
PMID:27011792
[Cited within: 2]

Sepsis is frequently complicated by coagulopathy and, in about 35 % of severe cases, by disseminated intravascular coagulation (DIC). In Japan, aggressive treatment of septic DIC is encouraged using antithrombin and recombinant thrombomodulin. The macrophages, monocytes, and neutrophils are a source of TF and participate in the direct activation of the coagulation cascade in the early phases of sepsis. And activated factor X (FXa), which is involved in hemostasis, thrombogenesis, inflammation, and cellular immune responses, induces TF expression in human peripheral monocytes and, conversely, that inhibition of FXa activity reduces TF expression. Both inflammation and coagulation play an important role in DIC due to sepsis. In addition to inflammatory cytokines (TNF-α, IL-1 and so on), HMGB1 has recently been shown to mediate the lethal late phase of sepsis and caused coagulopathy. TM not only binds HMGB1 but also aids the proteolytic cleavage of HMGB1 by thrombin. There have been many reports of the efficacy of recombinant TM and antithrombin for treatment of septic DIC from Japan. Further investigation of the efficacy of recombinant TM and AT in countries other than Japan, as well as the monitoring of medical costs incurred during hospitalization, will help validate the use of TM and AT for treatment of septic DIC.
Endothelin-mediated gut microcirculatory dysfunction during porcine endotoxaemia
DOI:10.1093/bja/aeq217
PMID:20710019
[Cited within: 2]

The potent vasoconstrictor endothelin-1 has been implicated in the pathogenesis of the microcirculatory dysfunction seen in sepsis. The mixed endothelin receptor antagonist tezosentan and the selective endothelin A-receptor antagonist TBC3711 were used to investigate the importance of the different endothelin receptors in modulating splanchnic regional blood flow and microvascular blood flow in endotoxaemia.Eighteen anaesthetized pigs were i.v. infused with endotoxin (Escherichia coli lipopolysaccharide, serotype 0111:b4) for 300 min. After 120 min, six animals received tezosentan and six animals received TBC3711. Six animals served as endotoxin-treated controls. Laser Doppler flowmetry was used to measure microcirculatory blood flow in the liver and ileum. Superior mesenteric artery flow (SMA(FI)) and portal vein flow (PV(FI)) were measured with ultrasonic flow probes, and air tonometry was used to measure Pco₂ in the ileal mucosa.TBC3711 did not improve splanchnic regional blood flow or splanchnic microvascular blood flow compared with endotoxin-treated controls. Tezosentan increased PV(FI) (P<0.05), but SMA(FI) was not improved compared with the other groups. In the tezosentan group, microvascular blood flow in the ileal mucosa (MCQ(muc)) improved and mucosal-arterial Pco₂ gap decreased (P<0.05 for both) compared with endotoxin-treated controls and the TBC3711 group.Tezosentan improved MCQ(muc) without any concomitant increase in SMA(FI), implying a direct positive effect on the microcirculation. TBC3711 was not effective in improving regional splanchnic blood flow or splanchnic microvascular blood flow. Dual endothelin receptor antagonism was necessary to improve MCQ(muc), indicating a role for the endothelin B-receptor in mediating the microcirculatory failure in the ileal mucosa.
Regulation of intestinal blood flow
PMID:10945962
[Cited within: 2]

The gastrointestinal system anatomically is positioned to perform two distinct functions: to digest and absorb ingested nutrients and to sustain barrier function to prevent transepithelial migration of bacteria and antigens. Alterations in these basic functions contribute to a variety of clinical scenarios. These primary functions intrinsically require splanchnic blood flow at both the macrovascular and microvascular levels of perfusion. Therefore, a greater understanding of the mechanisms that regulate intestinal vascular perfusion in the normal state and during pathophysiological conditions would be beneficial. The purpose of this review is to summarize the current understanding regarding the regulatory mechanisms of intestinal blood flow in fasted and fed conditions and during pathological stress.Copyright 2000 Academic Press.
Mechanisms of endotoxin-induced intestinal injury in a hyperdynamic model of sepsis
DOI:10.1097/00005373-199305000-00010 URL [Cited within: 2]
Effect of rhubarb pre-treatment on intestinal microcirculation in septic rats
DOI:10.1142/S0192415X14500761 URL [Cited within: 2]
Evaluation of sublingual and gut mucosal microcirculation in sepsis: a quantitative analysis
DOI:10.1097/CCM.0b013e3181b029c1
PMID:19770750
[Cited within: 2]

