INTRODUCTION
Acute pulmonary embolism (PE) is an emergent cardiopulmonary disease caused by thrombotic blockage of the pulmonary artery. Reported mortality rates are as high as 25%.[1] Evaluating the prognosis of acute PE has shifted from examining mortality to deterioration in the short term,[2] particularly in non-risk acute PE patients.[3,4] This deterioration can be assessed by the occurrence of adverse events.[5]
Bova score and risk stratification are two widely used methods to predict the risk of deterioration.[2,6] In addition, identification of the thrombus location using computerized tomography pulmonary arteriography (CTPA) can also help to evaluate the risk of deterioration.[7-9] Saddle main pulmonary artery (MPA) embolism, defined as thrombus straddling the bifurcation of the MPA trunk,[10] is a special subtype of thrombus location. In general, the presentation of saddle MPA embolism is a sign of poor short-term prognosis.[10,11] However, some studies have reported no correlation between saddle MPA embolism and deterioration.[5,12,13] The low incidence (2.6%-9.0%) of saddle MPA embolisms,[5,12] and the inconsistent baseline and imbalance of PE severity between saddle and non-saddle MPA embolism contribute to this controversy.[13]
Propensity score matching (PSM) analysis is suitable for studies with low rates of occurrence,[14] such as the incidence of saddle MPA embolism in acute PE. It reduces selection bias, as it balances the differences in clinical features and increases the statistical strength of retrospective studies.[15,16] To accurately assess the efficacy of saddle MPA embolism for predicting deterioration, we adjusted the baseline characteristics, balanced the acute PE severity with Bova score,[6] and performed risk stratification, which included the simple PE severity index (s-PESI)[2] by PSM. After PSM, we analyzed whether saddle MPA embolism represented a high risk for deterioration in non-high-risk acute PE patients.
METHODS
Study design and setting
We conducted this retrospective study on non-high-risk acute PE patients between January 2011 and October 2019.
Study criteria
Totally 858 non-high-risk acute PE patients who presented to the Shengjing Hospital of China Medical University were reviewed retrospectively. Non-high-risk acute PE patients were defined as patients who were admitted to the hospital: (1) without a history of cardiopulmonary resuscitation; (2) with systolic blood pressure ≥90 mmHg (1 mmHg=0.133 kPa); (3) no need for vasopressors; (4 ) without end-organ hypotension as defined by systolic pressure drop of <40 mmHg lasting for ≤15 minutes.[2] The inclusion criteria were: a diagnosis of acute PE by CTPA and aged ≥18 years. The exclusion criteria were: (1) definitive diagnosis of cor pulmonale and heart failure; (2) chronic and recurring PE; (3) presence of pulmonary artery tumor; (4) current pregnancy; (5) absence of CTPA data or echocardiography; (6) absence of cardiac troponin I (cTn-I) and N-terminal pro-brain natriuretic peptide (NT-proBNP); and (7) receiving thrombolysis before admission to the hospital.
Clinical data
Demographic and baseline characteristics, such as heart rate and systolic pressure, were collected from medical records. Deterioration was defined as the occurrence of adverse events, including PE-related shock, need for mechanical ventilation and cardiopulmonary resuscitation, life-saving hemodynamic support, and thrombolysis, within the first 30 days of admission to the hospital.[5] The date of occurrence of deterioration was also recorded.
Pulmonary embolism severity
Right ventricular dysfunction (RVD) was confirmed by echocardiography. Risk stratification was evaluated using the s-PESI, cTn-I, NT-proBNP, and RVD.[2] Patients were divided into the intermediate-high-risk, intermediate-low risk, and low-risk groups.[2] The Bova score was evaluated according to systolic pressure, heart rate, cTn-I, and RVD.[6] Patients were then divided according to Bova score (stage I, stage II, and stage III) (supplementary Tables 1, 2, and 3).
CTPA acquisition
A 64 detector-row computerized tomography system (Aquilion KV-120; Toshiba Medical Systems Corporation, Tokyo, Japan; parameters: 380 mA, 120 kV) was used to perform pulmonary angiography with 1-mm thick sections. The iodinated nonionic contrast agent (100 mL) was injected into the antecubital vein using an automatic dual-tube high-pressure injector (Ulrich REF XD 2051; Ulrich Medical GmbH, Germany) at a rate of 4 mL/second from the thoracic inlet to the upper abdomen.
