Dear editor,
Massive hemoptysis (MH) sometimes causes a fatal condition;[1] thus, prompt diagnosis and treatment for MH are crucial. However, a wide range of diseases (e.g., infectious diseases, malignancies, and systemic autoimmune diseases) is associated with the development of MH,[2] which occasionally causes difficulty with identifying the specific etiology. The administering of drugs increases the difficulty, and MH may have other etiologies, such as conditions resulting from interventions.
The clinical management of atrial fibrillation (AF) consists of a multimodal approach, including medical, interventional, and surgical treatments.[3] As a widely used intervention in the treatment of AF, radiofrequency catheter ablation (RFCA) may introduce many complications. Pulmonary vein stenosis (PVS) is an underrecognized and often misdiagnosed syndrome associated with this procedure.[4] However, the diagnosis and management of MH cases due to PVS have rarely been addressed.[5]
CASE
A 41-year-old man presented to our emergency department (ED) with cough and hemoptysis. He had complained of hemoptysis 22 days before and had a history of AF. Four months ago, he had been hospitalized in a community hospital and undergone RFCA for AF. He had been taking dabigatran until 40 days before the onset of hemoptysis. His chest radiograph taken in our ED was unremarkable, but fibro-laryngoscopy revealed fresh blood stains in the posterior area of the epiglottis. The patient received azithromycin and hemostatic therapy medication with carbazochrome sodium sulfonate, hemocoagulase agkistrodon, and Yunnan white powder, but the treatment was ineffective. At the initial medical evaluation, his blood pressure was 118/74 mmHg (1 mmHg=0.133 kPa), heart rate 105 beats per minute, respiratory rate 18 breaths per minute, and body temperature 36.7 °C. His oxygen saturation, when breathing room air, was 100%. Physical examination was generally normal, with no rales in the lungs or murmurs of the heart.
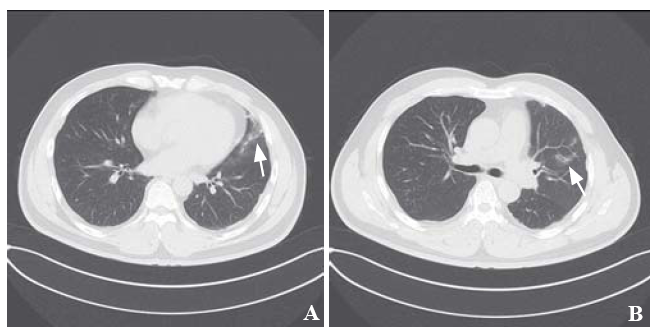
Chest computed tomography (CT) revealed scattered ground-glass opacities (GGOs) and patching effusions in the left upper lobe (Figures 1 A and B). Laboratory tests showed elevated white blood cells and monocytes (Table 1). The coagulation test was normal. An autoimmune panel was negative for the antinuclear antibody, anti-DNA antibody, anti-smooth muscle antibody, and antineutrophil cytoplasmic antibodies. An infective workup was also negative for any cultures of beta-D-glucan and tuberculosis (TB).
Figure 1.
Figure 1.
Chest CT. Both fi gures (A and B) revealed scattered GGOs and patching eff usions (shown by arrows) in the left upper lobe. CT: computed tomography; GGOs: ground glass opacities.
Table 1 Laboratory data
| Variables | Reference range | On admission |
|---|---|---|
| White blood cell (cell/μL) | 3,500-9,500 | 11,370 |
| Monocytes (cell/μL) | 100-600 | 620 |
| Hemoglobin (g/dL) | 13.0-17.5 | 14.0 |
| Platelet count (cell/μL) | 125,000-350,000 | 218,000 |
| Prothrombin time (%) | 80-150 | 85 |
| Activated partial thromboplastin time (s) | 28-42 | 32 |
| Fibrinogen (mg/dL) | 200-440 | 3.4 |
| Fibrin degradation products (μg/mL) | <5 | <2.5 |
| NT-pro-BNP (pg/mL) | 12-133 | <70 |
Serial hemoglobin measurements revealed a progressive decline from 14 g/dL on admission to 11.7 g/dL the next day. Consequently, bronchial arteriography was performed, which showed no significant bronchial arterial hemorrhage, but suggested that the left bronchial artery was thickened and tortuous. The interventional radiologists decided to perform a bronchial artery embolization (BAE) on the left lobe suspected of culprit vessel with coils. Two hours after BAE, the patient developed another episode of MH (200 mL).