To determine the relationship between sublingual and intestinal mucosal microcirculatory perfusion.Observational, experimental study.University-affiliated large animal laboratory.Ten fasted, anesthetized, mechanically ventilated, male pigs randomized to a sham group (n = 3) or to a hyperdynamic septic shock group (n = 7) in which cholangitis was induced by direct infusion of Escherichia coli into the common bile duct. This model was developed because it is not accompanied by changes in intra-abdominal pressure.The sublingual and intestinal microcirculations were simultaneously assessed at 4-hr intervals for up to 12 hrs with a modified orthogonal polarization spectral device and functional microvessel density and erythrocyte velocity were measured quantitatively. In sham animals, both regions maintained a stable functional microvessel density and erythrocyte velocity throughout the study period. In contrast, in septic animals, already after 4 hrs of sepsis, functional microvessel density was markedly decreased (>50%) in the sublingual and gut regions; mean erythrocyte velocity decreased dramatically and similarly in both regions, from 1022 +/- 80 to 265 +/- 43 mum/sec in the sublingual region and from 1068 +/- 45 to 243 +/- 115 mum/sec in the gut (p < 0.001, at T12). There was a significant correlation between the sublingual and gut microcirculations in septic animals (r = 0.92, p < 0.0001).The severity and the time course of microcirculatory changes were similar in the sublingual and in the gut region in this clinically relevant model of severe sepsis. These findings support the sublingual region as an appropriate region to monitor the microcirculation in sepsis.
Microcirculation and mitochondria in sepsis: getting out of breath
DOI:10.1097/ACO.0b013e328328d31a URL [Cited within: 2]
Gut microcirculatory and mitochondrial effects of hyperdynamic endotoxaemic shock and norepinephrine treatment
DOI:10.1093/bja/aer379
PMID:22157851
[Cited within: 2]

Microcirculatory and mitochondrial dysfunction are important factors in the development of septic shock. In this study, we investigated the effects of fluid resuscitated endotoxaemic shock and norepinephrine treatment on intestinal microcirculation and mitochondrial function in sheep.Eight anaesthetized sheep received an i.v. infusion of endotoxin. After 24 h, mean arterial pressure (MAP) was restored to baseline levels with a norepinephrine infusion. Five sheep served as sham experiments. Central and regional haemodynamics were monitored, and ileal microcirculation was evaluated with laser Doppler and sidestream dark-field videomicroscopy techniques. Gut mucosal acidosis was assessed by air tonometry, and ileal wall biopsies were analysed for mitochondrial activity.After 24 h of endotoxaemia, the animals had developed hyperdynamic shock with systemic and mucosal acidosis. Although superior mesenteric artery (SMA) flow was higher than the baseline values, ileal microcirculatory perfusion and mitochondrial complex I activity decreased. After norepinephrine was started, SMA flow, ileal microcirculation, and mucosal acidosis remained unchanged. Although no statistically significant difference could be demonstrated, norepinephrine increased mitochondrial complex I activity in five of the six animals from which ileal biopsies were taken.Although fluid resuscitated endotoxaemic shock increased regional blood flow, microcirculatory and mitochondrial alterations were still present. Restoring MAP with norepinephrine did not affect ileal microcirculation or mucosal acidosis, indicating that perfusion pressure manipulation is of limited importance to the intestinal microcirculation in established endotoxaemic shock.
Inhibition of GPR 55 improves dysregulated immune response in experimental sepsis
DOI:10.3233/CH-189320 URL [Cited within: 2]
Tetrahydrobiopterin improves microcirculation in experimental sepsis
DOI:10.3233/CH-160207 URL [Cited within: 3]
Endothelin A and B receptors: potential targets for microcirculatory-mitochondrial therapy in experimental sepsis
DOI:10.1097/SHK.0000000000001414 URL [Cited within: 3]
Effects of dexmedetomidine on intestinal microcirculation and intestinal epithelial barrier in endotoxemic rats
DOI:10.1097/ALN.0000000000001135 URL [Cited within: 3]
Effects of endotoxin absorber hemoperfusion on microcirculation in septic pigs
DOI:S0022-4804(16)30576-5
PMID:28501124
[Cited within: 2]