MPA reconstruction and thrombus location
The MPA was reconstructed using Mimics Medical software (version 19.0, Mimics Medical software, Belgium) with CTPA data. The MPA was divided into four parts: MPA trunk, MPA bifurcation, left pulmonary artery (LPA), and right pulmonary artery (RPA).[7,8] Based on the thrombus location, the acute PE patients were divided into four types: (1) MPA embolism (thrombus at LPA, RPA, bilateral pulmonary arteries, or bifurcation); (2) saddle MPA embolism (thrombus at bifurcation, or at bifurcation and extending into the LPA and RPA); (3) non-saddle MPA embolism (thrombus at the RPA or LPA and no thrombus at bifurcation); and (4) non-MPA embolism (no thrombus at MPA, but thrombus at peripheral pulmonary artery) (supplementary Figures 1A, B, and C). The correlation among the types of thrombus locations is summarized in supplementary Figure 2.
PSM
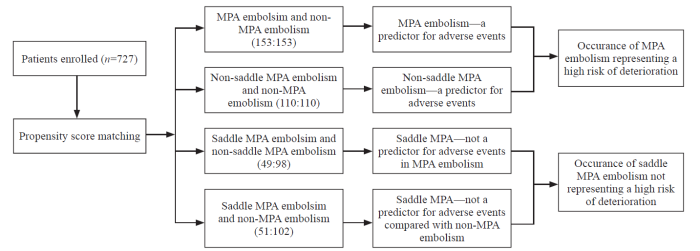
To investigate the predictive ability of MPA embolism and saddle MPA embolism for deterioration, baseline characteristics were normalized by age, sex, Bova score, and risk stratification, and four subgroups of PSM individuals were developed: (1) to evaluate whether MPA embolism was a sign for a high risk of deterioration, MPA embolism and non-MPA embolism patients were matched; (2) after excluding the interference of saddle MPA embolism, to evaluate whether non-saddle MPA embolism was still a sign for a high risk of deterioration, non-saddle MPA embolism and non-MPA embolism patients were matched; (3) to evaluate whether saddle MPA embolism was a sign for a high risk of deterioration in MPA embolism patients, saddle MPA embolism and non-saddle MPA embolism patients were matched; (4) to evaluate whether saddle MPA embolism was a sign for a high risk of deterioration compared with non-MPA embolism, saddle MPA embolism and non-MPA embolism patients were matched (Figure 1).
Figure 1.
Figure 1.
Flowchart of propensity score matching. MPA: main pulmonary artery.
Thrombus splintering
To investigate differences between deterioration and non-deterioration patients with saddle MPA embolisms using CTPA data, the right and left pulmonary regions were divided into ten and eight segments, respectively, from the third stage pulmonary artery. If the thrombus was located at one pulmonary artery or at a relatively downstream pulmonary artery, 1 point was recorded, and 0 point was recorded for other locations. The scores of each pulmonary artery were added up. Thrombus splintering was evaluated by the total number of points in the 18 pulmonary arteries. This method is the Qanadli Scoring technique.[17,18]
Analysis
Quantitative variables with normal distributions were expressed as the mean±standard deviation (SD) and analyzed with Student’s t-test. Categorical variables and their differences were analyzed with the χ2 test. To decrease the selection bias and potential confounding factors, we estimated the propensity score by means of logistic regression analysis and performed 1:1/1:2 nearest-neighbour individual matching based on the logit of propensity score using calipers of a width equal to 0.1 by age, sex, Bove score, and risk stratification.[14] A Cox model was used to analyze the correlation between the variables and deterioration in univariate and multivariate analyses, and hazard ratios (HRs) were calculated. Variables with P-value <0.10 in the univariate analysis were included in the multivariate analysis.[16] Kaplan-Meier analysis was used to compare the risk of deterioration. Receiver operating characteristic (ROC) curves were used to confirm identification ability. Two-tailed P values <0.05 were considered statistically significant. Statistical analyses were performed using R software version 3.3.2 (http://www.R-project.org).