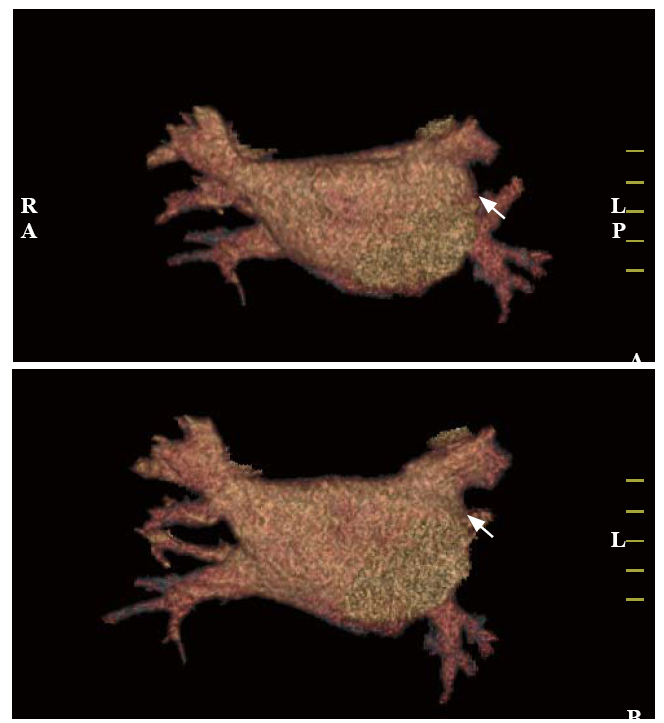
Taking the standard diagnostic approach, the bleeding source remained unexplained in the patient. Another possibility was that the hemoptysis and GGOs were caused by underlying cardiovascular diseases. However, this patient had no evidence of congestive heart failure, pulmonary edema, rheumatic heart disease, or pulmonary hypertension. Considering the medical history of RFCA 4 months ago, PVS was suspected. Contrast-enhanced multi-slice CT venography with three-dimensional reconstruction was conducted, which found left superior PVS (Figures 2 A and B). Pulmonary vein (PV) diameters (mean reference range[6]) were reported as follows: right superior PV 20.5 mm (13.0-15.0 mm), left superior PV 13.8 mm (16.0-17.0 mm), right inferior PV 20.4 mm (16.0-17.0 mm), and left inferior PV 13.4 mm (14.0-15.0 mm). The final diagnosis was PVS post RFCA for AF.
Figure 2.
Figure 2.
Contrast-enhanced multi-slice CT venography with three-dimensional reconstruction. Both figures (A and B) revealed detected left superior PVS (shown by arrows). CT: computed tomography; PVS: pulmonary venous stenosis.
Because the massive hemorrhage persisted, urgent surgical intervention was performed. During the surgical procedure, it was confirmed that the left superior PV and its branch wall were obviously thickened, and the lumen was almost occluded; the lumen of the left lower PV was enlarged, and its entrance to the atrium was significantly narrowed. Pericardial patch plasty restored the pulmonary venous drainage, veno-venous extracorporeal membrane oxygenation (ECMO) supported the patient’s breathing and circulation, and a temporary epicardial pacemaker provided cardiac pacing. On day 5 after surgery, the patient was successfully weaned off ECMO. On postoperative day 7, the patient was extubated from the ventilator. On postoperative day 17, the patient was discharged with no event.
DISCUSSION
MH is rare, accounting for less than 1%-5% of all cases of hemoptysis.[7] However, MH is life-threatening due to the influence of blood upon oxygen exchange.[8] In geographical areas with a high incidence of TB, TB remains a common etiology of hemoptysis (24.8%).[9] Otherwise, pulmonary malignancy (19.1%) is the most frequent cause of hemoptysis. Pneumonia (18.6%), bronchiectasis (14.9%), and acute bronchitis (13.7%) are frequent etiologies in developed countries.[2] MH may have other etiologies, such as conditions resulting from interventions.