Endotoxins contribute to systemic inflammatory response and microcirculatory dysfunctions under conditions of sepsis. Polymyxin B hemoperfusion (PMX-HP) is used to remove circulating endotoxins and improve clinical outcomes. This study aims to investigate the effect of PMX-HP on microcirculation in septic pigs.By using a septic pig model, we tested the hypothesis that PMX-HP can correct intestinal microcirculation, tissue oxygenation saturation, and histopathologic alterations. A total of 18 male pigs were divided into three groups: (1) sham; (2) sepsis (fecal peritonitis); and (3) sepsis + PMX-HP groups. A sidestream dark field video microscope was used to record microcirculation throughout the terminal ileal mucosa, colon mucosa, kidney surface, and sublingual area. A superficial tissue oxygenation monitor employing the light reflectance spectroscopy technique was used to measure the tissue oxygen saturation. Hematoxylin and eosin staining was used for histologic examination.The perfused small vessel density and tissue oxygen saturation of the ileal mucosa at 6 h were higher in the sepsis + PMX-HP group than those in the sepsis group. The fluid amount and norepinephrine infusion rate between the sepsis group and sepsis + PMX-HP groups did not differ significantly. The histologic score for the ileal mucosa was lower in the sepsis + PMX-HP group than that in the sepsis group. Finally, the urine output was higher in the sepsis + PMX-HP group than it was in the sepsis group.This study demonstrates that PMX-HP attenuates microcirculatory dysfunction, tissue desaturation, and histopathologic alterations in the ileal mucosa in septic pigs.Copyright © 2016 Elsevier Inc. All rights reserved.
Vancomycin and to lesser extent tobramycin have vasomodulatory effects in experimental endotoxemia in the rat
DOI:10.3233/CH-2010-1331 URL [Cited within: 3]
Enoxaparin sodium prevents intestinal microcirculatory dysfunction in endotoxemic rats
Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human translational ischemia-reperfusion model
DOI:10.1097/MCG.0b013e3182a87e3e
PMID:24100750
[Cited within: 2]

Intestinal fatty acid-binding protein (I-FABP) is a useful marker in the detection of intestinal ischemia. However, more insight into the test characteristics of I-FABP release is needed. This study aimed to investigate the relationship between plasma I-FABP levels and the severity of ischemic mucosal injury, and define the clinical usefulness of systemic I-FABP following ischemia.In a human experimental model, 6 cm of the jejunum, to be removed for surgical reasons, was selectively exposed to either 15, 30, or 60 minutes of ischemia (I) followed by 30 and 120 minutes of reperfusion (R). Blood and tissue was sampled at all time points. Arteriovenous (V-A) concentration differences of I-FABP were measured. Tissue sections were stained with hematoxylin/eosin, and villus height was measured to score epithelial damage.Histologic analysis showed only minor reversible intestinal damage following 15 I and 30 I; however, severe irreversible epithelial damage was observed in the jejunum exposed to 60 I. I-FABP V-A differences paralleled the degree of tissue damage over time [7.79 (± 1.8) ng/mL, 128.6 (± 44.2) ng/mL, 463.3 (± 139.8) ng/mL for 15 I, 30 I and 60 I, respectively]. A good correlation was found between histologic epithelial damage and V-A I-FABP (r=-0.82, P<0.001). Interestingly, systemic I-FABP levels were significantly increased after 60 I of this short small intestinal segment.This study demonstrates the relationship between the duration of ischemia and the extent of tissue damage, which is reflected by I-FABP V-A plasma levels. In addition, systemic I-FABP levels appear valuable in detecting irreversible intestinal ischemia-reperfusion damage.
Serum D-lactate levels as a predictor of intestinal ischemia-reperfusion injury
DOI:10.1007/s003830050436 URL [Cited within: 2]
Diamine oxidase in relation to diamine and polyamine metabolism
DOI:10.1007/BF02005768 URL [Cited within: 2]
Acute administration of antibiotics modulates intestinal capillary perfusion and leukocyte adherence during experimental sepsis
DOI:10.1016/j.ijantimicag.2013.02.024 URL [Cited within: 2]
Argatroban administration reduces leukocyte adhesion and improves capillary perfusion within the intestinal microcirculation in experimental sepsis
DOI:10.1160/TH10-04-0241 URL [Cited within: 2]
Effects of balanced hydroxyethyl starch solutions on gut mucosal microcirculation and exhaled nitric oxide in septic rats: a randomised, animal study
DOI:10.1097/EJA.0b013e3283614048 URL [Cited within: 2]
Effects of crystalloids and colloids on liver and intestine microcirculation and function in cecal ligation and puncture induced septic rodents
DOI:10.1186/1471-230X-12-179 URL [Cited within: 2]
Effects of sepsis on hippocampal volume and memory function
DOI:10.5847/wjem.j.1920-8642.2020.04.004 URL [Cited within: 2]
The effects of vasoactive drugs on intestinal functional capillary density in endotoxemic rats: intravital video-microscopy analysis
DOI:10.1213/ANE.0b013e3181c88af1 URL [Cited within: 3]
Statins prevent cognitive impairment after sepsis by reverting neuroinflammation, and microcirculatory/endothelial dysfunction
DOI:10.1016/j.bbi.2016.11.006 URL [Cited within: 2]
Impact of lipid modulation on the intestinal microcirculation in experimental sepsis
DOI:10.1016/j.mvr.2018.05.009 URL [Cited within: 2]
Expert consensus on diagnosis and treatment of septic shock with integrated traditional Chinese and Western medicine
Anti-inflammatory effects of a novel iron chelator, DIBI, in experimental sepsis
DOI:10.3233/CH-179205 URL [Cited within: 2]
Effect of deletion of cIAP 2 on intestinal microcirculation in mouse endotoxemia and polybacterial sepsis
DOI:10.1097/SHK.0000000000000132 URL [Cited within: 2]
Differential effects of selective and nonselective potassium channel inhibitors in ovine endotoxemic shock (macrocirculation) and in a rat model of septic shock (microcirculation)
DOI:10.1097/SHK.0000000000001113 URL [Cited within: 2]
Experimental TLR4 inhibition improves intestinal microcirculation in endotoxemic rats
DOI:10.1016/j.mvr.2015.06.004 URL [Cited within: 2]
Desmopressin improves intestinal functional capillary density and decreases leukocyte activation in experimental endotoxemia
DOI:10.1016/j.mvr.2013.09.001
PMID:24035754
[Cited within: 2]