RESULTS
Demographic, baseline characteristics, and pulmonary embolism severity
Of the 727 patients enrolled, 70 presented with deterioration, including 38 males and 32 females. Their average age was 59.91±15.29 years. Of the remaining 657 patients who did not present with deterioration, 328 were males, 329 were females, and their average age was 59.71±15.72 years. In terms of thrombus location, the prevalence of saddle MPA embolisms and MPA embolisms were higher in patients with deterioration than in those with non-deterioration (P<0.001 and P<0.001, respectively). There was a higher proportion of patients with Bova II and Bova III grades in patients with deterioration compared to those without deterioration (P=0.002 and P<0.001, respectively). There was also a higher proportion of patients with intermediate-high risk in patients with deterioration compared to those without deterioration (P<0.001) (Table 1).
Table 1 Baseline and other characteristics before propensity score matching (n=727)
| Variables | With deterioration (n=70) | Without deterioration (n=657) | P-value |
|---|---|---|---|
| Age, years, mean±SD | 59.91±15.29 | 59.71±15.72 | 0.920 |
| Sex, male/female | 38/32 | 328/329 | 0.490 |
| Main pulmonary artery embolism (+/-),n (%) | 43 (61.4) | 135 (20.5) | <0.001 |
| Saddle main pulmonary artery embolism (+/-), n (%) | 14 (20.2) | 37 (5.6) | <0.001 |
| Bova I, n (%) | 25 (35.7) | 560 (85.2) | <0.001 |
| Bova II, n (%) | 16 (22.9) | 70 (10.7) | 0.002 |
| Bova III, n (%) | 29 (41.4) | 27 (4.1) | <0.001 |
| Risk stratification (low risk), n (%) | 12 (17.1) | 132 (20.1) | 0.560 |
| Risk stratification (intermediate-low risk), n (%) | 11 (15.7) | 131 (19.9) | 0.400 |
| Risk stratification (intermediate-high risk), n (%) | 47 (67.1) | 94 (14.3) | <0.001 |
Results of PSM
A total of 178 patients were diagnosed with MPA embolism. Among them, 51 patients were diagnosed with saddle MPA embolism (12 peripheral embolisms and 39 non-peripheral embolisms), and 127 patients were diagnosed with non-saddle MPA embolism. Totally 549 patients were diagnosed with non-MPA embolism. After PSM, the four groups were formed based on demographics, baseline characteristics, and pulmonary embolism severity (supplementary Tables 4, 5, 6, and 7).
Efficacy of MPA embolism for predicting deterioration
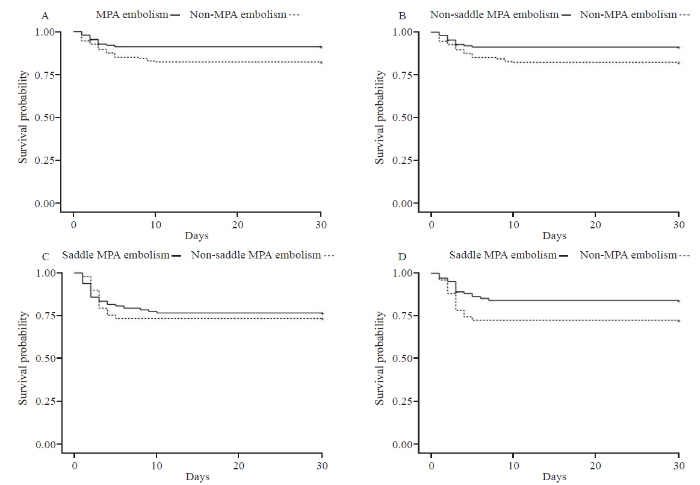
When 153 MPA embolism patients and 153 non-MPA embolism patients were matched, univariate and multivariate analyses revealed that MPA embolism and intermediate-high risk were predictors of deterioration (HR 1.96, 95% confidence interval [CI] 1.01-3.83, P=0.047 and HR 5.85, 95% CI 1.98-17.30, P=0.001, respectively) (Table 2). The Kaplan-Meier survival curve for MPA embolism revealed that MPA embolism patients had a higher risk of deterioration than non-MPA embolism patients (log-rank test=5.23, P=0.022) (Figure 2A).
