INTRODUCTION
Trauma is the leading cause of death for people aged under 45 years in developed nations.[1,2] Among injured patients, hemorrhage is the second leading cause of death after central nervous system (CNS) injury and the number one preventable cause of death.[3,4] Hemorrhage is complicated in 25%-30% of patients by trauma-induced coagulopathy (TIC).[5] TIC is a complex, multifactorial, and pathological process characterized by hypocoagulability and hyperfibrinolysis.[6] TIC carries a poor prognosis, and trauma patients with TIC are at a four-fold risk of mortality compared to trauma patients without TIC.[6] Tranexamic acid (TXA) is an antifibrinolytic agent, which has been hypothesized to reduce mortality in hemorrhagic trauma patients by retarding the hyperfibrinolysis seen in TIC.[7,8]
The Clinical Randomisation of an Anti-fibrinolytic in Significant Hemorrhage-2 (CRASH-2) is the largest randomized control trial (RCT) examining circulatory resuscitation for trauma patients to date and concludes a statistically significant reduction in all-cause mortality in the TXA group (14.5%) compared to the placebo group (16.0%), with a relative risk (RR) of 0.91 (95% confidence interval [CI] 0.85-0.97, P=0.0035).[9] The benefits were restricted to patients receiving TXA within 3 hours of injury, and the trial concluded that TXA administration within 3 hours of injury was safe and cost-effective and may result in a mortality benefit to injured patients.[9] Based on these conclusions, many nations around the world have implemented TXA into their massive transfusion protocols, and recommended routine use of TXA for all trauma patients at risk of hemorrhage.
Implementation of TXA post-CRASH-2 has been enthusiastic within the UK. However, this uptake has not been universal in other nations, and currently there exists significant geographical variance in the use of TXA for trauma patients.[10] A postulated reason for this variation relates to generalisability with many patients in CRASH-2 enrolled from developing nations, which commonly lack ready access to advanced processes and interventions in mature trauma systems.[11,12]
Geographically, Ireland is the closest nation to the UK. Both countries collect clinical audit data on major trauma in the Trauma Audit and Research Network (TARN) registry. The Irish arm of the audit is governed by the National Office of Clinical Audit (NOCA) and called the Major Trauma Audit (MTA). Furthermore, Ireland is currently in the midst of developing a coordinated national trauma network similar to the recently introduced and successful UK model.[13]
The aim of this study was to assess TXA use for severe trauma patients with hemorrhagic shock in Ireland after publication of CRASH-2 trial results and examine the association between TXA administration and mortality. We hypothesized that TXA administration, after adjustment for potential known confounders, would be associated with reduced mortality at hospital discharge.
METHODS
Study design
A retrospective cohort study was conducted using data derived from the MTA via the NOCA.
TARN is an academically led, independent, and prospective trauma registry for injured patients presenting to hospitals in the UK and Ireland. At present, TARN is the largest trauma registry in Europe capturing data from all injured patients in the UK and Ireland.
NOCA is an Irish clinical audit program established in 2012 designed to be an ongoing measure of clinical practice in Ireland.[14] NOCA is funded by the Health Service Executive Improvement Division and is governed by an independent voluntary board and operationally supported by the Royal College of Surgeons in Ireland.[14,15] Upon establishment, NOCA engaged the services of TARN as a data registry for Irish trauma patients entitled the MTA. Currently, 26 trauma-receiving hospitals in Ireland give feedback data to TARN.[14] NOCA publishes an annual MTA and has shown a continued increase in the uptake of the TARN database each year, as well as increased robustness of the data collected.[15]
NOCA supports research within an ethical framework. It distributes data for high-quality research, internally to primary NOCA based researchers, and externally to collaborative national and international research partners.
Inclusion criteria
All injured patients presenting to one of the 26 trauma-receiving hospitals in Ireland between January 2013 and December 2018 and evidence of hemorrhagic shock on presentation were included in this study. Hemorrhagic shock was defined as clinically significant hypotension and blood transfusion. The CRASH-2 trial enrolled patients with systolic blood pressure (SBP) <90 mmHg (1 mmHg=0.133 kPa) or heart rate >110 beats/minute or clinically adjudged risk of significant hemorrhage. TARN defines clinically significant hypotension as SBP <110 mmHg and thus indicates TXA for all patients with SBP <110 mmHg. This is due to previous studies showing that trauma patients with SBP <110 mmHg have a two-fold increase in mortality.[16] Although all patients with SBP are indicated TXA as per the TARN protocol and key performance indicators (KPIs), we defined clinically significant hypotension as SBP <100 mmHg to ensure a significant population sample size while also making the results of our examination more generalizable to the original CRASH-2 study population. For patients who had an initial SBP missing in the ED, we imputed the pre-hospital SBP. Blood product transfusion is recognized as the gold standard of management for hemorrhagic trauma patients.[17] In CRASH-2, only half of the patients enrolled actually received blood product transfusion or damage control surgery. This has led some experts to question if all of the CRASH-2 trial patients were actually suffering clinically significant hemorrhage. This may have resulted in an underestimation of the benefits of TXA in the CRASH-2 trial as some patients enrolled may have had no chance of benefit. For this study, to ensure the selection of patients with confirmed hemorrhage, the requirement of blood transfusion was added as an inclusion criterion.
