INTRODUCTION
Septic shock, a life-threatening organ dysfunction caused by the dysregulated host response to infection, is characterized by severe circulatory, cellular, and metabolic abnormalities.[1] It has been regarded as a formidable clinical challenge associated with mortality 30% to 40%.[2,3] Septic shock is a common clinical syndrome, but has pronounced heterogeneity such as variable infection sites and sources, pathogen species, and host comorbidities.[4] There has been an increasing emphasis on evidence-based adjunct therapy beyond hemodynamic support and antimicrobial therapy.[5,6]
Corticosteroids have been used in the treatment of patients with septic shock for more than half a century.[7] Till now, nearly thirty randomized controlled trials (RCTs) have evaluated the efficacy of corticosteroids in these patients but yielded different results, including two well-known RCTs published in the year 2018.[8,9] Twelve systematic reviews since 2018 have been conducted to try to address the discrepancy in these previous trials by classifying the doses of steroids and the severity of shock.[10,11,12,13,14,15,16,17,18,19,20,21]
However, these studies and reviews have not yet addressed the heterogeneity of the patient population, such as the immunological state of a patient, which is another important clinical aspect and may result in significant enrollment bias.
Recently, focusing on immunocompromised patients with septic shock, we performed an observational cohort study, and found that corticosteroid therapy had adverse effects on survival, hemodynamic stability, and hospital duration in the selected population.[22] Therefore, we aim to perform a systematic review which eliminated the impact of immune status to assess the benefits and risks of corticosteroids in septic shock, and to identify the exact group of patients who may benefit from corticosteroid treatment.
METHODS
Search strategy
We systematically performed electronic search of Medline via PubMed, Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, and EMBASE from inception to March 12, 2020. We combined MeSH and title/abstract keywords, such as “steroids”, “glucocorticoids”, “corticosteroids”, “prednisolon”, “methylprednisolon”, “prednison”, “dexamethasone”, “triamcinolon”, “fludrocortisone”, “betamethasone”, “hydrocortisone”, “sepsis”, and “shock, septic” to identify all RCTs comparing corticosteroids with a control group for immunocompetent patients with septic shock.
Study selection
Two authors independently identified the trials for inclusion based on their titles and abstracts, and evaluated the full texts of the papers.
Eligibility criteria
(1) Population. Immunocompetent adult patients with septic shock, defined based on the definition of included trials, were eligible for inclusion. Sepsis patients without circulatory failure were excluded. The immunocompetent patient was defined as the exclusion of one or more immunocompromised underlying conditions, including immunosuppression, immunodeficiency, immunosuppressive therapy, human immunodeficiency virus positive or acquired immune deficiency syndrome, advanced or end-stage neoplasm, and organ transplant recipients. (2) Intervention. All types of corticosteroids were included, regardless of the formula, dose, start time, and duration of treatment. (3) Control. The control group was allowed for the following interventions: placebo, saline, or no intervention. (4) Outcomes. The primary outcome was short-term mortality during intensive care unit (ICU) or hospital stay. The “short term” was defined as the mortality on day 28 or day 30. The secondary outcomes included mortality variables, the number of patients with shock reversal (stable hemodynamic status more than 24 hours after withdrawal of vasopressor therapy) within 28 days, and time to shock reversal. The safety outcomes included infection, gastrointestinal bleeding, and hyperglycemia. (5) Type of study. All trials included were RCTs, irrespective of language or publication status.
Data extraction and quality assessment
Characteristics of participants, study design, and outcomes for analyses were extracted following a standardized data extraction form by two reviewers independently. Two investigators independently assessed the risk of bias according to the Cochrane Handbook for Systematic Review of Intervention to assign a value of “high”, “low”, or “unclear” for each trial.
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to evaluate the quality of evidence associated with each major outcome and present the results in the summary of findings (SoFs) table.
Statistical analysis
All statistical analyses were performed on Review Manager 5.3 software and trial sequential analysis (TSA) v.0.9.5.10 beta.[23] We presented results as relative risk ratio (RR) for dichotomous data and mean difference (MD) for continuous data, which were pooled using the Mantel-Haenszel (M-H) and inverse variance method, respectively. Both RR and MD were provided with 95% confidence interval (CI). Heterogeneity was assessed by the Chi-square test with significance set at a P-value of 0.05, and quantitatively by inconsistency (I2) statistics. We reported all results from a more conservative random-effect model taking into consideration clinical heterogeneity. Subgroup analyses were also performed for all outcomes based on the trial quality.
TSA
We performed TSA to assess the increased risk of random errors due to the relatively sparse data and repeated significance testing. The result was displayed on a TSA diagram with a TSA-adjusted CI and an adjusted level of statistical significance. TSA was used to appropriately reduce the risk of a wrong conclusion in a meta-analysis that did not achieve the required information size (RIS). TSA-adjusted CI was calculated by the random-effect model for diversity (D2) with 5% risk of type I error and a power of 80%. For the estimate of the RIS, we set the intervention effect of a 15% relative risk reduction (RRR), and calculated the control event incidence from the conventional meta-analysis.
RESULTS
Study characteristics
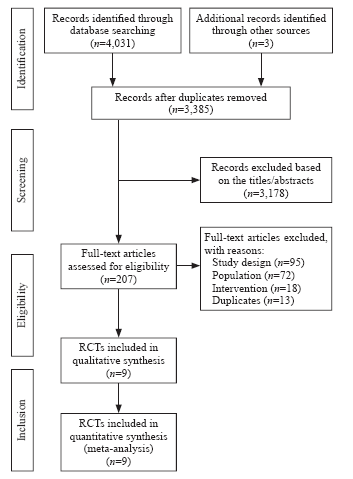
Of the 4,034 records identified in our research, full texts of 207 records were reviewed, and 27 trials initially included were assessed for patients by immune status. Ultimately, nine RCTs were included in our systematic review.[24,25,26,27,28,29,30,31,32] The results of the search and selection flow diagram were shown in Figure 1. The detailed descriptions of the included trials were presented in Table 1. Nine RCTs with a total of 1,298 participants were finally analyzed, comprising 667 in the corticosteroid group and 631 in the control group.[24,25,26,27,28,29,30,31,32]
Figure 1.
Figure 1.
Flow diagram showing results of the search and selection of eligible studies. RCT: randomized controlled trial; study design: not RCT; population: no exclusion of immunosuppression or not septic shock; intervention: not corticosteroids.
Table 1 Characteristics of included RCTs comparing corticosteroids versus control in immunocompetent patients with septic shock
| Study | Design and study place | Sample size (corticosteroids /control) | Excluded population (major selection criteria) | Intervention | Outcomesa |
|---|---|---|---|---|---|
| Annane et al[29] (2002) | Multicenter (19 sites), France | 150/149 | Advanced form of cancer or AIDS infection | IV hydrocortisone 50 mg bolus q6h and po fludrocortisone 50 μg qd versus placebo for seven days | ICU mortality, 28-day mortality, hospital mortality, one-year mortality, seven-day mortality,b shock reversal, and safety outcomes |
| Briegel et al[30] (1999) | One center, Germany | 20/20 | End-stage neoplasm, organ transplant recipients | IV hydrocortisone 100 mg loading, followed by 0.18 mg/(kg·h) continuous infusion until shock reversal, then reduced to 0.08 mg/(kg·h) for six days, then tapered off versus placebo (physiologic saline solution) | Shock reversal, 28-day mortality,b ICU mortality, hospital mortality, one-year mortality, seven-day mortality,b and safety outcomes |
| Cicarelli et al[27] (2007) | One center, Brazil | 14/15 | Immunosuppression therapy, end stage neoplasm with a life expectancy of less than three months | IV dexamethasone 0.2 mg/kg q36h for three doses versus placebo (0.9% physiological saline solution) | Seven-day mortality, 28-day mortality, and shock reversal |
| Doluee et al[32] (2018) | One center, Iran | 56/52 | Malignancy | IV hydrocortisone 50 mg q6h versus placebo (saline in the same volume) for seven days | Twenty-eight-day mortality |
| Luce et al[31] (1988) | One center, USA | 38/37 | Severe immunodeficiency and AIDS | IV methylprednisolone 30 mg/kg q6h for four doses versus mannitol placebo | Incidence of ARDS, hospital mortality, and safety outcomes |
| Lv et al[24] (2017) | One center, China | 58/60 | Immunosuppression | IV hydrocortisone 200 mg/d for six days, then tapered off versus placebo (normal saline) | Hospital mortality, 28-day mortality, shock reversal, and length of stay in ICU and hospital |
| Oppert et al[28] (2005) | One center, Germany | 18/23 | HIV positive or recipients of organ transplants | IV hydrocortisone 50 mg bolus, followed by 0.18 mg/(kg·h) continuous infusion until shock reversal, then tapered off versus placebo | Time to cessation of vasopressor support, 28-day mortality, and shock reversal |