Table 2 Prognostic factors associated with deterioration
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| MPA embolism to non-MPA embolism, n=306 (153:153) | ||||
| MPA embolism | 2.13 (1.10-4.12) | 0.025* | 1.96 (1.01-3.83) | 0.047* |
| Saddle MPA embolism | 1.75 (0.83-3.68) | 0.140 | - | - |
| Bova score II | 1.64 (0.75-3.58) | 0.220 | 0.57 (0.22-1.44) | 0.230 |
| Bova score III | 5.17 (2.51-10.65) | <0.001* | 1.51 (0.61-3.74) | 0.370 |
| Risk stratification (intermediate-low risk) | 1.35 (0.43-4.17) | 0.610 | 1.45 (0.46-4.54) | 0.520 |
| Risk stratification (intermediate-high risk) | 5.28 (2.19-12.76) | <0.001* | 5.85 (1.98-17.30) | 0.001* |
| Non-saddle MPA embolism to non-MPA embolism,n=220 (110:110) | ||||
| MPA embolism | 2.33 (1.06-5.12) | 0.034* | 2.34 (1.07-5.15) | 0.034* |
| Saddle MPA embolism | - | - | - | - |
| Bova score II | 2.22 (0.84-5.83) | 0.011* | 0.97 (0.31-3.03) | 0.950 |
| Bova score III | 6.93 (2.99-16.06) | <0.001* | 2.80 (0.92-8.54) | 0.070 |
| Risk stratification (intermediate-low risk) | 3.99 (0.99-15.95) | 0.050 | 3.72 (0.91-14.25) | 0.068 |
| Risk stratification (intermediate-high risk) | 9.30 (2.76-31.30) | <0.001* | 5.99 (1.36-26.45) | 0.018* |
| Saddle MPA embolism to non-saddle MPA embolism,n=147 (49:98) | ||||
| MPA embolism | - | - | - | - |
| Saddle MPA embolism | 1.13 (0.57-2.23) | 0.720 | - | - |
| Bova score II | 1.28 (0.50-3.31) | 0.610 | 0.67 (0.21-2.09) | 0.490 |
| Bova score III | 4.00 (1.88-8.49) | <0.001* | 1.85 (0.63-5.43) | 0.260 |
| Risk stratification (intermediate-low risk) | 2.01 (0.52-7.76) | 0.310 | 2.12 (0.54-8.42) | 0.280 |
| Risk stratification (intermediate-high risk) | 4.92 (1.49-16.25) | 0.009* | 3.94 (0.87-17.73) | 0.074 |
| Saddle MPA embolism to non-MPA embolism, n=153 (51:102) | ||||
| MPA embolism | - | - | - | - |
| Saddle MPA embolism | 1.90 (0.93-3.87) | 0.080 | 1.92 (0.93-3.99) | 0.079 |
| Bova score II | 1.79 (0.71-4.51) | 0.220 | 0.60 (0.21-1.72) | 0.340 |
| Bova score III | 6.72 (2.82-16.00) | <0.001* | 1.99 (0.71-5.53) | 0.190 |
| Risk stratification (intermediate-low risk) | 1.26 (0.21-7.57) | 0.800 | 1.21 (0.20-7.38) | 0.830 |
| Risk stratification (intermediate-high risk) | 6.97 (1.65-29.42) | 0.008* | 7.06 (1.38-36.09) | 0.019* |
MPA: main pulmonary artery; HR: hazard ratio; CI: confidence interval; *P<0.05.
Figure 2.
Figure 2.
Kaplan-Meier curves. A: following PSM, MPA embolism and non-MPA embolism patients were compared (log-rank test=5.23, P=0.022); B: following PSM and exclusion of saddle MPA embolism patients, non-saddle MPA embolism and non-MPA embolism patients were compared (log-rank test=4.70, P=0.030); C: following PSM, saddle MPA embolism and non-saddle MPA embolism patients are compared (log-rank test=1.20, P=0.729); D: following PSM, saddle MPA embolism and non-MPA embolism patients were compared (log-rank test=3.17, P=0.077); MPA: main pulmonary artery; PSM: propensity score matching.