Exclusion criteria
We excluded patients for whom there was no recorded data on whether TXA had been administered or not.
Data analysis
Patients with SBP <100 mmHg, receiving transfusion with blood products and confirmed data on TXA status, were sub-grouped into two cohorts based on TXA administration. Variables intended for multivariable analysis initially were: shock index (SI), age, sex, temperature, respiratory rate (RR), heart rate (HR), Injury Severity Score (ISS), Glasgow Coma Scale (GCS), mechanism of injury (categorical description) and mechanism of injury (binary description - blunt or penetrating). These variables were chosen as they had previously been shown to correlate with shock and/or mortality outcomes.[18-24] Temperature and RR were excluded from multivariable analysis as no RR in ED or temperature was recorded in the TARN database. Arrival mode was added as a variable for analysis as it was reliably recorded, and we believed that it was fitting in a study examining care prior to implementation of a nationwide trauma system.
Variables were summarized using mean and standard deviation (SD) if continuously and normally or near-normally distributed. Median and interquartile range (IQR) were used if the data were skewed. Ordinal and discrete variables were summarized using median and IQR. Categorical data such as the mechanism of injury were summarized using count and proportion.
Means were compared using Student’s t-test, medians were compared using the Wilcoxon Rank Sum test, and the Chi-square test was used to compare proportions, unless the count in a cell was <5, in which case, Fisher’s exact test was used. A P-value of <0.05 was considered to be statistically significant.
Baseline variables of both cohorts were summarized and analyzed for comparability.
All variables demonstrating an association with mortality (P<0.10) as listed in Table 1 were entered into a multivariable regression model to determine independent association with mortality. Results were presented using adjusted odds ratio (OR) and 95% confidence interval (CI). Additional stepwise regression to arrive at a reduced model was not performed due to the potential absence of important confounders.
Table 1 Demographics and baseline variables
| Variables | TXA not administered (n=101) | TXA administered (n=133) | P-value |
|---|---|---|---|
| Age, n (%) 0-10 years 11-20 years 21-40 years 41-65 years >65 years | 10 (9.90) 6 (5.94) 27 (26.73) 22 (21.78) 36 (35.64) | 1 (0.75) 9 (6.77) 50 (37.39) 40 (30.08) 33 (24.81) | 0.002 |
| Sex (male), n (%) | 55 (54.46) | 99 (74.44) | 0.001 |
| Injury mechanism, n (%) Vehicle Penetrating High fall Low fall Other blunt Other | 19 (18.81) 14 (13.86) 12 (11.88) 44 (43.56) 9 (8.91) 3 (2.97) | 68 (51.13) 26 (19.55) 15 (11.28) 17 (12.78) 6 (4.51) 1 (0.75) | <0.001 |
| Penetrating trauma, n (%) | 15 (14.85) | 30 (22.56) | 0.140 |
| Ambulance/helicopter, n (%) | 81 (80.20) | 128 (96.24) | <0.001 |
| ED SBPa, mmHg | 77.9±20.5 | 75.5±21.1 | 0.390 |
| ED SI >1, n (%) | 58 (57.43) | 94 (70.68) | 0.030 |
| ED GCSb, n (%) 3-8 9-12 13-15 | 24 (23.76) 7 (6.93) 67 (66.36) | 38 (28.57) 12 (9.02) 82 (61.65) | 0.610 |
| ED pulse ratea, beats/minute | 88.7±32.8 | 96.4±35.7 | 0.100 |
| ISS, n (%) 0-12 13-25 >25 | 31 (30.69) 46 (45.54) 24 (23.76) | 29 (21.80) 38 (28.57) 66 (49.62) | <0.001 |
Figures above were recorded as count with (%) indicating the proportion of patients from each cohort; a: data were presented as mean±standard deviation; b: this data was missing for some patients; P-values for count and proportion were calculated using Chi-square test or Fisher’s exact; P-values for mean and standard deviation were calculated using Student’s t-test. TXA: tranexamic acid; ED: emergency department; SBP: systolic blood pressure; SI: shock index; GCS: Glasgow Coma Scale; ISS: Injury Severity Score.