PVS is defined as ≥20% luminal narrowing of PVs.[3] Jin et al[10] reported that when venous pressure suddenly rose for some reason, the venous dilatation or varicose formation caused by elevated pulmonary venous pressure may trigger rupture. Histological examination of PVS in patients after RFCA shows obviously thickened vein walls resulting in cardiomyocyte death followed by myofibroblast proliferation.[11]
This patient’s course highlighted three important clinical issues.
First, PVS after RFCA for AF might be an etiology of MH. PVS is an underrecognized syndrome that is a reported complication of RFCA in patients with AF; the published incidence of PVS varies widely from 0% to 38%.[4] As a result, the diagnosis of PVS is often delayed. Emergency department (ED) physicians should be alert to surgical complications, especially for patients with newly developed respiratory symptoms after RFCA. Therefore, a detailed medical history and a complete physical examination may provide diagnostic information for the origin of a case of hemoptysis.[12]
Second, according to the clinical condition of a patient’s MH, the appropriate examination should be chosen based on safety and efficiency. Especially in unstable MH, the primary focus is patient stabilization and resuscitation.[13] The initial assessment should include complete laboratory tests and imaging.[10] Multi-slice spiral chest CT angiography (MCTA) may be a noninvasive and useful examination to diagnose hemoptysis, localize bleeding, and detect potential vascular malformation.[5,6] Hirshberg et al[14] reported that bronchoscopy was another examination used to locate the bleeding spot with sufficient suction, and provided the possibility for endoscopic intervention. Unfortunately, during the COVID-19 pandemic, bronchoscopy has been underutilized due to conservative hospital policy. Yet, it is estimated that 90% of cases of MH emanate from the bronchial vascular system.[15] Therefore, bronchial artery embolization (BAE) is now universally accepted as an effective and first-line treatment of MH, especially in the setting of failed noninvasive procedures.[7]
Third, there are several therapeutic options to restore pulmonary venous drainage. Nonsurgical therapeutic options for management of patients include stent implantation and invasive balloon angioplasty.[3] Currently, a percutaneous dilatation with balloon angioplasty of the narrowed PV represents the first-line therapy for symptomatic PVS. At long-term follow-up, stent implantation seems to be superior to balloon angioplasty alone.[16] However, in an emergency situation, lobectomy or pneumonectomy could be a life-saving procedure performed for acute MH or complete PV occlusion.[12] Other surgical treatment options for patients with hemoptysis caused by acquired PVS include venoplasty or a pericardial patch plasty.[5]
CONCLUSIONS
We report a case of MH caused by PVS after RFCA for AF. PVS should be considered in the differential diagnosis of patients with hemoptysis. In particular, patients with a history of interventional therapy for AF are predisposed to pulmonary venous obstruction. Contrast-enhanced multi-slice CT venography with three-dimensional reconstruction is an important method for the detection of PVS. Early diagnosis and reasonable treatment based on the patient’s condition can improve a patient prognosis.
Funding: None.
Ethics approval: Not needed.
Conflicts of interests: The authors have no conflict of interest.
Contributors: All authors have substantial contributions to the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; and final approval of the version to be published.
Reference
Managing massive hemoptysis
DOI:S0012-3692(19)31386-8
PMID:31374211
[Cited within: 1]

Massive hemoptysis is a medical emergency with high mortality presenting several difficult diagnostic and therapeutic challenges. The origin of bleeding and underlying etiology often is not immediately apparent, and techniques for management of this dangerous condition necessitate an expedient response. Unlike hemorrhage in other circumstances, a small amount of blood can rapidly flood the airways, thereby impairing oxygenation and ventilation, leading to asphyxia and consequent cardiovascular collapse. Of paramount importance is early control of the patient's airway and immediate isolation of hemorrhage in an attempt to localize and control bleeding. A coordinated team response is essential to guarantee the best chances of patient survival. Prompt control of the airway and steps to limit the spread of hemorrhage take precedence. Bronchial artery embolization, rigid and flexible bronchoscopy, and surgery all serve as potential treatment options to provide definitive control of hemorrhage. Several adjunctive therapies described in recent years may also assist in the control of bleeding; however, their role is less defined in life-threatening hemoptysis and warrants additional studies. In this concise review, we emphasize the steps necessary for a systematic approach in the management of life-threatening hemoptysis.Copyright © 2019 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Observational, multicentre study on the epidemiology of haemoptysis