| Sprung et al[26] (2008) | Multicenter (52 sites), Europe and Israel | 251/248 | Immunosuppression | IV hydrocortisone 50 mg q6h for five days, then tapered to 50 mg q12h for three days, then 50 mg QD for three days versus placebo | Mortality in ICU and hospital, 28-day mortality, one-year mortality, shock reversal, length of stay in ICU and hospital, and safety outcomes |
| Wan et al[25] (2014) | One center, China | 62/27 | Advanced form of cancer or HIV infection | IV hydrocortisone 50 mg q6h for seven days or five days versus saline | Shock reversal, 28-day mortality, seven-day mortality, length of stay in ICU, and safety outcomes |
RCTs: randomized controlled trials; AIDS: acquired immunodeficiency syndrome; IV: intravenous; ICU: intensive care unit; HIV: human immunodeficiency virus; a: only primary outcome of included trials and outcomes analyzed in this meta-analysis were presented in the table; b: data were calculated by Kaplan-Meier curves.
Mortality
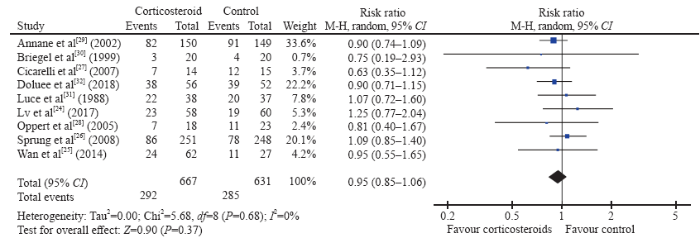
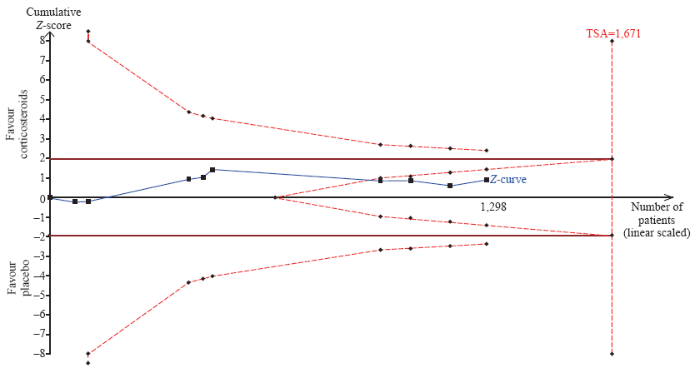
The short-term mortality in the corticosteroid and the control groups was 43.8% (292/667) and 45.2% (285/631), respectively. The pooled analysis revealed no statistically significant effects of corticosteroids (RR 0.95, 95% CI 0.85 to 1.06, P=0.37, I2=0%, TSA-adjusted CI 0.83 to 1.09, moderate-certainty evidence) (Figures 2 and 3, Table 2). TSA with RRR 15% produced an incidence of 45.1% and 38.3% in the control and corticosteroid groups, respectively. The cumulative Z-curves crossed the futility area, which excluded an effect size of 15% RRR or larger (Figure 3).
Figure 2.
Figure 2.
Forest plot of all trials for short-term mortality. CI: confidence interval; M-H: Mantel-Hansen; df: degrees of freedom.
Figure 3.
Figure 3.
Trial sequential analysis of all trials for short-term mortality. TSA: trial sequential analysis. The required information size was 1,671 patients. The incidence in the control arm of 45.1% with a relative risk reduction of 15.0% produced an incidence of 38.3% in the corticosteroid group. The TSA-adjusted 95% confidence interval for a relative risk of 0.95 was 0.83 to 1.09 and the cumulative Z-curves crossed futility area.
Table 2 Summary of findings for all included RCTs (grading of recommendations assessment, development, and evaluation)
| Outcomes | Anticipated absolute effects a (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with control | Risk with corticosteroids | ||||
| Short-term mortality | 452 per 1,000 | 429 per 1,000 (384-479) | RR 0.95 (0.85-1.06) | 1,298 (nine RCTs) | ⊕⊕⊕⊝ moderateb |
| Long-term mortality | 606 per 1,000 | 582 per 1,000 (528-649) | RR 0.96 (0.87-1.07) | 816 (three RCTs) | ⊕⊕⊕⊕ high |
| Seven-day mortality | 412 per 1,000 | 280 per 1,000 (210-371) | RR 0.68 (0.51-0.90) | 457 (four RCTs) | ⊕⊕⊝⊝ lowb, c |
| Time to shock reversal | Ranging from 75.81 to 91.2 hours | MD -21.56 (-32.95 to -10.16) | 263 (four RCTs) | ⊕⊕⊕⊝ moderateb | |
| Shock reversal | 648 per 1,000 | 700-1,000 (642-765) | RR 1.08 (0.99-1.18) | 997 (five RCTs) | ⊕⊕⊕⊝ moderateb |
| Infection | 257 per 1,000 | 280 per 1,000 (224-352) | RR 1.09 (0.87-1.37) | 894 (four RCTs) | ⊕⊕⊕⊝ moderatec |
| Gastrointestinal bleeding | 88 per 1,000 | 100 per 1,000 (69-143) | RR 1.14 (0.79-1.63) | 927 (four RCTs) | ⊕⊕⊝⊝ lowb, c |
| Hyperglycemia | 657 per 1,000 | 749 per 1,000 (676-834) | RR 1.14 (1.03-1.27) | 539 (two RCTs) | ⊕⊕⊕⊝ moderateb |
RCTs: randomized controlled trials; CI: confidence interval; MD: mean difference; ICU: intensive care unit; RR: relative risk; a: the risk in the corticosteroid group (and its 95% confidence interval) was based on the assumed risk in the control group and the relative effect of the corticosteroid (and its 95% CI); b: downgraded one level for serious risk of bias; c: downgraded one level for serious imprecision.
For the long-term mortality, the pooled estimate of RR for 1-year mortality for corticosteroids compared with control was 0.96 (95% CI 0.87 to 1.07, P=0.49, I2=0%, high-certainty evidence). Compared with placebo or the control group, corticosteroids lowered the 7-day mortality (RR 0.68, 95% CI 0.51 to 0.90, P<0.01, I2=0%, low-certainty evidence) in initial meta-analysis. However, the TSA-adjusted CI of the random-effect model was 0.39 to 1.16 without the TSA monitoring boundary being crossed, which was not statistically significant and indicated that the effect was uncertain.
Shock reversal
The conventional analysis revealed a statistically significant shortening of time to shock reversal in favor of corticosteroids (MD -21.56 hours, 95% CI -32.95 to -10.16, P<0.01, I2=0%, TSA-adjusted CI -33.33 to -9.78, moderate-certainty evidence). For shock reversal within 28 days, there was no significant difference between the corticosteroid group and the control group.
Safety outcomes
Corticosteroids likely increased the rates of hyperglycemia (RR 1.14, 95% CI 1.03 to 1.27, P=0.01, I2=0%, TSA-adjusted CI 1.00 to 1.30, moderate-certainty evidence). However, the side effects of corticosteroids on infection and gastrointestinal bleeding were not significant.
Subgroup analyses for outcomes based on trial quality
Subgroup analyses for outcomes were performed according to the risk of bias. The results did not demonstrate a beneficial effect of corticosteroids in reducing short-term mortality in the subgroup of high-quality trials (RR 0.89, 95% CI 0.74 to 1.08, P=0.24; I2=0%). For other mortality outcomes, results from trials at the low risk of bias did not substantially differ from the results of all trials.
Study quality
DISCUSSION
In this meta-analysis of nine RCTs with 1,298 patients with septic shock, we found no benefits of corticosteroids on either short-term mortality or long-term mortality. Our pooled analysis revealed that the administration of corticosteroids resulted in shorter time to shock reversal compared with the control group.
To the best of our knowledge, this is the first systematic review or meta-analysis to assess the efficacy and safety of corticosteroids in patients with septic shock based on the patients’ immune status. Previous reviews mainly enrolled patients with sepsis or septic shock and performed subgroup analyses based on the trial quality, the doses and regimens of corticosteroids, and the severity of diseases.[10,11,12,13,14,15] However, there was no differentiation or discussion of the immunological status of patients.
There is a consensus on the definitions for the immunocompetent state and the immunocompromised status: the former is usually defined as the exclusion of the latter. There were some variations in the definition of “immunocompromised” in each of the aforementioned studies.
The mechanism of corticosteroids in septic shock may be its ability to down-regulate the pro-inflammatory response.[33] However, the balance between the immune enhancement and suppression is highly dependent on the immune activation of the host as well as the dose and duration of corticosteroid therapy.[33,34] Immunocompetent patients may exhibit a profound hyper-inflammatory response followed by the cascade of events in the early stage of the disease, when the application of corticosteroids for control of systemic inflammatory response syndrome may be beneficial. The theory might partially account for the findings that corticosteroids significantly reduced the time to shock reversal. While signs of compensated anti-inflammatory response syndrome may predominate in the whole stages of immunocompromised patients, the assignment of corticosteroids might strengthen the immunosuppression resulting in the accelerated deterioration of septic shock.[34,35] Although corticosteroids did not reduce the short-term and long-term mortalities in immunocompetent patients with septic shock, it was helpful in shock reversal without increasing the risk of infection. Given the findings, the administration of corticosteroids could be considered in immunocompetent patients suffering from septic shock to achieve hemodynamic stability.[36]
Our study has several limitations. Firstly, the systematic review was not registered in the International Prospective Register of Systematic Reviews (PROSPERO), and no protocol has been published. Secondly, we tried to contact the authors of included trials to gather data on immunocompetent persons, but many trials were excluded because of the lack of detailed data on the patients’ immune status. Thirdly, there were many “unclear” ratings for risk of bias assessments, although we attempted to contact trial authors to clarify these ambiguities.
CONCLUSIONS
Corticosteroid therapy is not associated with the lower short- or long-term mortalities compared with placebo in immunocompetent patients with septic shock. However, corticosteroids significantly shorten the time to shock reversal without increasing the risk of infection. The patient’s immune status should also be considered during clinical treatment and clinical trials in future.
Funding: This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (2020-I2M-C&T-B-014), CAMS Teaching Reform Research Fund (2018zlgc0101), and CAMS Online Open Course Construction Fund (J2009022861).
Ethical approval: Not needed.
Conflicts of interests: The authors indicated no potential conflicts of interest.
Contributors: XL proposed and wrote the paper. All authors have reviewed and approved the final version of manuscript for publication.
Reference
DOI:10.1001/jama.2016.0287
URL
PMID:26903338
[Cited within: 1]