The discriminative ability of the model was assessed using the area under receiving operator curve (AUROC). The Hosmer-Lemeshow test was reported for goodness of fit (GOF). Multi-collinearity among included variables was assessed using the variance inflation factor. Age, GCS, and ISS were grouped and analyzed as ordinal variables. Data analysis was performed using Stata Version 15.1 (College Station, USA).
Ethics
This study was conducted in compliance with the governing legislation of the Health Research Board of Ireland, complying with international European Union standards as well as the General Data Protection Regulation (GDPR) act of 2018. Further GDPR training was undertaken in conjunction with the Mater Misericordiae University Hospitals (MMUH) GDPR officer to ensure compliance with all privacy regulations. This training involved completion of online modules relating to GDPR and the ascertainment of a certificate on the fundamentals of GDPR. Approval by the MMUH ethics board with reference to the Health Service Executive National Consent Protocol was awarded before a data request was submitted to NOCA.[25] Once submitted, the data request was approved by the MTA governance committee.
RESULTS
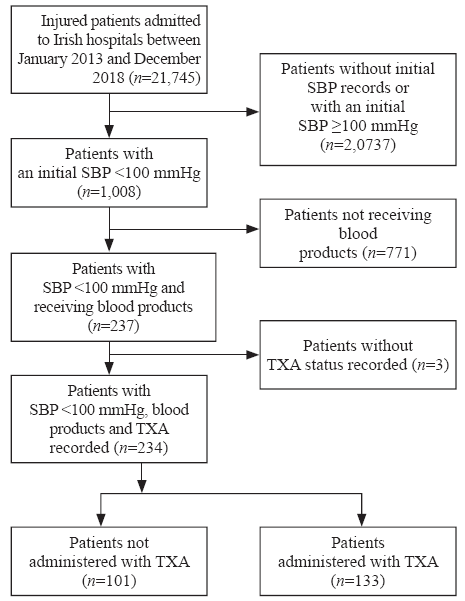
A total of 21,745 patients were included in the TARN trauma registry from Ireland between January 2013 and December 2018. Among them, 18,211 (83.7%) patients had an initial ED SBP ≥100 mmHg, and therefore, were excluded from this study. For 2,538 (11.7%) patients, the initial ED SBP was not recorded. Of these 2,538 patients, 12 had an initial pre-hospital SBP recorded that was <100 mmHg and were therefore included in the study. This resulted in a total of 1,008 (4.6%) patients presenting to an Irish ED between 2013 and 2018 with an initial SBP <100 mmHg. Of the 1,008 patients, three data entries did not include information relating to TXA. Of the 1,005 patients, 771 did not receive blood products and were thus excluded. This resulted in a final total of 234 (1.1%) patients. Of the patients that met our final inclusion criteria, 133 (56.8%; 95% CI 50.2%-63.3%) received TXA (Figure 1).
Figure 1.
Figure 1.
Inclusion and exclusion of patients.
Differences among variables sub-grouped by the primary exposure variables of interest are presented in Table 1.
There were 66 (28.2%; 95% CI 22.5%-34.4%) deaths at hospital discharge. Potential confounders are listed in Table 2 and after adjustment, TXA was not significantly associated with lower mortality (adjusted OR 0.86; 95% CI 0.31-2.38). Variables associated with mortality at hospital discharge were male sex, lower initial systolic BP, and higher injury severity.
Table 2 Association between variables and mortality
| Variables | Adjusted OR (95% CI) | P-value |
|---|---|---|
| TXA | 0.86 (0.31-2.38) | 0.770 |
| Male sex | 3.30 (1.14-9.50) | 0.027 |
| Helicopter transport | 12.90 (0.47-326.70) | 0.130 |
| ED SBP | 0.96 (0.93-0.98) | 0.002 |
| ED pulse | 0.96 (0.90-1.02) | 0.220 |
| ED GCS | 0.98 (0.92-1.03) | 0.490 |
| ISS | 1.07 (1.04-1.11) | <0.001 |
OR: odds ratio; CI: confidence interval; TXA: tranexamic acid; ED: emergency department; SBP: systolic blood pressure; GCS: Glasgow Coma Scale; ISS: Injury Severity Score.
