Dear editor,
Transcatheter aortic valve replacement (TAVR) is a safe and effective treatment for severe aortic valve stenosis and aortic regurgitation. Multiple randomized trials have proved its therapeutic advantages in patients at any level of surgical risks.[1,2,3] However, new-onset conduction disorders, including atrioventricular block (AVB) and left bundle branch block (LBBB), are the most common complications after TAVR procedure,[4] and might lead to deteriorated hemodynamics and worsened clinical outcome. Our center predominantly uses the self-expanding valves, with an incidence of new-onset conduction disorder following TAVR at around 10%. After TAVR, complete AVB usually requires pacemaker implantation, and the presence of new-onset LBBB is associated with increased risk of heart failure, complete AVB, and mortality.[5,6] We have a standard protocol to closely observe the post-TAVR electrocardiogram (ECG) changes and identify the ones that require permanent pacemaker implantation. However, conventional pacing mode, with right ventricular lead placed at either the apex or septum, is not capable of correcting QRS width, thus failing to restore the contraction sequence of the ventricular wall. His bundle pacing (HBP) is a new trend to achieve more physiological QRS complexes and ventricular contraction. In a multicenter study of 16 patients who developed new-onset LBBB following TAVR to explore the feasibility and safety of HBP, notably narrowed QRS was achieved in 11 patients (69%), but they only recruited patients with balloon-expandable valves, in addition to a small sample size.[7] In this case report, we successfully implanted a permanent pacemaker with HBP technique to correct TAVR-induced AVB in an old female patient.
CASE
A 69-year-old female patient presented for recurrent chest pain and shortness of breath. She had an unremarkable coronary angiography. An echocardiogram revealed severe aortic regurgitation with a mean pressure gradient of regurgitated jet at 11 mmHg (1 mmHg=0.133 kPa) and a maximum velocity at 2.32 m/s. The baseline left ventricular ejection fraction (LVEF) with biplane Simpson’s method was 71%. She had a history of well-controlled hypertension. Due to her recurrent and worsening symptoms, we advised her to consider surgical aortic valve replacement. However, the patient refused open-heart surgery and asked for alternative methods. Based on this, our heart team suggested mini-invasive trans-femoral TAVR. An informed consent form was obtained from the patient and her family. After a comprehensive pre-TAVR assessment, a 29-mm VenusA Valve (Venus Medtech, Hangzhou, China) was selected (Figure 1). The TAVR procedure was performed on 28 November 2019. After balloon pre-dilation, we released the VenusA Valve at the bottom of aortic sinus. The post-procedural digital subtraction angiography (DSA) showed that the mean depth of the implanted valve was 5.53 mm. The prosthetic valve successfully attenuated the aortic regurgitation with the mean pressure gradient decreased to 2 mmHg, and the maximum velocity dropped to 0.8 m/s. Meanwhile, we found a new-onset third-degree AVB with widened QRS. After one week, there was no improvement in her complete AVB. On the 9th day after TAVR, we implanted a dual-chamber permanent pacemaker with HBP technique. The right ventricular pacing lead (3830, Medtronic Inc., Minneapolis, USA) was screwed into the septum at the level of His bundle, using a fixed curve sheath (C315HIS, Medtronic). The QRS duration and morphology were recorded and analyzed through an intracardiac electrogram. At the first time, the classical “W” type morphology of QRS complexes was not observed at lead V1. We adjusted the pacing lead according to the anatomical landmark of His bundle and intracardiac electrogram. Finally, we screwed the lead at the optimal position. When we paced the 3830 lead, there was no right bundle branch block (RBBB) recruitment, which indicated a successful non-selective HBP. The atrial lead was screwed into the right appendage with a 2088TC lead (Abbott, Chicago, USA), and then connected to PM2224 dual-chamber pacemaker (Abbott, Chicago, USA). Post-HBP ECG showed sinus rhythm, ventricular pacing on atrial sense-ventricular pacing (AS-VP), and the QRS width at 107 ms.
Figure 1.
Figure 1.
The VenusA Valve.
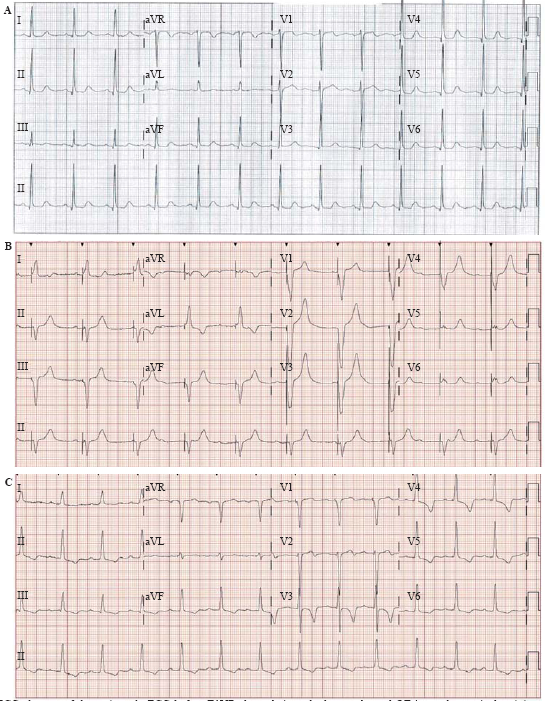
At the one-month follow-up visit, the patient was asymptomatic with improved exercise tolerance, at New York Heart Association (NYHA) function class I. Computed tomography (CT) revealed the prosthetic valve and pacing leads were in a good position. An echocardiogram showed LVEF of 55.1%. Aortic valve area was 2.22 cm2, with a mean pressure gradient of 2 mmHg and a maximum velocity of 1.18 m/s. An ECG showed AS-VP with QRS width at 104 ms (Figure 2).