Blood flow to the intestine is decreased in sepsis in favor of vital organs resulting in ischemic damage of the gut mucosa. Once the mucosa is damaged, increased translocation of intestinal bacteria to the systemic circulation may occur. This in turn aggravates the inflammatory response contributing to the development of multi-organ failure. Desmopressin is a synthetic analog of vasopressin, an anti-diuretic hormone which has been shown to induce vasodilation and is thought to be implicated in immunomodulation. In this study, we investigate the effects of desmopressin on the intestinal microcirculation during sepsis in an experimental endotoxemia model in rats using intravital microscopy. In addition, we investigate the effects of desmopressin on systemic inflammation.Forty Lewis rats were subdivided into four groups, where rats received intravenous saline (control), desmopressin (1μg/kg/ml), lipopolysaccharide (5mg/kg) or lipopolysaccharide followed by desmopressin. Inflammatory response was assessed by quantifying the number of temporary and firmly adherent leukocytes in submucosal venules. Capillary perfusion was determined by assessing the number of functional, non-functional and dysfunctional capillaries in the intestinal wall layers (muscularis longitudinalis, muscularis circularis and mucosa). Additionally, inflammatory cytokine levels were determined by multiplex assays.The number of firmly adhering leukocytes in V1 venules of rats receiving lipopolysaccharide and treated with desmopressin was significantly reduced compared to lipopolysaccharide only group (LPS: 259±25.7 vs. LPS+DDAVP: 203±17.2; n/mm(2); p<0.05). Additionally, desmopressin treatment improved impaired intestinal microcirculation by improving functional capillary density following lipopolysaccharide administration in all examined layers of the intestinal wall. We also observed a significant decrease in TNF-α levels in rats which received desmopressin in endotoxemia compared to untreated rats (LPS: 383±64.2; LPS+DDAVP: 261.3±22; pg/ml; p<0.05).Desmopressin administration improved intestinal capillary perfusion and reduced inflammatory response in rat endotoxemia.© 2013.
Hypercapnia-induced amelioration of the intestinal microvascular oxygenation in sepsis is independent of the endogenous sympathetic nervous system
DOI:10.1097/SHK.0000000000000920
PMID:28650926
[Cited within: 2]