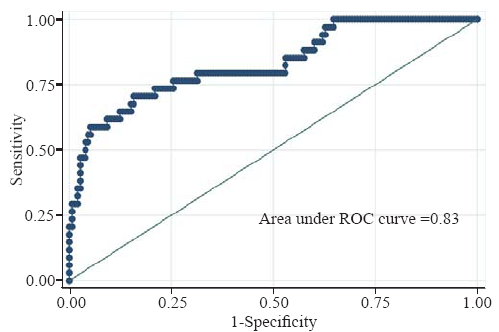
The model demonstrated good discrimination (Figure 2) with AUROC 0.83 (95% CI 0.75-0.91). The P-value for Hosmer-Lemeshow GOF was 0.21. The mean variance inflation factor was 1.24. There was no significant interaction between ED SBP and ISS (P-value for interaction term=0.60).
Figure 2.
Figure 2.
ROC curve for logistic regression model used.
DISCUSSION
After publication of CRASH-2 trial results, TXA was administered to 57% of injured patients in hemorrhagic shock arriving at EDs in Ireland. TXA was administered to patients with high SI and higher ISS more commonly, with significant baseline differences among cohorts. When adjusted for known confounders, TXA administration was not associated with improved survival to hospital discharge.
In this selected cohort of injured patients who were hypotensive and received blood transfusion, the proportion of patients that received TXA was lower than expected. Coats et al[26] found that since the publication of CRASH-2, 68.7% of injured patients in the UK who received blood products also received TXA. This finding was in keeping with current literature that shows uptake of TXA post-publication of CRASH-2 has not been universal.[10]
One reason that may contribute to the lower implementation of TXA in Ireland compared to the UK, is the lack of a coordinated national trauma system in Ireland. Without a coordinated national system, no national statutory body exists to standardize and provide governance for trauma patients in Ireland. Many individual hospitals and national bodies, e.g., Irish Association for Emergency Medicine (IAEM), have proposed and implemented guidelines on TXA use. However, this approach is neither universally mandated nor adopted throughout all 26 trauma-receiving hospitals in Ireland. Therefore, it is difficult to estimate how many of the 26 separate units have a major hemorrhage protocol (MHP) in place and have TXA administration included as an integral constituent. Ireland is currently in the process of restructuring to implement a coordinated national trauma system.[27] This will see two strategically placed Major Trauma Centers (MTC) in Ireland as hubs to a number of smaller spoke trauma units throughout the country. As part of this major reorganization of services, a National Trauma Office will be implemented, which will provide key statutory oversight for national guidelines and major policies including TXA use for major trauma patients as a part of MHP.
While our hypothesis of improved outcomes associated with TXA administration was not proven, the differential administration of TXA to more severely injured patients may have been a contributing factor. While the attempt to account for confounders resulted in a model of good fit and discrimination, it is possible that unknown confounders were present. Retrospective cohort studies examining the use of TXA have previously reported a wide heterogeneity of outcomes, and results have not always been consistent with the findings of the CRASH-2 trial. For example, while Valle et al[28] failed to show significant benefit from TXA, other studies such as the Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Trial[29] and a study reported by Shiraishi et al[30] demonstrated the possible benefits of TXA administration.
This study was limited in being a retrospective analysis of registry data and the possibility of unknown confounders. However, it provides a framework for describing current management within the Irish trauma system and assessing quality improvement strategies. Specification by strict inclusion criteria to select a population with bleeding and shock was designed to maximize any potential effect, however it resulted in a small sample size. Furthermore, information on delivery of TXA and timing of injury were not available. The timing of TXA administration has been associated with potential harm when administered >3 hours of injury.[31] In addition, data on secondary outcomes such as thromboembolic events were not available. Whether TXA results in increased thromboembolic events in the trauma setting is still unknown. The CRASH-2 trial did not find an increase in thromboembolic events, however, retrospective cohort studies have previously shown an increase in propensity-matched cohorts.[32,33] In our dataset, the incidence of venous thromboembolism (VTE) was poorly recorded with only 17 (7.2%) data entries and improved data collection will assist secondary analyses in future.