Figure 2.
Figure 2.
ECG changes of the patient. A: ECG before TAVR showed sinus rhythm, prolonged QT interval, ventricular sinister high potential, PR interval 174 ms, QRS 96 ms; B: post-TAVR ECG with temporary pacemaker showed AVB III, QRS 148 ms; C: ECG with HBP showed ventricular sinister high potential, PR interval 243 ms, QRS 107 ms; ECG: electrocardiogram; TAVR: transcatheter aortic valve replacement. AVB III: third degree atrioventricular block; HBP: His-bundle pacing.
At the one-year follow-up visit, the patient remained asymptomatic and active in daily activities. The LVEF with biplane Simpson’s method was 69.6%, and the prosthetic valve area was 2.19 cm2. There was no obvious regurgitation of the prosthetic valve identified. Cardiac CT also showed a stable position of the prosthetic valve and the pacing leads.
DISCUSSION
New-onset AVB is a common and severe post-TAVR complication, and impairs the outcomes of TAVR. Irreversible AVB mandates permanent pacemaker implantation. The conventional ventricular pacemaker is wildly used in patients with post-TAVR AVB, but it increases the risks of ventricular dyssynchrony and heart failure due to widened QRS duration. The HBP technique is superior to conventional right ventricular pacing in terms of narrowing QRS width and restoring ventricular synchrony, which ultimately improves the outcomes.
The anatomical structure of interventricular septum and the depth of valve implantation are associated with post-TAVR conduction disorders. In our case, the patient’s interventricular septum thickness was 1.4 mm as shown in CT, and the mean valve implantation depth was 5.32 mm, which might lead to the new-onset post-TAVR AVB. Also, the patient’s His bundle area was narrower and longer than the usual AVB patients.
In this report, the patient underwent HBP after TAVR due to a new-onset AVB with widened QRS. At one-month and one-year follow-up visits, she felt well with a good cardiac performance.
This successful case of post-TAVR HBP implies that the HBP can be an efficient way to correct TAVR-induced conduction system abnormities, especially in those with a widened QRS. In the future, we still need more cases of post-TAVR HBP to better understand the pros and cons of this novel technique.
Funding: None.
Ethical approval: The ethics committee of our center approved the study protocol.
Conflicts of interests: The authors declare that they have no competing interests.
Contributors: All authors have substantial contributions to the acquisition, analysis, or interpretation of data for the work. All authors read and approved the final version of the submitted manuscript.
Reference
Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl
[J]
Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl
[J]
5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial
DOI:10.1016/S0140-6736(15)60308-7
URL
PMID:25788234
[Cited within: 1]

BACKGROUND: The Placement of Aortic Transcatheter Valves (PARTNER) trial showed that mortality at 1 year, 2 years, and 3 years is much the same with transcatheter aortic valve replacement (TAVR) or surgical aortic valve replacement (SAVR) for high-risk patients with aortic stenosis. We report here the 5-year outcomes. METHODS: We did this randomised controlled trial at 25 hospitals, in Canada (two), Germany (one), and the USA (23). We used a computer-generated randomisation sequence to randomly assign high-risk patients with severe aortic stenosis to either SAVR or TAVR with a balloon-expandable bovine pericardial tissue valve by either a transfemoral or transapical approach. Patients and their treating physicians were not masked to treatment allocation. The primary outcome of the trial was all-cause mortality in the intention-to-treat population at 1 year, we present here predefined outcomes at 5 years. The study is registered with ClinicalTrials.gov, number NCT00530894. FINDINGS: We screened 3105 patients, of whom 699 were enrolled (348 assigned to TAVR, 351 assigned to SAVR). Overall mean Society of Thoracic Surgeons Predicted Risk of Mortality score was 11.7%. At 5 years, risk of death was 67.8% in the TAVR group compared with 62.4% in the SAVR group (hazard ratio 1.04, 95% CI 0.86-1.24; p=0.76). We recorded no structural valve deterioration requiring surgical valve replacement in either group. Moderate or severe aortic regurgitation occurred in 40 (14%) of 280 patients in the TAVR group and two (1%) of 228 in the SAVR group (p<0.0001), and was associated with increased 5-year risk of mortality in the TAVR group (72.4% for moderate or severe aortic regurgitation vs 56.6% for those with mild aortic regurgitation or less; p=0.003). INTERPRETATION: Our findings show that TAVR as an alternative to surgery for patients with high surgical risk results in similar clinical outcomes. FUNDING: Edwards Lifesciences.
Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis.
[J]DOI:10.1016/j.jacc.2014.04.033 URL [Cited within: 1]
Mortality and heart failure hospitalization in patients with conduction abnormalities after transcatheter aortic valve replacement
DOI:10.1016/j.jcin.2018.10.053
URL
PMID:30621978
[Cited within: 1]

OBJECTIVES: The aim of this study was to assess mortality and rehospitalization in patients with new bundle branch block (BBB) and/or permanent pacemaker (PPM) after transcatheter aortic valve replacement (TAVR). BACKGROUND: Previous studies have provided inconsistent results on the clinical impact of new BBB or new PPM after TAVR. METHODS: A total of 816 consecutive patients without pre-procedural BBB or PPM undergoing TAVR between 2007 and 2017 were followed for 5 years or until data extraction in September 2017. Data on vital status and hospitalization were obtained through national registries. RESULTS: Within 30 days post-TAVR, new BBB without PPM and new PPM occurred in 247 (30.3%) and 132 (16.2%) patients, respectively, leaving 437 patients (53.6%) without conduction abnormalities. Median follow-up was 2.5 years (interquartile range: 1.0 to 4.9 years). One-year all-cause mortality was increased for new BBB (hazard ratio [HR]: 2.80; 95% confidence interval [CI]: 1.18 to 3.67) but not for new PPM (HR: 1.64; 95% CI: 0.72 to 3.74) compared with patients with no conduction abnormalities. The risk for late all-cause mortality (>/=1 year after TAVR) was higher both for patients with new BBB (HR: 1.79; 95% CI: 1.24 to 2.59) and for those with new PPM (HR: 1.58; 95% CI: 1.01 to 2.46) compared with patients with no conduction abnormalities. Patients with new BBB (HR: 1.47; 95% CI: 1.02 to 2.12) and new PPM (HR: 1.66; 95% CI: 1.09 to 2.54) had a higher risk for heart failure hospitalization and reduced left ventricular ejection fraction (p < 0.0001 for both groups) during follow-up. CONCLUSIONS: New BBB and new PPM developed frequently after TAVR. New BBB was associated with increased early and late all-cause mortality, whereas new PPM was associated with late all-cause mortality. Furthermore, both new BBB and new PPM increased the risk for heart failure hospitalizations.
Impact of new-onset left bundle branch block and periprocedural permanent pacemaker implantation on clinical outcomes in patients undergoing transcatheter aortic valve replacement: a systematic review and meta-analysis
DOI:10.1161/CIRCINTERVENTIONS.115.003635
URL
PMID:27169577
[Cited within: 1]

BACKGROUND: Available data on the clinical impact of new-onset left bundle branch block (LBBB) and permanent pacemaker implantation (PPI) after transcatheter aortic valve replacement (TAVR) remains controversial. We aimed to evaluate the impact of (1) periprocedural new-onset LBBB or PPI post-TAVR on cardiac mortality and all-cause 1-year mortality and (2) new-onset LBBB on the need for PPI at 1-year follow-up. METHODS AND RESULTS: We performed a systematic search from PubMed and EMBASE databases for studies reporting raw data on new-onset LBBB post-TAVR and the need for PPI or mortality at 1-year follow-up, or on 1-year mortality according to the need for periprocedural PPI post-TAVR. Data from 17 studies, including 4756 patients (8 studies) and 7032 patients (11 studies) for the evaluation of the impact of new-onset LBBB and periprocedural PPI post-TAVR were sourced, respectively (with 2 studies used for both outcomes). New-onset LBBB post-TAVR was associated with a higher risk of PPI (risk ratio [RR], 2.18; 95% confidence interval [CI], 1.28-3.70) and cardiac death (RR, 1.39; 95% CI, 1.04-1.86) during follow-up, as well with a tendency toward an increase in all-cause mortality (RR, 1.21; 95% CI, 0.98-1.50). Periprocedural PPI post-TAVR was not associated with any increased risk of all-cause mortality at 1 year (RR, 1.03; 95% CI, 0.9-1.18), yet a tendency toward a protective effect on cardiac death was observed (RR, 0.78; 95% CI, 0.60-1.03). CONCLUSIONS: New-onset LBBB post-TAVR is a marker of an increased risk of cardiac death and need for PPI at 1-year follow-up. The need for PPI early post-TAVR did not increase the risk of death.
Feasibility of His‐bundle pacing in patients with conduction disorders following transcatheter aortic valve replacement.
[J]DOI:10.1111/jce.v31.4 URL [Cited within: 1]