Insufficient microvascular oxygenation (μHBO2) of the intestinal mucosa worsens outcome of septic patients. Hypercapnia ameliorates μHBO2, mediated via endogenous vasopressin release. Under physiological conditions, blockade of the endogenous sympathetic nervous system abolishes this protective effect of hypercapnia. The aim of our study was therefore to evaluate the role of the endogenous sympathetic nervous system during hypercapnia on intestinal μHBO2 under septic conditions.We randomized 80 male Wistar rats into eight groups. Sepsis was induced via colon ascendens stent peritonitis. The animals were subjected to 120 min of normocapnic (pCO2 35 mm Hg-45 mm Hg) or moderate hypercapnic (pCO2 65 mm Hg-75 mm Hg) ventilation 24 h after surgery. Animals received sympathetic blockade (hexamethonium 15 mg · kg (bolus) followed by 15 mg · kg · h (infusion) intravenously) or the same volume as vehicle (NaCl 0.9%). Microcirculatory oxygenation (μHBO2) and perfusion (μflow) were recorded using tissue reflectance spectrophotometry and laser Doppler.In septic animals, μHBO2 decreased during normocapnia (-8.9 ± 4%) and increased during hypercapnia (+7.8 ± 7.5%). The additional application of hexamethonium did not influence these effects. μHBO2 declined in normocapnic septic animals treated with hexamethonium similar to normocapnia alone (-6.1 ± 5.4%) and increased in hypercapnic animals treated with hexamethonium similar to hypercapnia alone (+7.9 ± 11.7%). Furthermore, hypercapnic ventilation ameliorated microcirculatory perfusion (μflow) irrespective of whether animals received hexamethonium (from 113 ± 54 [AU] to 206 ± 87 [AU]) or vehicle (from 97 ± 37 [AU]-169 ± 52 [AU]).The amelioration of the intestinal microcirculation during hypercapnia in sepsis is independent of the endogenous sympathetic nervous system.
Vasopressin V1A receptors mediate the stabilization of intestinal mucosal oxygenation during hypercapnia in septic rats
DOI:10.1016/j.mvr.2016.03.002
PMID:26969105
[Cited within: 2]

Microvascular oxygen saturation (μHBO2) plays an essential role in the development and outcome of sepsis. Hypercapnia (HC) improves the microvascular oxygenation of the mucosa in both healthy and septic animals. Vasopressin V1A receptor blockade prevents this positive effect under otherwise physiological conditions. The aim of this study was to investigate the effects and mechanisms of the vasopressin system during hypercapnia under septic conditions.80 rats were randomized into 8 groups (N=10). Colon ascendens stent peritonitis (CASP) or sham surgery was performed on 40 animals each to establish a moderate polymicrobial sepsis or sham control, respectively. 24h after sepsis induction the animals were subjected to 120min of volume-controlled and pressure-limited ventilation with either normocapnic (pCO2 35-45mmHg) or moderate hypercapnic (pCO2 of 65-75mmHg) ventilation targets. Animals received either vasopressin V1A receptor blockade (SR 49059, 1mgkg(-1) i.v.) or vehicle solution (dimethyl sulfoxide, 1%). Blood pressure, heart rate, pO2 and pCO2 were measured and microcirculatory oxygenation (μHBO2) and microcirculatory flow (μflow) were recorded using tissue reflectance spectrophotometry. Oxygen supply (μDO2) and consumption (μVO2) were calculated from intermittent blood gas analysis.In septic animals, μHBO2 declined during normocapnia (-11±10.3) but remained unchanged during hypercapnia. μHBO2 declined with vasopressin V1A receptor blockade both during normocapnia (-7.4±10.6) and hypercapnia (-9.2±9.8). Microcirculatory oxygen consumption was significantly reduced by hypercapnia in septic animals (-2.4·10(5) [AU]±2.4·10(5) [AU]). In sham animals, μHBO2 and μVO2 did not change.Vasopressin V1A receptors mediate the beneficial effects of hypercapnia on microcirculatory oxygenation during sepsis. The effects of vasopressin on μHBO2 might be related to decreased oxygen consumption during hypercapnia.Copyright © 2016. Published by Elsevier Inc.
Physostigmine reverses disturbances of the intestinal microcirculation during experimental endotoxemia
DOI:10.3233/CH-131743 URL [Cited within: 2]
Combination of dehydroepiandrosterone and orthovanadate administration reduces intestinal leukocyte recruitment in models of experimental sepsis
DOI:10.1016/j.mvr.2014.07.010
PMID:25086183
[Cited within: 2]