External validation of CRASH-2 trial findings may enable better translation. Several RCTs are currently underway examining the use of TXA for injured patients in countries with mature trauma systems in an attempt to externally validate the findings of the CRASH-2 trial. The Pre-hospital Anti-fibrinolytics for Traumatic Coagulopathy and Hemorrhage Study (PATCH) is an international, multicenter, double-blinded, placebo-controlled trial examining TXA in advanced trauma systems based out of Australia and New Zealand. PATCH will enroll 1,200 severely injured patients deemed to be at risk of acute traumatic coagulopathy (ATC) using the coagulopathy of severe trauma (COAST) scoring system. The primary outcome of the PATCH trial will be the proportion of patients with a favourable outcome at 6 months, as defined by Glasgow Outcome Scale-Extended (GOS-E) of 5-8, with recruitment expected to complete in the second half of 2020.[11]
Study of Tranexamic Acid during Air Medical Prehospital transport (STAAMP) was a US-based multicentre, double-blinded, placebo-controlled trial examining TXA in trauma patients. The primary outcome of all cause mortality difference was not demonstrated. However, the study generated the hypothesis that TXA may be of benefit in subgroups of patient who receive the drug early or have initial hypotension.[34]
Tranexamic Acid Mechanisms and Pharmacokinetics in Traumatic Injury (TAMPITI) was a US-based RCT. By analyzing pharmacokinetic, pharmacodynamic, and immune parameter samples of patients randomized to three arms (placebo, 2 g TXA intravenous, 4 g TXA intravenous), the TAMPITI Trial concluded minimal immunomodulatory effects with respect to leukocyte phenotypes and circulating cytokine levels after TXA administration.[35]
CONCLUSIONS
Among injured patients in Ireland presenting with hypotension and managed with blood transfusion, TXA was administered to 56.8% of patients who were severely injured. However, a mortality benefit could not be demonstrated, which may be due to the low proportion of patients receiving TXA. We recommend ongoing efforts to standardize the care of injured patients across Ireland with development of a national coordinated trauma system using robust guidelines combined with ongoing surveillance of TXA administration.
ACKNOWLEDGEMENTS
We would like to thank Maydul Islam for extraction of data. We would like to acknowledge all of the audit coordinators and clinical leads in the Irish hospitals who submit data to the Major Trauma Audit governed by the National Office of Clinical Audit.
Funding: None.
Ethical approval: Approval by the Mater Misericordiae University Hospital (MMUH) ethics board with reference to the Health Service Executive National Consent Protocol was awarded.
Conflicts of interests: Biswadev Mitra is a chief investigator in the PATCH-Trauma trial indicating a belief in equipoise between TXA and placebo for injured patients. No other authors have conflicts to declare.
Contributors: KW wrote the first draft. All authors contributed to the study and to further drafts.
Reference
The acute management of trauma hemorrhage: a systematic review of randomized controlled trials
DOI:10.1186/cc10096 URL [Cited within: 1]
Epidemiology and contemporary patterns of trauma deaths: changing place, similar pace, older face
PMID:17899256
[Cited within: 1]

The epidemiology of trauma deaths in Europe is less than well investigated. Thus, our goal was to study the contemporary patterns of trauma deaths within a defined population with an exceptionally high trauma autopsy rate.This was a retrospective evaluation of 260 consecutive trauma autopsies for which we collected demographic, pre-hospital and in-hospital data. Patients were analyzed for injury severity by standard scoring systems (Abbreviated Injury Scale [AIS], Revised Trauma Score [RTS], and Injury Severity Score [ISS]), and the Trauma and Injury Severity Scale [TRISS] methodology.The fatal trauma incidence was 10.0 per 100,000 inhabitants (17.4 per 100,000 age-adjusted > or = 55 years). Blunt mechanism (87%), male gender (75%), and pre-hospital deaths (52%) predominated. Median ISS was 38 (range: 4-75). Younger patients (<55 years) who died in the hospital were more often hypotensive (SBP < 90 mmHg; p = 0.001), in respiratory distress (RR < 10/min, or > 29/min; p < 0.0001), and had deranged neurology on admission (Glasgow Coma Score [GCS] < or = 8; p < 0.0001), compared to those > or = 55 years. Causes of death were central nervous system (CNS) injuries (67%), exsanguination (25%), and multiorgan failure (8%). The temporal death distribution is model-dependent and can be visualized in unimodal, bimodal, or trimodal patterns. Age increased (r = 0.43) and ISS decreased (r = -0.52) with longer time from injury to death (p < 0.001). Mean age of the trauma patients who died increased by almost a decade during the study period (from mean 41.7 +/- 24.2 years to mean 50.5 +/- 25.4 years; p = 0.04). The pre-hospital:in-hospital death ratio shifted from 1.5 to 0.75 (p < 0.007).While pre-hospital and early deaths still predominate, an increasing proportion succumb after arrival in hospital. Focus on injury prevention is imperative, particularly for brain injuries. Although hemorrhage and multiorgan failure deaths have decreased, they do still occur. Redirected attention and focus on the geriatric trauma population is mandated.