To exclude the influence of saddle MPA embolism, 110 non-saddle MPA embolism patients and 110 non-MPA embolism patients were matched. The univariate and multivariate analyses revealed that MPA embolism and intermediate-high risk were independent predictors of deterioration (HR 2.34, 95% CI 1.07-5.15, P=0.034 and HR 5.99, 95% CI 1.36-26.45, P=0.018, respectively) (Table 2). The Kaplan-Meier survival curve for MPA embolism revealed that the MPA embolism patients had a higher risk of deterioration than non-MPA embolism patients (log-rank test=4.70, P=0.030) (Figure 2B).
Efficacy of saddle MPA embolism for predicting deterioration
To evaluate whether saddle MPA was an independent predictor of deterioration in MPA embolism patients, 49 saddle MPA embolism patients were matched with 98 non-saddle MPA embolism patients. We found that saddle MPA embolism was not an independent predictor of adverse events in MPA embolism patients (Table 2). There was no statistical significance between saddle MPA embolism and non-saddle MPA embolism patients on the Kaplan-Meier survival curve (log-rank test=1.20, P=0.729) (Figure 2C).
To evaluate whether saddle MPA patients had a higher risk of deterioration compared to non-MPA embolism patients, 51 saddle MPA patients and 102 non-MPA patients were matched. There was no statistical significance between the groups in either the univariate or multivariate analysis (Table 2). There was no statistical significance between saddle MPA embolism and non-MPA embolism patients (log-rank test=3.17, P=0.077) (Figure 2D).
Thrombus stability of saddle MPA embolism
The thrombus splintering was 4.02±3.83 points in all saddle MPA embolism patients, 7.50±3.06 points in saddle MPA embolism patients with deterioration, and 2.70±3.20 points in saddle MPA embolism patients without deterioration. These differences were statistically significant between deterioration and non-deterioration patients (P<0.001). Thrombus splintering was a discriminatory factor for deterioration (the area under the ROC curve 0.866, 95% CI 0.735-0.997, P<0.001) and the optimal cut-off value was 5.5 points (sensitivity 85.7% and specificity 86.5%) (Figure 3).
Figure 3.
Figure 3.
ROC curve of thrombus splitting for prediction of deterioration in saddle MPA embolism patients (the area under the ROC 0.866, 95% CI 0.735-0.997, P<0.001). MPA: main pulmonary artery; ROC: receiver operating characteristic; CI: confidence interval.
DISSCUSION
Deterioration is related to circulation collapse in non-high-risk patients with acute PE.[19] Heart rate, systolic pressure, RVD, cTn-I, and NT-proBNP are all recognized as predictors of such circulation collapse.[2] A combination of several predictors is useful for identifying patients at a high risk of deterioration, including Bova scores[6] and risk stratification.[2] If a novel method could improve the predictive ability of deterioration in non-high-risk acute PE patients in conjunction with these two methods above, it would be a beneficial supplement for the management of acute PE patients. Identification of thrombus location meets this requirement. Therefore, we designed this study to evaluate the correlation between thrombus location and deterioration using PSM for age, sex, Bova scores, and risk stratification. The results of our study revealed that MPA embolism was a sign of a high risk of deterioration, regardless of the inclusion or exclusion of saddle MPA embolism. Saddle MPA embolism may not be a sign of a high risk of deterioration. Although there is no difference between saddle MPA embolism and non-MPA embolism in the risk of deterioration, this statistically non-significant difference is not enough to say saddle MPA embolisms are as safe as non-MPA embolisms. We suggest that the saddle MPA embolism should be considered at a similar risk of deterioration as MPA embolism, but it is not necessary to consider it as a special subtype with a higher risk of deterioration than MPA embolism.