DOI:10.1183/13993003.01813-2017 URL [Cited within: 2]
Long-term outcomes of surgical ablation for atrial fibrillation: impact of ablation lesion sets
DOI:10.1016/j.jacasi.2021.06.001 URL [Cited within: 3]
Severe pulmonary vein stenosis resulting from ablation for atrial fibrillation
PMID:27793993
[Cited within: 2]

The frequency of pulmonary vein stenosis (PVS) after ablation for atrial fibrillation has decreased, but it remains a highly morbid condition. Although treatment strategies including pulmonary vein dilation and stenting have been described, the long-term impacts of these interventions are unknown. We evaluated the presentation of severe PVS, and examined the risk for restenosis after intervention using either balloon angioplasty (BA) alone or BA with stenting.This was a prospective, observational study of 124 patients with severe PVS evaluated between 2000 and 2014.All 124 patients were identified as having severe PVS by computed tomography in 219 veins. One hundred two patients (82%) were symptomatic at diagnosis. The most common symptoms were dyspnea (67%), cough (45%), fatigue (45%), and decreased exercise tolerance (45%). Twenty-seven percent of patients experienced hemoptysis. Ninety-two veins were treated with BA, 86 were treated with stenting, and 41 veins were not treated. A 94% acute procedural success rate was observed and did not differ by initial management. Major procedural complications occurred in 4 of the 113 patients (3.5%) who underwent invasive assessment, and minor complications occurred in 15 patients (13.3%). Overall, 42% of veins developed restenosis including 27% of veins (n=23) treated with stenting and 57% of veins (n=52) treated with BA. The 3-year overall rate of restenosis was 37%, with 49% of BA-treated veins and 25% of stented veins developing restenosis (hazard ratio, 2.77; 95% confidence interval, 1.72-4.45; P<0.001). After adjustment for age, CHA2DS2-VASc score, hypertension, and the time period of the study, there was still a significant difference in the risk of restenosis for BA versus stenting (hazard ratio, 2.46; 95% confidence interval, 1.47-4.12; P<0.001).The diagnosis of PVS is challenging because of nonspecific symptoms and the need for dedicated pulmonary vein imaging. There is no difference in acute success by type of initial intervention; however, stenting significantly reduces the risk of subsequent pulmonary vein restenosis in comparison with BA.© 2016 American Heart Association, Inc.
Haemoptysis due to pulmonary venous stenosis
DOI:10.1183/09059180.00003713 URL [Cited within: 3]
Visualization of pulmonary vein stenosis after radio frequency ablation using multi-slice computed tomography: initial clinical experience in 33 patients
PMID:15982498
[Cited within: 2]

Radio frequency ablation (RFA) of the pulmonary veins (PV) is an established technique for treatment of atrial fibrillation (AF). However, stenoses within the treated areas are well known complications. Thus, a reliable non-invasive diagnosis of PV stenosis would be an important step forward in the care of these patients (pts). Aim of the present study was the diagnostic accuracy of new multi-slice detected computed tomography (MSCT) in visualization of PV and in detecting PV stenosis.A total of 33 pts (17 male, 16 female, mean age 57+/-10.2 years [40-71]) were included. Retrospectively ECG-gated CT angiography (CTA) was performed within 1 day to a maximum of 380 days after RFA with a MSCT scanner. Interpretation of the scan was performed on conventional contrast enhanced axial slices and on 3D volume rendering images (maximum intensity projection: MIP, multi-planar reconstruction: MPR). Lesion severity was determined on a semi-quantitative scale (mild: <20%, intermediate: 20-50%, severe >50%) and compared to conventional angiography which had been performed at the beginning and at the end of RFA.MSCTA was applied without any complications, and all treated pulmonary veins (n=73) could be visualized. Diagnostic image quality was obtained in all examinations. A significant stenosis was detected by conventional angiography in 26/73 (36%) PV (2/73 (3%) severe, 14/73 (19%) intermediate, 10/73 (14%) mild). Using MSCTA, only 13 stenosis in 73 treated PV could be visualized (1/73 (1%) severe, 6/73 (8%) intermediate, 6/73 (8%) mild).Multi-slice-detector CT is able to visualize PV and to detect PV stenoses. However, stenosis severity seems to be underestimated and not all lesions could be accurately detected. Larger studies have to be performed to further assess the diagnostic accuracy and clinical reliability of this new non-invasive method and to focus on the incidence of PV stenosis following RFA especially in long-time follow up.