IMPORTANCE: Definitions of sepsis and septic shock were last revised in 2001. Considerable advances have since been made into the pathobiology (changes in organ function, morphology, cell biology, biochemistry, immunology, and circulation), management, and epidemiology of sepsis, suggesting the need for reexamination. OBJECTIVE: To evaluate and, as needed, update definitions for sepsis and septic shock. PROCESS: A task force (n = 19) with expertise in sepsis pathobiology, clinical trials, and epidemiology was convened by the Society of Critical Care Medicine and the European Society of Intensive Care Medicine. Definitions and clinical criteria were generated through meetings, Delphi processes, analysis of electronic health record databases, and voting, followed by circulation to international professional societies, requesting peer review and endorsement (by 31 societies listed in the Acknowledgment). KEY FINDINGS FROM EVIDENCE SYNTHESIS: Limitations of previous definitions included an excessive focus on inflammation, the misleading model that sepsis follows a continuum through severe sepsis to shock, and inadequate specificity and sensitivity of the systemic inflammatory response syndrome (SIRS) criteria. Multiple definitions and terminologies are currently in use for sepsis, septic shock, and organ dysfunction, leading to discrepancies in reported incidence and observed mortality. The task force concluded the term severe sepsis was redundant. RECOMMENDATIONS: Sepsis should be defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. For clinical operationalization, organ dysfunction can be represented by an increase in the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score of 2 points or more, which is associated with an in-hospital mortality greater than 10%. Septic shock should be defined as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone. Patients with septic shock can be clinically identified by a vasopressor requirement to maintain a mean arterial pressure of 65 mm Hg or greater and serum lactate level greater than 2 mmol/L (>18 mg/dL) in the absence of hypovolemia. This combination is associated with hospital mortality rates greater than 40%. In out-of-hospital, emergency department, or general hospital ward settings, adult patients with suspected infection can be rapidly identified as being more likely to have poor outcomes typical of sepsis if they have at least 2 of the following clinical criteria that together constitute a new bedside clinical score termed quickSOFA (qSOFA): respiratory rate of 22/min or greater, altered mentation, or systolic blood pressure of 100 mm Hg or less. CONCLUSIONS AND RELEVANCE: These updated definitions and clinical criteria should replace previous definitions, offer greater consistency for epidemiologic studies and clinical trials, and facilitate earlier recognition and more timely management of patients with sepsis or at risk of developing sepsis.
Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3)
DOI:10.1001/jama.2016.0289
URL
PMID:26903336
[Cited within: 1]