Previous studies have shown a poor short-term prognosis in MPA embolism patients.[7,8] Our study supported this view. Regardless of the inclusion or exclusion of saddle MPA embolism, patients with MPA embolism had a higher risk of deterioration than those with non-MPA embolism in our study. In regards to anatomy, it was in series between MPA and relative peripheral pulmonary arteries, and it was in parallel among the peripheral pulmonary arteries. MPA embolism occurs only if its downstream pulmonary artery provides the resistant point. This resistant point is provided by the bifurcation of the pulmonary artery in different grades of the pulmonary artery or the peripheral pulmonary artery with a similar size to the thrombus.[8,13] Based on the physiopathology above, in general,MPA embolism is combined with peripheral pulmonary embolism (non-MPA embolism). If the thrombus was located at the MPA, dominantly subsegment pulmonary arteries would also be blocked[8] and severe inflammation[20] would be activated, causing the peripheral pulmonary artery to constrict and aggravate the severity.[21] This is why MPA embolism presents a higher risk of deterioration than non-MPA embolism.
There has been no consensus on the correlation between saddle MPA embolism and deterioration in non-high-risk acute PE. A study has revealed that saddle MPA embolism represents a poor short-term prognosis,[22] but in our study, saddle MPA was not a sign of a high risk of deterioration. Several factors may have led to different conclusions. First, the diversity of enrolled patients can cause different conclusions. Most studies, including ours, chose non-high-risk patients as the study population. These studies may underestimate the incidence rate of saddle MPA embolism because some high-risk acute PE patients with saddle MPA embolism might have a quick death and not be able to complete the CTPA for diagnosis.[13] In high-risk patients with acute PE, shock is the first distinguishable characteristic for making a clinical decision regarding where the thrombus is located in the pulmonary artery. Therefore, identification of the thrombus location is clinically significant only in non-high-risk acute PE patients. Second, due to the low incidence of saddle MPA embolism, previous studies could not compare saddle MPA embolism to other thrombus locations at the same baseline and PE severity. To avoid this imbalance, we used PSM to evaluate the correlation between saddle MPA embolism and deterioration to estimate the risk of deterioration. Although the saddle MPA embolism represents clot location both in MPA and MPA bifurcation, MPA bifurcation section is larger than other section.[23] If a thrombus is mainly located at the MPA bifurcation and only spitted into a few fragments, it would only mildly impede pulmonary circulation. Meanwhile, if the thrombus is located at the MPA bifurcation, combining many fragments, it would seriously impede pulmonary circulation. We inferred only saddle MPA embolism with many fragments, which leads to multi-pulmonary artery blockages and presents a high risk of deterioration. To test this hypothesis, we calculated the accumulated amount of peripheral pulmonary arteries with the thrombus as a measure of thrombus splintering. This revealed that most saddle MPA embolisms showed low thrombus splintering and only a few fragments in the peripheral pulmonary artery. Saddle MPA embolisms with a large number of thrombus fragments showed a high risk of deterioration.
There were several limitations to our study. Although we matched saddle MPA embolism patients with others, the number of patients with saddle MPA embolism remained small. Furthermore, this study was retrospective. These factors may limit the generalizability and statistical strength of the results. Although we did attempt to minimize selection bias using PSM for age, sex, Bova score, and risk stratification, larger studies are needed to reduce this potential bias.
CONCLUSIONS
In non-high-risk acute PE patients, the presentation of MPA embolism is associated with a higher risk of deterioration compared to non-MPA embolisms. Saddle MPA embolism is not a sign of a high risk of deterioration unless combined with thrombi blockage in multi-peripheral pulmonary arteries.
Funding: The study was supported by the 345 Talent Project, Shengjing Hospital of China Medical University.
Ethical approval: This research was approved by the Institutional Review Board of Shengjing Hospital of China Medical University and the requirement for informed consent was waived owing to the retrospective nature of the research (202PS292K).
Conflicts of interests: There is no conflict of interest.
Contributors: MZ: conception and design; DJ: provision of study materials, collection and assembly of data, and manuscript writing; CJ: data analysis and interpretation; all authors: final approval of manuscrip.
All the supplementary files in this paper are available at http://wjem.com.cn.