Acute traumatic coagulopathy
DOI:10.1097/01.TA.0000069184.82147.06 URL [Cited within: 1]
Acute coagulopathy of trauma: mechanism, identification and effect
DOI:10.1097/MCC.0b013e3282f1e78f URL [Cited within: 2]
Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial
DOI:10.1016/S0140-6736(10)60835-5 URL [Cited within: 2]
Geographical variance in the use of tranexamic acid for major trauma patients
DOI:10.3390/medicina55090561 URL [Cited within: 2]
Tranexamic acid for trauma: filling the ‘GAP’ in evidence
DOI:10.1111/emm.2014.26.issue-2 URL [Cited within: 2]
Tranexamic acid use in United States trauma centers: a national survey
DOI:10.1016/0002-9610(51)90370-4 URL [Cited within: 1]
Changing the system—major trauma patients and their outcomes in the NHS (England) 2008-17
DOI:10.1016/j.eclinm.2018.07.001 URL [Cited within: 1]
Systolic blood pressure below 110 mmHg is associated with increased mortality in blunt major trauma patients: multicentre cohort study
DOI:10.1016/j.resuscitation.2011.04.021 URL [Cited within: 1]
Injury mechanisms, patterns and outcomes of older polytrauma patients: an analysis of the Dutch Trauma Registry
Importance of respiratory rate for the prediction of clinical deterioration after emergency department discharge: a single-center, case-control study
DOI:10.1002/ams2.2017.4.issue-2 URL
Hypothermia as a predictor for mortality in trauma patients at admittance to the intensive care unit
DOI:10.4103/0974-2700.185276 URL
The utility of a shock index ≥1 as an indication for pre-hospital oxygen carrier administration in major trauma
DOI:10.1016/j.injury.2013.01.010 URL
Major trauma in geriatric patients
Abbreviated injury scale unification: the case for a unified injury system for global use
DOI:10.1097/00005373-199908000-00016 URL
Outcome predictors for severely brain-injured patients directly admitted or transferred from emergency departments to a trauma center
DOI:10.5847/wjem.j.1920-8642.2020.02.010 PMID:32076479 [Cited within: 1]
Implementation of tranexamic acid for bleeding trauma patients: a longitudinal and cross-sectional study
DOI:10.1016/j.jemermed.2007.06.015 URL [Cited within: 1]
Trauma systems around the world: a systematic overview
DOI:10.1097/TA.0000000000001633
PMID:28715361
[Cited within: 1]

Implementation of trauma care systems has resulted in improved patient outcomes, but international differences obviously remain. Improvement of care can only be established if we recognize and clarify these differences. The aim of the current review is to provide an overview of the recent literature on the state of trauma systems globally.The literature review over the period 2000 to 2016 was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines. Prehospital care, acute hospital care and quality assurance were classified using the World Health Organization Trauma System Maturity Index in four levels from I (least mature) to IV (most mature).The search yielded 93 articles about trauma systems in 32 countries: 23 high-income (HI), 8 middle-income (MI) countries and 1 low-income (LI) country. Trauma-related mortality was highest in the MI and LI countries. Level IV prehospital care with Advanced Life Support was established in 19 HI countries, in contrast to the MI and LI countries where this was only reported in Brazil, China, and Turkey. In 18 HI countries, a Level III/IV hospital-based trauma system was implemented, whereas in nine LI- and MI countries Level I/II trauma systems were seen, mostly lacking dedicated trauma centers and teams. A national trauma registry was implemented in 10 HI countries.Despite the presence of seemingly sufficient resources and the evidence-based benefits of trauma systems, only nine of the 23 HI countries in our review have a well-defined and documented national trauma system. Although 90% of all lethal traumatic injuries occur in middle and LI countries, according to literature which our study is limited to, only few of these countries a hold formal trauma system or trauma registry. Much can be gained concerning trauma systems in these countries, but unfortunately, the economic situation of many countries may render trauma systems not at their top priority list.Systematic review, level III.
Do all trauma patients benefit from tranexamic acid?
DOI:10.1097/TA.0000000000000242
PMID:24854303
[Cited within: 1]

This study tested the hypothesis that early routine use of tranexamic acid (TXA) reduces mortality in a subset of the most critically injured trauma intensive care unit patients.Consecutive trauma patients (n = 1,217) who required emergency surgery (OR) and/or transfusions from August 2009 to January 2013 were reviewed. At surgeon discretion, TXA was administered at a median of 97 minutes (1-g bolus then 1-g over 8 hours) to 150 patients deemed high risk for hemorrhagic death. With the use of propensity scores based on age, sex, traumatic brain injury (TBI), mechanism of injury, systolic blood pressure, transfusion requirements, and Injury Severity Score (ISS), these patients were matched to 150 non-TXA patients.The study population was 43 years old, 86% male, 54% penetrating mechanism of injury, 25% TBI, 28 ISS, with 22% mortality. OR was required in 78% at 86 minutes, transfusion was required in 97% at 36 minutes, and 75% received both. For TXA versus no TXA, more packed red blood cells and total fluid were required, and mortality was 27% versus 17% (all p < 0.05). The effects of TXA were similar in those with or without TBI, although ISS, fluid, and mortality were all higher in the TBI group. Mortality associated with TXA was influenced by the timing of administration (p < 0.05), but any benefit was eliminated in those who required more than 2,000-mL packed red blood cells, who presented with systolic blood pressure of less than 120 mm Hg or who required OR (all p < 0.05).For the highest injury acuity patients, TXA was associated with increased, rather than reduced, mortality, no matter what time it was administered. This lack of benefit can probably be attributed to the rapid availability of fluids and emergency OR at this trauma center. Prospective studies are needed to further identify conditions that may override the benefits from TXA.Therapeutic study, level IV.
Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study
DOI:10.1001/archsurg.2011.287 URL [Cited within: 1]
Effectiveness of early administration of tranexamic acid in patients with severe trauma
DOI:10.1002/bjs.10497
PMID:28230248
[Cited within: 1]

A reduction in mortality with the early use of tranexamic acid has been demonstrated in severely injured patients who are bleeding. However, the modest treatment effect with no reduction in blood transfusion has raised concerns. The aim of the present study was to estimate the effectiveness of regular use of tranexamic acid in severely injured patients.This multicentre observational study used retrospectively collected data from consecutive injured patients (Injury Severity Score at least 16) treated in 15 Japanese academic institutions in 2012. A propensity score-matched analysis compared patients who did or did not receive tranexamic acid administration within 3 h of injury. Study outcomes included 28-day all-cause and cause-specific mortality, and need for blood transfusion.Of 796 eligible subjects, 281 were treated with tranexamic acid. Propensity score matching selected a total of 500 matched subjects (250 in each group). Tranexamic acid administration was associated with lower 28-day mortality (10·0 versus 18·4 per cent; difference -8·4 (95 per cent c.i. -14·5 to -2·3) per cent) and lower 28-day mortality from primary brain injury (6·0 versus 13·2 per cent; difference -7·2 (-12·3 to -2·1) per cent). However, there was no significant difference between groups in the need for blood transfusion (33·2 versus 34·8 per cent; difference -1·6 (-9·9 to 6·7) per cent).Early tranexamic acid use was associated with reduced mortality in severely injured patients, in particular those with a primary brain injury.© 2017 BJS Society Ltd Published by John Wiley & Sons Ltd.
The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients
Evaluation of military use of tranexamic acid and associated thromboembolic events
DOI:10.1001/jamasurg.2017.3821
PMID:29071337
[Cited within: 1]

Since publication of the CRASH-2 and MATTERs studies, the US military has included tranexamic acid (TXA) in clinical practice guidelines. While TXA was shown to decrease mortality in trauma patients requiring massive transfusion, improper administration and increased risk of venous thromboembolism remain a concern.To determine the appropriateness of TXA administration by US military medical personnel based on current Joint Trauma System clinical practice guidelines and to determine if TXA administration is associated with venous thromboembolism.This cohort study of US military casualties in US military combat support hospitals in Afghanistan and a single US-based tertiary military treatment facility within the continental United States was conducted from 2011 to 2015, with follow-up through initial hospitalization and readmissions.Data collected for all patients included demographic information as well as Injury Severity Score; receipt of blood products, TXA, and/or a massive transfusion; and admission hemodynamics.Variance from guidelines in TXA administration and venous thromboembolism. Tranexamic acid overuse was defined as a hemodynamically stable patient receiving TXA but not a massive transfusion, underuse was defined as a patient receiving a massive transfusion but not TXA, and TXA administration was considered delayed when given more than 3 hours after injury.Of the 455 identified patients, 443 (97.4%) were male, and the mean (SD) age was 25.3 (4.8) years. A total of 173 patients (38.0%) received a massive transfusion, and 139 (30.5%) received TXA in theater. Overuse occurred in 18 of 282 patients (6.4%) and underuse in 46 of 173 (26.6%) receiving massive transfusions, and delayed administration was found in 6 of 145 patients (4.3%) receiving TXA. Overuse increased at 3.3% per quarter (95% CI, 4.0-9.9; P < .001; R2 = 0.340) and underuse decreased at -4.4% per quarter (95% CI, -4.5 to -3.6; P < .001; R2 = 0.410). Tranexamic acid administration was an independent risk factor for venous thromboembolism (odds ratio, 2.58; 95% CI, 1.20-5.56; P = .02).Military medical personnel decreased missed opportunities to appropriately use TXA but also increased overuse. In addition, TXA administration was an independent risk factor for venous thromboembolism. A reevaluation of the use of TXA in combat casualties should be undertaken.