IMPORTANCE: Septic shock currently refers to a state of acute circulatory failure associated with infection. Emerging biological insights and reported variation in epidemiology challenge the validity of this definition. OBJECTIVE: To develop a new definition and clinical criteria for identifying septic shock in adults. DESIGN, SETTING, AND PARTICIPANTS: The Society of Critical Care Medicine and the European Society of Intensive Care Medicine convened a task force (19 participants) to revise current sepsis/septic shock definitions. Three sets of studies were conducted: (1) a systematic review and meta-analysis of observational studies in adults published between January 1, 1992, and December 25, 2015, to determine clinical criteria currently reported to identify septic shock and inform the Delphi process; (2) a Delphi study among the task force comprising 3 surveys and discussions of results from the systematic review, surveys, and cohort studies to achieve consensus on a new septic shock definition and clinical criteria; and (3) cohort studies to test variables identified by the Delphi process using Surviving Sepsis Campaign (SSC) (2005-2010; n = 28,150), University of Pittsburgh Medical Center (UPMC) (2010-2012; n = 1,309,025), and Kaiser Permanente Northern California (KPNC) (2009-2013; n = 1,847,165) electronic health record (EHR) data sets. MAIN OUTCOMES AND MEASURES: Evidence for and agreement on septic shock definitions and criteria. RESULTS: The systematic review identified 44 studies reporting septic shock outcomes (total of 166,479 patients) from a total of 92 sepsis epidemiology studies reporting different cutoffs and combinations for blood pressure (BP), fluid resuscitation, vasopressors, serum lactate level, and base deficit to identify septic shock. The septic shock-associated crude mortality was 46.5% (95% CI, 42.7%-50.3%), with significant between-study statistical heterogeneity (I2 = 99.5%; tau2 = 182.5; P < .001). The Delphi process identified hypotension, serum lactate level, and vasopressor therapy as variables to test using cohort studies. Based on these 3 variables alone or in combination, 6 patient groups were generated. Examination of the SSC database demonstrated that the patient group requiring vasopressors to maintain mean BP 65 mm Hg or greater and having a serum lactate level greater than 2 mmol/L (18 mg/dL) after fluid resuscitation had a significantly higher mortality (42.3% [95% CI, 41.2%-43.3%]) in risk-adjusted comparisons with the other 5 groups derived using either serum lactate level greater than 2 mmol/L alone or combinations of hypotension, vasopressors, and serum lactate level 2 mmol/L or lower. These findings were validated in the UPMC and KPNC data sets. CONCLUSIONS AND RELEVANCE: Based on a consensus process using results from a systematic review, surveys, and cohort studies, septic shock is defined as a subset of sepsis in which underlying circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than sepsis alone. Adult patients with septic shock can be identified using the clinical criteria of hypotension requiring vasopressor therapy to maintain mean BP 65 mm Hg or greater and having a serum lactate level greater than 2 mmol/L after adequate fluid resuscitation.
Effect of low high-density lipoprotein levels on mortality of septic patients: a systematic review and meta-analysis of cohort studies. World
[J]
Classification of patients with septic shock: are we there yet?
[J]
Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016
DOI:10.1007/s00134-017-4683-6
URL
PMID:28101605
[Cited within: 1]