Life-threatening hemoptysis
DOI:10.1055/s-0040-1714386 URL [Cited within: 1]
Localization of bleeding sites in patients with hemoptysis based on their chest computed tomography findings: a retrospective cohort study
DOI:10.1186/s12890-016-0322-1 URL [Cited within: 1]
Chinese expert recommendation for diagnosis and treatment of massive hemoptysis
DOI:10.1159/000502156 URL [Cited within: 2]
Pulmonary vein stenosis: expression of receptor tyrosine kinases by lesional cells
PMID:16533697
[Cited within: 1]

Primary pulmonary vein stenosis (PVS) is a progressive disorder of infants. Although catheter based intervention and chemotherapy are used to manage the disorder, the benefit of these approaches is reduced considerably by restenosis. The nature of the intimal cells causing the occlusive lesions in PVS is poorly understood.Seven PVS cases were studied with antibodies for smooth muscle actin (SMA), muscle-specific actin (MSA), monoclonal desmin, S100 protein, CD31, CD34, CD45RO, CD68, CD99, Ki-67 (MIB-I), and with antibodies directed against several receptor tyrosine kinases (RTK), including platelet-derived growth factor alpha and beta receptor (PDGFR-alpha and -beta), epidermal growth factor receptor (EGFR), fibroblast growth factor receptor (FGFR), vascular endothelial growth factor 1 and 2 receptor (VEGFR), and stem cell factor receptor (c-kit).Lesional cells stained strongly and diffusely with SMA and MSA, but not for macrophage, lymphocyte, endothelial markers, or for Ki-67. RTK expression was strong and diffuse for PDGFR-alpha and -beta, FGFR, and VEGFR-2. Lesional cells stained for VEGF and PDGF beta receptor was phosphorylated.The histologic appearance, and the strong diffuse immunoreactivity for smooth muscle markers, indicates that the intimal lesional cells are myofibroblast-like. Expression of various receptor tyrosine kinases and some ligands suggests an autocrine or paracrine role of these proteins in the pathogenesis of the intimal occlusive lesion in PVS.
Bilateral pulmonary vein stenting for treatment of massive hemoptysis caused by pulmonary vein stenosis following catheter ablation for atrial fibrillation
ACR appropriateness criteria® hemoptysis
DOI:10.1097/RTI.0b013e3181e35b0c URL [Cited within: 1]
Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital
PMID:9266882
[Cited within: 1]

Hemoptysis, an important and alarming symptom, often indicates serious disease. This study was designed to assess the different causes of hemoptysis, the relative importance of the different diagnostic modalities employed, and the outcome in an Israeli population cohort.A retrospective analysis of 208 patients with hemoptysis at the Hadassah University Hospital, Jerusalem, Israel between January 1980 and August 1995.Bronchiectasis (20%), lung cancer (19%), bronchitis (18%), and pneumonia (16%) accounted for most causes of hemoptysis. In contrast to older studies, active tuberculosis was a rare finding (1.4%). Bronchiectasis and bleeding diathesis were major causes of moderate to severe hemoptysis while bronchitis and lung cancer were commonly associated with milder degrees of bleeding. CT scan was the most sensitive diagnostic test when employed alone, with a positive yield of 67%. However, it failed to locate at least three cases of lung cancer. When combining a CT study together with a bronchoscopy, the positive yield increased to 93%. The mortality rate for patients with mild to moderate hemoptysis was low (2.5% and 6%, respectively), while patients with massive hemoptysis had high mortality rates (38%). Patients with lung cancer or bleeding diathesis had higher mortality rates compared with the rest of the cohort.Hemoptysis is a common symptom with a good prognosis in most cases. However, patients exhibiting massive bleeding or those with lung malignancy and patients with bleeding diathesis had a poorer prognosis. Patients older than 50 years with a positive smoking history need an extensive evaluation and follow-up to exclude lung carcinoma. The combined use of bronchoscopy and chest CT has the best yield in evaluating hemoptysis.
Hemoptysis: bronchial and nonbronchial systemic arteries at 16-detector row CT
DOI:10.1148/radiol.2341032079 URL [Cited within: 1]
Balloon angioplasty versus stenting for pulmonary vein stenosis after pulmonary vein isolation for atrial fibrillation: a meta-analysis