Tranexamic acid administration is associated with an increased risk of posttraumatic venous thromboembolism
DOI:10.1097/TA.0000000000002061
PMID:30239375
[Cited within: 1]

Tranexamic acid (TXA) is used as a hemostatic adjunct for hemorrhage control in the injured patient and reduces early preventable death. However, the risk of venous thromboembolism (VTE) has been incompletely explored. Previous studies investigating the effect of TXA on VTE vary in their findings. We performed a propensity matched analysis to investigate the association between TXA and VTE following trauma, hypothesizing that TXA is an independent risk factor for VTE.This retrospective study queried trauma patients presenting to a single Level I trauma center from 2012 to 2016. Our primary outcome was composite pulmonary embolism or deep vein thrombosis. Mortality, transfusion, intensive care unit and hospital lengths of stay were secondary outcomes. Propensity matched mixed effects multivariate logistic regression was used to determine adjusted odds ratio (aOR) and 95% confidence intervals (95% CI) of TXA on outcomes of interest, adjusting for prespecified confounders. Competing risks regression assessed subdistribution hazard ratio of VTE after accounting for mortality.Of 21,931 patients, 189 pairs were well matched across propensity score variables (standardized differences <0.2). Median Injury Severity Score was 19 (interquartile range, 12-27) and 14 (interquartile range, 8-22) in TXA and non-TXA groups, respectively (p = 0.19). Tranexamic acid was associated with more than threefold increase in the odds of VTE (aOR, 3.3; 95% CI, 1.3-9.1; p = 0.02). Tranexamic acid was not significantly associated with survival (aOR, 0.86; 95% CI, 0.23-3.25; p = 0.83). Risk of VTE remained elevated in the TXA cohort despite accounting for mortality (subdistribution hazard ratio, 2.42; 95% CI, 1.11-5.29; p = 0.03).Tranexamic acid may be an independent risk factor for VTE. Future investigation is needed to identify which patients benefit most from TXA, especially given the risks of this intervention to allow a more individualized treatment approach that maximizes benefits and mitigates potential harms.Therapeutic, level III.
Tranexamic acid during prehospital transport in patients at risk for hemorrhage after injury: a double-blind, placebo-controlled, randomized clinical trial
The immunologic effect of early intravenous two and four gram bolus dosing of Tranexamic Acid Compared to Placebo in Patients with Severe Traumatic Bleeding (TAMPITI): a randomized, double-blind, placebo-controlled, single-center trial
DOI:10.3389/fimmu.2020.02085
PMID:33013880
[Cited within: 1]

The hemostatic properties of tranexamic acid (TXA) are well described, but the immunological effects of TXA administration after traumatic injury have not been thoroughly examined. We hypothesized TXA would reduce monocyte activation in bleeding trauma patients with severe injury.This was a single center, double-blinded, randomized controlled trial (RCT) comparing placebo to a 2 g or 4 g intravenous TXA bolus dose in trauma patients with severe injury. Fifty patients were randomized into each study group. The primary outcome was a reduction in monocyte activation as measured by human leukocyte antigen-DR isotype (HLA-DR) expression on monocytes 72 h after TXA administration. Secondary outcomes included kinetic assessment of immune and hemostatic phenotypes within the 72 h window post-TXA administration.The trial occurred between March 2016 and September 2017, when data collection ended. 149 patients were analyzed (placebo, = 50; 2 g TXA, = 49; 4 g TXA, = 50). The fold change in HLA-DR expression on monocytes [reported as median (Q1-Q3)] from pre-TXA to 72 h post-TXA was similar between placebo [0.61 (0.51-0.82)], 2 g TXA [0.57 (0.47-0.75)], and 4 g TXA [0.57 (0.44-0.89)] study groups ( = 0.82). Neutrophil CD62L expression was reduced in the 4 g TXA group [fold change: 0.73 (0.63-0.97)] compared to the placebo group [0.97 (0.78-1.10)] at 24 h post-TXA ( = 0.034). The fold decrease in plasma IL-6 was significantly less in the 4 g TXA group [1.36 (0.87-2.42)] compared to the placebo group [0.46 (0.19-1.69)] at 72 h post-TXA ( = 0.028). There were no differences in frequencies of myeloid or lymphoid populations or in classical complement activation at any of the study time points.In trauma patients with severe injury, 4 g intravenous bolus dosing of TXA has minimal immunomodulatory effects with respect to leukocyte phenotypes and circulating cytokine levels.www.ClinicalTrials.gov, identifier NCT02535949.Copyright © 2020 Spinella, Thomas, Turnbull, Fuchs, Bochicchio, Schuerer, Reese, Coleoglou Centeno, Horn, Baty, Shea, Meledeo, Pusateri, Levy, Cap and Bochicchio.