Reference
Prognostic value of cardiovascular parameters in computed tomography pulmonary angiography in patients with acute pulmonary embolism
DOI:10.1183/13993003.02611-2017 URL [Cited within: 1]
ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC)
DOI:10.1183/13993003.01647-2019 URL [Cited within: 8]
Risk stratification in normotensive acute pulmonary embolism patients: focus on the intermediate-high risk subgroup
DOI:10.1177/2048872619846506 URL [Cited within: 1]
The PERT concept: a step-by-step approach to managing pulmonary embolism
DOI:10.1016/j.chest.2020.07.065 URL [Cited within: 1]
Saddle pulmonary embolism in hemodynamically stable patients: to lyse or not to lyse? An issue in no guidelines land
DOI:10.1016/j.ejim.2017.09.002 URL [Cited within: 4]
Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism
DOI:10.1378/chest.14-2551 URL [Cited within: 4]
Central emboli rather than saddle emboli predict adverse outcomes in patients with acute pulmonary embolism
DOI:10.1016/j.thromres.2014.08.027 URL [Cited within: 3]
A decision tree built with parameters obtained by computed tomographic pulmonary angiography is useful for predicting adverse outcomes in non-high-risk acute pulmonary embolism patients
DOI:10.1186/s12931-019-1160-5 URL [Cited within: 4]
Venous thromboembolism in the emergency department: A survey of current best practice awareness in physicians and nurses in China
DOI:10.5847/wjem.j.1920-8642.2019.01.001 URL [Cited within: 1]
Saddle pulmonary embolism: laboratory and computed tomographic pulmonary angiographic findings to predict short-term mortality
DOI:10.1016/j.hlc.2016.02.019 URL [Cited within: 2]
Short term clinical outcome of acute saddle pulmonary embolism
PMID:12591851 [Cited within: 1]
Saddle vs non-saddle pulmonary embolism: clinical presentation, hemodynamics, management, and outcomes
DOI:10.1016/j.mayocp.2017.07.014 URL [Cited within: 2]
Saddle pulmonary embolism: is it as bad as it looks? A community hospital experience
DOI:10.1097/CCM.0b013e31822571b2 URL [Cited within: 4]
Combined transcatheter arterial chemoembolization and radiofrequency ablation versus hepatectomy for recurrent hepatocellular carcinoma after initial surgery: a propensity score matching study
DOI:10.1007/s00330-017-5166-4 URL [Cited within: 2]
Clinical characteristics and prognostic factors of patients with intrahepatic cholangiocarcinoma with fever: a propensity score matching analysis
DOI:10.1634/theoncologist.2018-0268 URL [Cited within: 1]
Microwave ablation versus resection for hepatocellular carcinoma within the Milan criteria: a propensity-score analysis
New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography
DOI:10.2214/ajr.176.6.1761415 URL [Cited within: 1]
Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography
DOI:10.1148/radiol.12110802 URL [Cited within: 1]
Characterization of hemodynamically stable acute heart failure patients requiring a critical care unit admission: Derivation, validation, and refinement of a risk score
DOI:10.1016/j.ahj.2017.03.014 URL [Cited within: 1]
Fibrinolysis and inflammation in venous thrombus resolution
DOI:10.3389/fimmu.2019.01348
PMID:31258531
[Cited within: 1]

Clinical observations and accumulating laboratory evidence support a complex interplay between coagulation, inflammation, innate immunity and fibrinolysis in venous thromboembolism (VTE). VTE, which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), and the subsequent complications of post-thrombotic syndrome (PTS), are significant causes of morbidity and mortality in patients. Clinical risk factors for VTE include cancer, major trauma, surgery, sepsis, inflammatory bowel disease, paralysis, prolonged periods of immobility, and aging. Abnormalities in venous blood flow or stasis initiates the activation of endothelial cells, and in concert with platelets, neutrophils and monocytes, propagates VTE in an intact vein. In addition, inflammatory cells play crucial roles in thrombus recanalization and restoration of blood flow via fibrinolysis and vascular remodeling. Faster resolution of the thrombus is key for improved disease prognosis. While in the clinical setting, anticoagulation therapy is successful in preventing propagation of venous thrombi, current therapies are not designed to inhibit inflammation, which can lead to the development of PTS. Animal models of DVT have provided many insights into the molecular and cellular mechanisms involved in the formation, propagation, and resolution of venous thrombi as well as the roles of key components of the fibrinolytic system in these processes. Here, we review the recent advances in our understanding of fibrinolysis and inflammation in the resolution of VTE.