Dehydroepiandrosterone (DHEA) was shown to improve the immune function and survival in experimental sepsis. This study examined the effect of DHEA on intestinal leukocyte recruitment during experimental sepsis, considering factors of gender (male, female and ovariectomized female animals) and combined treatment using orthovanadate (OV) in two models of sepsis.Male rats underwent colon ascendens stent peritonitis (CASP) or endotoxemia. DHEA was administered after induction of experimental sepsis. Changes in leukocyte adherence and capillary perfusion (measured as intestinal functional capillary density - FCD) were assessed using intravital microscopy. While DHEA increased baseline leukocyte adherence in control animals, DHEA reduced leukocyte adherence and increased FCD in male animals with CASP. These effects were also observed in DHEA-treated ovariectomized female rats with CASP. Similarly, the administration of DHEA reduced the number of adherent leukocytes to intestinal venules by 30% in the endotoxemia model. The combined treatment of DHEA and OV significantly reduced adherence of leukocytes to intestinal venules and improved FCD.Our results indicate that DHEA is able to reduce intestinal leukocyte recruitment induced by experimental sepsis. Combination of DHEA with OV inhibits leukocyte adherence to intestinal endothelium, similar to what is achieved by the single administration of DHEA but with significantly improved FCD. These findings suggest a potential role for DHEA and OV in clinical sepsis.Copyright © 2014 Elsevier Inc. All rights reserved.
Experimental cannabinoid 2 receptor-mediated immune modulation in sepsis
Estradiol receptors agonists induced effects in rat intestinal microcirculation during sepsis
DOI:10.1016/j.mvr.2012.10.002
PMID:23063870
[Cited within: 2]

The steroid hormone estradiol is suggested to play a protective role in intestinal injury during systemic inflammation (sepsis). Our aim was to determine the effects of specific estradiol receptor (ER-α and ER-ß) agonists on the intestinal microcirculation during experimental sepsis. Male and sham ovariectomized female rats were subjected to sham colon ascendens stent peritonitis (CASP), and they were compared to male and ovariectomized female rats underwent CASP and either estradiol receptor α (ER-α) agonist propyl pyrazole triol (PPT), estradiol receptor ß (ER-ß) agonist diarylpropiolnitrile (DPN), or vehicle treatment. Intravital microscopy was performed, which is sufficiently sensitive to measure changes in the functional capillary density (FCD) as well as the major steps in leukocyte recruitment (rolling and adhesion). The leukocyte extravasations were also quantified by using histological paraffin sections of formalin fixed intestine. We found that either DPN (ER-β) or PPT (ER-α) significantly reduced (P<0.05) sepsis-induced leukocyte-endothelial interaction (rolling, adherent leukocytes and neutrophil extravasations) and improved the intestinal muscular FCD. [PPT: Female; Leukocyte rolling (n/min): V(3) 3.7±0.7 vs 0.8±0.2, Leukocyte adhesion(n/mm(2)): V(3) 131.3±22.6 vs 57.2±13.5, Neutrophil extravasations (n/10000 μm(2)): 3.1±0.7 vs 6 ±1. Male; Leukocyte adhesion (n/mm(2)): V(1) 154.8±19.2 vs 81.3±11.2, V(3) 115.5±23.1 vs 37.8±12]. [DPN: Female; neutrophil extravasations (n/10000 μm(2)) 3.8±0.6 vs 6 ±1. Male; Leukocyte adhesion (n/mm(2)) V(1) 154.8±19.2 vs 70±10.5, V(3) 115.5±23.1 vs 52.8±9.6].Those results suggest that the observed effects of estradiol receptors on different phases of leukocytes recruitment with the improvement of the functional capillary density could partially explain the previous demonstrated salutary effects of estradiol on the intestinal microcirculation during sepsis. The observed activity of this class of compounds could open up a new avenue of research into the potential treatment of sepsis.Copyright © 2012 Elsevier Inc. All rights reserved.
Cannabinoid receptor 1 inhibition improves the intestinal microcirculation in experimental endotoxemia
DOI:10.3233/CH-131668 URL [Cited within: 2]
Inhibition of endocannabinoid degradation in experimental endotoxemia reduces leukocyte adhesion and improves capillary perfusion in the gut
Inhibition of lectin-like oxidized low-density lipoprotein receptor-1 reduces leukocyte adhesion within the intestinal microcirculation in experimental endotoxemia in rats
DOI:10.1186/cc9367 URL [Cited within: 2]