OBJECTIVE: To provide an update to
How I treat septic shock
DOI:10.1007/s00134-018-5401-8 URL PMID:30315330 [Cited within: 1]
The effectiveness of hydrocortisone in the management of severe infections
DOI:10.1001/jama.1963.63700060029012 URL [Cited within: 1]
Adjunctive glucocorticoid therapy in patients with septic shock. N Engl
[J]
Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl
[J]
Effect of adjunctive corticosteroids on clinical outcomes in adult patients with septic shock: a meta-analysis of randomized controlled trials and trial sequential analysis.
[J]
Do low-dose corticosteroids improve survival or shock reversal from septic shock in adults? Meta-analysis with trial sequential analysis.
[J]
Low-dose corticosteroids for adult patients with septic shock: a systematic review with meta-analysis and trial sequential analysis
DOI:10.1007/s00134-018-5197-6
URL
PMID:29761216
[Cited within: 2]

PURPOSE: To assess the effect of low dose corticosteroids on outcomes in adults with septic shock. METHODS: We systematically reviewed randomised clinical trials (RCTs) comparing low-dose corticosteroids to placebo in adults with septic shock. Trial selection, data abstraction and risk of bias assessment were performed in duplicate. The primary outcome was short-term mortality. Secondary and tertiary outcomes included longer-term mortality, adverse events, quality of life, and duration of shock, mechanical ventilation and ICU stay. RESULTS: There were 22 RCTs, including 7297 participants, providing data on short-term mortality. In two low risk of bias trials, the relative risk (RR) of short-term mortality with corticosteroid versus placebo was 0.98 [95% confidence interval (CI) 0.89-1.08, p = 0.71]. Sensitivity analysis including all trials was similar (RR 0.96; 95% CI 0.91-1.02, p = 0.21) as was analysis of longer-term mortality (RR 0.96; 95% CI 0.90-1.02, p = 0.18). In low risk of bias trials, the risk of experiencing any adverse event was higher with corticosteroids; however, there was substantial heterogeneity (RR 1.66; 95% CI 1.03-2.70, p = 0.04, I(2) = 78%). No trials reported quality of life outcomes. Duration of shock [mean difference (MD) -1.52 days; 95% CI -1.71 to -1.32, p < 0.0001], duration of mechanical ventilation (MD -1.38 days; 95% CI -1.96 to -0.80, p < 0.0001), and ICU stay (MD -0.75 days; 95% CI -1.34 to -0.17, p = 0.01) were shorter with corticosteroids versus placebo. CONCLUSIONS: In adults with septic shock treated with low dose corticosteroids, short- and longer-term mortality are unaffected, adverse events increase, but duration of shock, mechanical ventilation and ICU stay are reduced. PROSPERO registration no. CRD42017084037.
Corticosteroids in sepsis: an updated systematic review and meta-analysis
DOI:10.1097/CCM.0000000000003262
URL
PMID:29979221
[Cited within: 2]

OBJECTIVE: This systematic review and meta-analysis addresses the efficacy and safety of corticosteroids in critically ill patients with sepsis. DATA SOURCES: We updated a comprehensive search of MEDLINE, EMBASE, CENTRAL, and LILACS, and unpublished sources for randomized controlled trials that compared any corticosteroid to placebo or no corticosteroid in critically ill children and adults with sepsis. STUDY SELECTION: Reviewers conducted duplicate screening of citations, data abstraction, and, using a modified Cochrane risk of bias tool, individual study risk of bias assessment. DATA EXTRACTION: A parallel guideline committee provided input on the design and interpretation of the systematic review, including the selection of outcomes important to patients. We assessed overall certainty in evidence using Grading of Recommendations Assessment, Development and Evaluation methodology and performed all analyses using random-effect models. For subgroup analyses, we performed metaregression and considered p value less than 0.05 as significant. DATA SYNTHESIS: Forty-two randomized controlled trials including 10,194 patients proved eligible. Based on low certainty, corticosteroids may achieve a small reduction or no reduction in the relative risk of dying in the short-term (28-31 d) (relative risk, 0.93; 95% CI, 0.84-1.03; 1.8% absolute risk reduction; 95% CI, 4.1% reduction to 0.8% increase), and possibly achieve a small effect on long-term mortality (60 d to 1 yr) based on moderate certainty (relative risk, 0.94; 95% CI, 0.89-1.00; 2.2% absolute risk reduction; 95% CI, 4.1% reduction to no effect). Corticosteroids probably result in small reductions in length of stay in ICU (mean difference, -0.73 d; 95% CI, -1.78 to 0.31) and hospital (mean difference, -0.73 d; 95% CI, -2.06 to 0.60) (moderate certainty). Corticosteroids result in higher rates of shock reversal at day 7 (relative risk, 1.26; 95% CI, 1.12-1.42) and lower Sequential Organ Failure Assessment scores at day 7 (mean difference, -1.39; 95% CI, -1.88 to -0.89) (high certainty). Corticosteroids likely increase the risk of hypernatremia (relative risk, 1.64; 95% CI, 1.32-2.03) and hyperglycemia (relative risk, 1.16; 95% CI, 1.08-1.24) (moderate certainty), may increase the risk of neuromuscular weakness (relative risk, 1.21; 95% CI, 1.01-1.52) (low certainty), and appear to have no other adverse effects (low or very low certainty). Subgroup analysis did not demonstrate a credible subgroup effect on any of the outcomes of interest (p > 0.05 for all). CONCLUSIONS: In critically ill patients with sepsis, corticosteroids possibly result in a small reduction in mortality while also possibly increasing the risk of neuromuscular weakness.
Effect of low-dose hydrocortisone therapy in adult patients with septic shock: a meta-analysis with trial sequential analysis of randomized controlled trials.
[J]
The effectiveness and safety of corticosteroids therapy in adult critical ill patients with septic shock: a meta-analysis of randomized controlled trials
DOI:10.1097/SHK.0000000000001202
URL
PMID:29889815
[Cited within: 2]

OBJECTIVE: To investigate the effectiveness and safety of corticosteroids therapy in adult critical ill patients with septic shock. METHODS: The PUBMED, EMBASE, Web of Science, Cochrane Library databases were systematically searched from the inception dates to March 24, 2018. To identify randomized controlled trials that evaluating the role of corticosteroids therapy in adult critical ill patients with septic shock. The primary outcome was 28-day mortality. The second outcomes included 90-day mortality, intensive care unit (ICU) mortality, in-hospital mortality, length of stay in ICU, length of stay in hospital, reversal of shock, and superinfection. RESULTS: A total of 18 randomized controlled trials involving 8,128 adult critical ill patients with septic shock fulfilled the inclusion criteria. The outcomes of this meta-analysis showed that corticosteroids therapy did not significantly reduce the 28-day mortality [RR = 0.94; 95% CI, 0.84-1.05; Z = 1.07 (P = 0.285)]. However, corticosteroids therapy was associated with a significantly shorter length of stay in ICU [WMD = -1.55; 95% CI, -2.19 to -0.91; Z = 4.74 (P = 0.000)]. 90-day mortality, ICU mortality, in-hospital mortality, length of stay in hospital, reversal of shock, and superinfection had no significant difference between the corticosteroids therapy and placebo therapy (P > 0.05). Similar results were obtained in subgroups of trials stratified according to the dose of corticosteroids (high dose or low does). CONCLUSIONS: Based on the results of this meta-analysis, corticosteroids therapy was associated with a significantly shorter length of stay in ICU among adult critical ill patients with septic shock. The mortality was similar between the corticosteroids therapy and placebo.
Reevaluating the role of corticosteroids in septic shock: an updated meta-analysis of randomized controlled trials
DOI:10.1155/2019/3175047
URL
PMID:31281831
[Cited within: 1]