Quantitative CT evaluation of small pulmonary vessels in patients with acute pulmonary embolism
DOI:S1076-6332(17)30500-7
PMID:29331359
[Cited within: 1]

The objective of this study was to investigate the correlation between the computed tomography (CT) cross-sectional area (CSA) of small pulmonary vessels and the CT obstruction index in patients with acute pulmonary embolism (PE) and the correlation between the changes in these measurements after anticoagulant therapy.Fifty-two patients with acute PE were selected for this study. We measured the CSA less than 5 mm on coronal reconstructed images to obtain the percentage of the CSA (%CSA < 5). CT angiographic index was obtained based on the Qanadli method for the evaluation of the degree of pulmonary arterial obstruction. Spearman rank correlation analysis was used to evaluate the relationship between the initial and the follow-up values and changes in the %CSA < 5 and the CT obstruction index.There was no significant correlation between the %CSA < 5 and CT obstruction index on both initial (ρ = -0.03, P = 0.84) and follow-up (ρ = -0.03, P = 0.82) assessments. In contrast, there was a significant negative correlation between the changes in %CSA < 5 and the CT obstruction index (ρ = -0.59, P < 0.0001).Although the absolute %CSA < 5 and CT obstruction index were not significantly correlated, the changes in the values of the two parameters had a significant correlation. Changes in %CSA < 5, which can be obtained easily, can be used as biomarker of therapeutic response in patients with acute PE.Copyright © 2018 The Association of University Radiologists. Published by Elsevier Inc. All rights reserved.
Short-term mortality of patients with saddle pulmonary embolism: a single-center study
DOI:10.5543/tkda.2019.77292
PMID:31219452
[Cited within: 1]

Although hemodynamic instability has been identified as the most established mortality predictor in acute pulmonary embolism (PE), the debate is still open about the prognostic significance of saddle pulmonary embolism (SPE). This study determined the in-hospital mortality rate of SPE patients diagnosed via computed tomographic pulmonary angiography (CTPA) and compared these cases with non-SPE patients.The presence of SPE observed on CTPA was used to classify 492 consecutive patients into SPE and non-SPE groups. Different features were compared between the 2 groups, and independent predictors of in-hospital mortality in acute PE were identified.A total of 70 patients (14.2%) had SPE. In univariate analysis, the SPE group was seen to have a higher in-hospital mortality rate, as well as a lower oxygen saturation level and systolic and diastolic blood pressure in comparison with the non-SPE group (all p values <0.005). Multivariate analysis revealed that SPE was an independent predictor of in-hospital mortality in acute PE patients (Odds ratio: 9.21, 95% confidence interval: 3.40-24.89; p value <0.001).The results of this study indicated that SPE had a statistically significant importance in predicting in-hospital mortality and adverse events in PE patients. These findings were not consistent with many prior studies.
Four-dimensional computed tomography: a method of assessing right ventricular outflow tract and pulmonary artery deformations throughout the cardiac cycle
DOI:10.1007/s00330-010-1913-5
PMID:20680286
[Cited within: 1]

To characterise 3D deformations of the right ventricular outflow tract (RVOT)/pulmonary arteries (PAs) during the cardiac cycle and estimate the errors of conventional 2D assessments.Contrast-enhanced, ECG-gated cardiovascular computed tomography (CT) findings were retrospectively analysed from 12 patients. The acquisition of 3D images over 10 phases of the cardiac cycle created a four-dimensional CT (4DCT) dataset. The datasets were reconstructed and deformation measured at various levels of the RVOT/PAs in both space and time. Section planes were either static or dynamic relative to the motion of the structures.4DCT enabled measurement and characterisation of in vivo 3D changes of patients' RVOT/PA during the cardiac cycle. The studied patient population showed a wide range of RVOT/PA morphologies, sizes and dynamics that develop late after surgical repair of congenital heart disease. There were also significant differences in the measured cross-sectional areas of the structures between static and dynamic section planes (up to 150%, p<0.05) secondary to large 3D displacements and rotations.4DCT imaging data suggest high variability in RVOT/PA dynamics and significant errors in deformation measurements if 3D analysis is not carried out. These findings play an important role for the development of novel percutaneous approaches to pulmonary valve intervention.