What Is Known and Objective. To reevaluate the benefits and risks of corticosteroid treatment in adult patients with septic shock. Methods. This study was performed based on PRISMA guidelines. Randomized controlled trials (RCTs) of corticosteroids versus placebo were retrieved from PubMed, MEDLINE, EMBASE, Web of Science, the Cochrane Central RCTs, and ClinicalTrials.gov from January 1980 to April 2018. We also conducted a trial sequential analysis to indicate the possibility of type I or II errors and calculate the information size. Grading of Recommendations, Assessment, Development and Evaluation approach (GRADE) was applying to assess the certainty of evidence at the primary outcome level. Results. Twenty-one RCTs were identified and analyzed. Patients treated with corticosteroid had a 7% reduction in relative risk in 28-day all-cause mortality compared to controls (RR 0.93, 95% CI 0.88 to 0.99). However, there were no significant differences for the intensive care unit (ICU) mortality (RR 0.97, 95% CI 0.86 to 1.09) or in-hospital mortality (RR 1.01, 95% CI 0.92 to 1.11). Corticosteroids shortened the length of ICU stay by 1.04 days (RR -1.04, 95% CI -1.72 to -0.36) and the length of hospital stay by 2.49 days (RR -2.49, 95% CI -4.96 to -0.02). Corticosteroids increased the risk of hyperglycemia (RR 1.11, 95% CI 1.06 to 1.16) but not gastroduodenal bleeding (RR 1.06, 95% CI 0.82 to 1.37) or superinfection (RR 1.04, 95% CI 0.94 to 1.15). However, some date on secondary outcomes were unavailable because they were not measured or not reported in the included studies which may cause a lack of power or selective outcome reporting. The information size was calculated at 10044 patients. Trial sequential analysis showed that the meta-analysis was conclusive and the risk of type 2 error was minimal. What Is New and Conclusion. Corticosteroids are likely to be effective in reducing 28-day mortality and attenuating septic shock without increasing the rate of life-threatening complications. TSA showed that the risk of type II error in this meta-analysis was minimal and the result was conclusive.
Association of corticosteroid treatment with outcomes in adult patients with sepsis: a systematic review and meta-analysis
DOI:10.1001/jamainternmed.2018.5849
URL
PMID:30575845
[Cited within: 1]

Importance: Although corticosteroids are widely used for adults with sepsis, both the overall benefit and potential risks remain unclear. Objective: To conduct a systematic review and meta-analysis of the efficacy and safety of corticosteroids in patients with sepsis. Data Sources and Study Selection: MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched from inception until March 20, 2018, and updated on August 10, 2018. The terms corticosteroids, sepsis, septic shock, hydrocortisone, controlled trials, and randomized controlled trial were searched alone or in combination. Randomized clinical trials (RCTs) were included that compared administration of corticosteroids with placebo or standard supportive care in adults with sepsis. Data Extraction and Synthesis: Meta-analyses were conducted using a random-effects model to calculate risk ratios (RRs) and mean differences (MDs) with corresponding 95% CIs. Two independent reviewers completed citation screening, data abstraction, and risk assessment. Main Outcomes and Measures: Twenty-eight-day mortality. Results: This meta-analysis included 37 RCTs (N = 9564 patients). Eleven trials were rated as low risk of bias. Corticosteroid use was associated with reduced 28-day mortality (RR, 0.90; 95% CI, 0.82-0.98; I2 = 27%) and intensive care unit (ICU) mortality (RR, 0.85; 95% CI, 0.77-0.94; I2 = 0%) and in-hospital mortality (RR, 0.88; 95% CI, 0.79-0.99; I2 = 38%). Corticosteroids were significantly associated with increased shock reversal at day 7 (MD, 1.95; 95% CI, 0.80-3.11) and vasopressor-free days (MD, 1.95; 95% CI, 0.80-3.11) and with ICU length of stay (MD, -1.16; 95% CI, -2.12 to -0.20), the sequential organ failure assessment score at day 7 (MD, -1.38; 95% CI, -1.87 to -0.89), and time to resolution of shock (MD, -1.35; 95% CI, -1.78 to -0.91). However, corticosteroid use was associated with increased risk of hyperglycemia (RR, 1.19; 95% CI, 1.08-1.30) and hypernatremia (RR, 1.57; 95% CI, 1.24-1.99). Conclusions and Relevance: The findings suggest that administration of corticosteroids is associated with reduced 28-day mortality compared with placebo use or standard supportive care. More research is needed to associate personalized medicine with the corticosteroid treatment to select suitable patients who are more likely to show a benefit.
Can corticosteroids reduce the mortality of patients with severe sepsis? A systematic review and meta-analysis. Am
[J]
Effects and safety of separate low-dose hydrocortisone use in patients with septic shock: a meta-analysis. Hong Kong
[J]
Corticosteroids for treating sepsis in children and adults
Impact and beneficial critical points of clinical outcome in corticosteroid management of adult patients with sepsis: meta-analysis and grade assessment
DOI:10.3389/fphar.2019.01101
URL
PMID:31607929
[Cited within: 1]

Background: With new randomised pieces of evidence and the latest clinical practice guideline from the BMJ emerging in 2018, an updated analysis of best available evidence on the controversial effects of corticosteroids in sepsis is warranted. Objectives: To comprehensively evaluate whether corticosteroids are beneficial in reducing mortality and what cumulative dosage, daily dosage, and duration of corticosteroid treatment would enable adult patients with sepsis to reach the critical point of benefits. Methods: Ovid MEDLINE, Ovid EMbase, Cochrane Library, and LILACS database were searched until March 22, 2019. Results: Thirty RCTs with 8,836 participants were identified. Long course low-dose corticosteroid therapy could improve 28-day mortality (RR = 0.90, 95% CI = 0.84-0.97; high quality), intensive care unit mortality (RR = 0.87; 95% CI = 0.79-0.95; moderate quality), and in-hospital mortality (RR = 0.88, 95% CI = 0.79-0.997; high quality). However, we found no benefits for 90-day, 180-day, and 1-year mortality. Subgroup results of long course corticosteroid treatment in a population with septic shock and vasopressor-dependent septic shock, corticosteroid regimen with hydrocortisone plus fludrocortisone, corticosteroid dosing strategies including bolus dosing and infusion dosing, the strategies of abrupt discontinuation, timing of randomisation /=10, and sample size >/=500 were associated with a marginally reduction in 28-day mortality. Conclusions: This meta-analysis found that the long course low-dose and not short course high-dose corticosteroid treatment could marginally improve short-term 28-day mortality with high quality, especially septic shock and vasopressor-dependent septic shock, and it is recommended that long course (about 7 days) low-dose (about 200-300mg per day) hydrocortisone (or equivalent) with cumulative dose (at least about 1,000mg) may be a viable management option for overall patients with sepsis, and it can be also adapted to patient with septic shock alone. Early hydrocortisone plus fludrocortisone administration, via continuous infusion or bolus dosing, is also particularly important for the prognosis. Abrupt discontinuation of corticosteroids, as opposed to the conventional tapered discontinuation, may be considered as a desirable option in 28-day mortality. The safety profile of long course low-dose corticosteroid treatment, including adverse hyperglycaemia and hypernatraemia events, remains a concern, although these events could be easily treated. Clinical Trial Registration: PROSPERO, identifier CRD 42018092849.
Efficacy and safety of corticosteroids for septic shock in immunocompromised patients: a cohort study from MIMIC
User manual for trial sequential analysis(TSA)
Early initiation of low-dose hydrocortisone treatment for septic shock in adults: a randomized clinical trial. Am
[J]
Clinical trial of low-dose corticosteroid treatment for surgical postoperative patients with septic shock. Journal of
Hydrocortisone therapy for patients with septic shock. N Engl
[J]
Early dexamethasone treatment for septic shock patients: a prospective randomized clinical trial
DOI:10.1590/s1516-31802007000400009
URL
PMID:17992396
[Cited within: 3]

CONTEXT AND OBJECTIVE: Sepsis and septic shock are very common conditions among critically ill patients that lead to multiple organ dysfunction syndrome (MODS) and death. Our purpose was to investigate the efficacy of early administration of dexamethasone for patients with septic shock, with the aim of halting the progression towards MODS and death. DESIGN AND SETTING: Prospective, randomized, double-blind, single-center study, developed in a surgical intensive care unit at Hospital das Clinicas, Faculdade de Medicina da Universidade de Sao Paulo. METHODS: The study involved 29 patients with septic shock. All eligible patients were prospectively randomized to receive either a dose of 0.2 mg/kg of dexamethasone (group D) or placebo (group P), given three times at intervals of 36 hours. The patients were monitored over a seven-day period by means of the sequential organ failure assessment score. RESULTS: Patients treated with dexamethasone did not require vasopressor therapy for as much time over the seven-day period as did the placebo group (p = 0.043). Seven-day mortality was 67% in group P (10 out of 15) and 21% in group D (3 out of 14) (relative risk = 0.31, 95% confidence interval 0.11 to 0.88). Dexamethasone enhanced the effects of vasopressor drugs. CONCLUSIONS: Early treatment with dexamethasone reduced the seven-day mortality among septic shock patients and showed a trend towards reduction of 28-day mortality.
Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock
DOI:10.1097/01.ccm.0000186370.78639.23
URL
PMID:16276166
[Cited within: 4]

OBJECTIVES: To investigate the effect of low-dose hydrocortisone on time to shock reversal, the cytokine profile, and its relation to adrenal function in patients with early septic shock. DESIGN: Prospective, randomized, double-blind, single-center study. SETTING: Medical intensive care unit of a university hospital. PATIENTS: Forty-one consecutive patients with early hyperdynamic septic shock. INTERVENTIONS: After inclusion and a short adrenocorticotropic hormone test, all patients were randomized to receive either low-dose hydrocortisone (50-mg bolus followed by a continuous infusion of 0.18 mg/kg body of weight/hr) or matching placebo. After shock reversal, the dose was reduced to 0.06 mg/kg/hr and afterward slowly tapered. Severity of illness was estimated using Acute Physiology and Chronic Health Evaluation II score and Sequential Organ Failure Assessment score. MEASUREMENTS AND MAIN RESULTS: Time to cessation of vasopressor support (primary end point) was significantly shorter in hydrocortisone-treated patients compared with placebo (53 hrs vs. 120 hrs, p < .02). This effect was more profound in patients with impaired adrenal reserve. Irrespective of endogenous steroid production, cytokine production was reduced in the treatment group with lower plasma levels of interleukin-6 and a diminished ex vivo lipopolysaccharide-stimulated interleukin-1 and interleukin-6 production. Interleukin-10 levels were unaltered. Adverse events were not more frequent in the treatment group. CONCLUSIONS: Treatment with low-dose hydrocortisone accelerates shock reversal in early hyperdynamic septic shock. This was accompanied by reduced production of proinflammatory cytokines, suggesting both hemodynamic and immunomodulatory effects of steroid treatment. Hemodynamic improvement seemed to be related to endogenous cortisol levels, whereas immune effects appeared to be independent of adrenal reserve.
Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock
DOI:10.1001/jama.288.7.862
URL
PMID:12186604
[Cited within: 4]

CONTEXT: Septic shock may be associated with relative adrenal insufficiency. Thus, a replacement therapy of low doses of corticosteroids has been proposed to treat septic shock. OBJECTIVE: To assess whether low doses of corticosteroids improve 28-day survival in patients with septic shock and relative adrenal insufficiency. DESIGN AND SETTING: Placebo-controlled, randomized, double-blind, parallel-group trial performed in 19 intensive care units in France from October 9, 1995, to February 23, 1999. PATIENTS: Three hundred adult patients who fulfilled usual criteria for septic shock were enrolled after undergoing a short corticotropin test. INTERVENTION: Patients were randomly assigned to receive either hydrocortisone (50-mg intravenous bolus every 6 hours) and fludrocortisone (50- micro g tablet once daily) (n = 151) or matching placebos (n = 149) for 7 days. MAIN OUTCOME MEASURE: Twenty-eight-day survival distribution in patients with relative adrenal insufficiency (nonresponders to the corticotropin test). RESULTS: One patient from the corticosteroid group was excluded from analyses because of consent withdrawal. There were 229 nonresponders to the corticotropin test (placebo, 115; corticosteroids, 114) and 70 responders to the corticotropin test (placebo, 34; corticosteroids, 36). In nonresponders, there were 73 deaths (63%) in the placebo group and 60 deaths (53%) in the corticosteroid group (hazard ratio, 0.67; 95% confidence interval, 0.47-0.95; P =.02). Vasopressor therapy was withdrawn within 28 days in 46 patients (40%) in the placebo group and in 65 patients (57%) in the corticosteroid group (hazard ratio, 1.91; 95% confidence interval, 1.29-2.84; P =.001). There was no significant difference between groups in responders. Adverse events rates were similar in the 2 groups. CONCLUSION: In our trial, a 7-day treatment with low doses of hydrocortisone and fludrocortisone significantly reduced the risk of death in patients with septic shock and relative adrenal insufficiency without increasing adverse events.
Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study
DOI:10.1097/00003246-199904000-00025
URL
PMID:10321661
[Cited within: 4]

OBJECTIVE: To investigate the effects of stress doses of hydrocortisone on the duration of vasopressor therapy in human septic shock. DESIGN: Prospective, randomized, double-blind, single-center study. SETTING: Twenty-bed multidisciplinary intensive care unit in a 1400-bed university hospital. PATIENTS: Forty consecutive patients who met the ACCP/SCCM criteria for septic shock. An additional criterion for inclusion in the study was vasopressor support and high-output circulatory failure with a cardiac index of >4 L/min/m2 after fluid resuscitation (pulmonary capillary wedge pressure: 12-15 mm Hg) and without the use of positive inotropes such as dobutamine or dopexamine. The primary study end point was the time to cessation of vasopressor support (norepinephrine or epinephrine in any dose, dopamine > or = 6 microg/kg/min). Secondary study end points were the evolution of hemodynamics and the multiple organ dysfunction syndrome (MODS). The severity of illness at recruitment was graded using the Acute Physiology and Chronic Health Evaluation II and the Simplified Acute Physiology Score II scoring systems. MODS was described by the Sepsis-related Organ Failure Assessment score. INTERVENTIONS: All eligible patients were prospectively randomized to receive either stress doses of hydrocortisone or placebo. Hydrocortisone was started with a loading dose of 100 mg given within 30 mins and followed by a continuous infusion of 0.18 mg/ kg/hr. When septic shock had been reversed, the dose of hydrocortisone was reduced to 0.08 mg/kg/hr. This dose was kept constant for 6 days. As soon as the underlying infection had been treated successfully or sodium serum concentrations had increased to >155 mmol/L, the hydrocortisone infusion was tapered in steps of 24 mg/day. Physiologic saline solution was the placebo. MEASUREMENTS AND MAIN RESULTS: Hemodynamic and oxygen-derived variables were measured at previously defined time points over a study period of 5 days. Relevant clinical and laboratory measurements were registered for a study period of 14 days to assess the evolution of organ dysfunction. Baseline data at recruitment did not differ between the two groups. Shock reversal was achieved in 18 of the 20 patients treated with hydrocortisone vs. 16 of the 20 patients treated with placebo. Hydrocortisone significantly reduced the time to cessation of vasopressor support. The median time of vasopressor support was 2 days (1st and 3rd Quartiles, 1 and 6 days) in the hydrocortisone-treated group and 7 days (1st and 3rd Quartiles, 3 and 19 days) in the placebo group (p = .005 Breslow test). There was a trend to earlier resolution of the organ dysfunction syndrome in the hydrocortisone group. CONCLUSIONS: Infusion of stress doses of hydrocortisone reduced the time to cessation of vasopressor therapy in human septic shock. This was associated with a trend to earlier resolution of sepsis-induced organ dysfunctions. Overall shock reversal and mortality were not significantly different between the groups in this low-sized single-center study.
Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock
DOI:10.1164/ajrccm/138.1.62
URL
PMID:3202402
[Cited within: 4]

We conducted a prospective, randomized, double-blind study to determine whether high-dose methylprednisolone could prevent parenchymal lung injury, including the adult respiratory distress syndrome (ARDS), or improve mortality when administered early in septic shock. All patients already hospitalized in or newly admitted to the medical and surgical intensive care units at San Francisco General Hospital between September 1, 1983 and August 29, 1986 were eligible for admission to the study if they had either (1) an increase in temperature of 1.5 degrees C and a decrease in systolic blood pressure of 20 mm Hg or more from baseline values (in already hospitalized patients), or (2) a temperature greater than 38.5 degrees C or less than 35.5 degrees C and a systolic blood pressure of less than 90 mm Hg (in newly admitted patients). Patients meeting these criteria were excluded if they (1) had severe immunodeficiency, (2) were less than 18 or greater than 76 yr of age, (3) had multilobar roentgenographic infiltrates, or (4) were already receiving corticosteroids. Eighty-seven patients enrolled in the study received either methylprednisolone, 30 mg/kg per dose, or mannitol placebo for a total of 4 doses every 6 h, following the presumptive diagnosis of septic shock. Of these patients, 75 ultimately were determined on the basis of culture results to have actually had septic shock at the time of entry. Thirteen of the patients who received methylprednisolone developed ARDS, compared to 14 patients who received placebo. Lesser degrees of parenchymal lung injury did not differ between the 2 groups.(ABSTRACT TRUNCATED AT 250 WORDS)
The effect of physiologic dose of intravenous hydrocortisone in patients with refractory septic shock: a randomized control trial
DOI:10.15171/jept.2017.25 URL [Cited within: 4]
The role of glucocorticoids as adjunctive treatment for sepsis in the modern era
DOI:10.1016/S2213-2600(18)30265-0
URL
PMID:30006071
[Cited within: 2]

Glucocorticoids have been used as adjunctive therapy in patients with sepsis and septic shock for more than four decades. The rationale for the use of glucocorticoids is that this class of drugs downregulates the proinflammatory response and limits the anti-inflammatory response while preserving innate immunity. Between 1976 and 2017, 22 randomised placebo-controlled trials have been published evaluating the benefit of glucocorticoids in patients with community-acquired pneumonia, sepsis, and septic shock. These studies produced conflicting results. In 2018, two large randomised controlled trials (RCTs) were published evaluating the role of hydrocortisone in patients with septic shock. The Activated Protein C and Corticosteroids for Human Septic Shock (APROCCHSS) trial reported a reduction in 90-day mortality whereas the Adjunctive Corticosteroid Treatment in Critically Ill Patients with Septic Shock (ADRENAL) trial reported no mortality benefit. This Viewpoint critically appraises these two RCTs and evaluates the use of glucocorticoids in the treatment of sepsis and septic shock in the modern era.
Advances in the management of sepsis and the understanding of key immunologic defects
DOI:10.1097/ALN.0b013e31823422e8
URL
[Cited within: 2]

Anesthesiologists are increasingly confronting the difficult problem of caring for patients with sepsis in the operating room and in the intensive care unit. Sepsis occurs in more than 750,000 patients in the United States annually and is responsible for more than 210,000 deaths. Approximately 40% of all intensive care unit patients have sepsis on admission to the intensive care unit or experience sepsis during their stay in the intensive care unit. There have been significant advances in the understanding of the pathophysiology of the disorder and its treatment. Although deaths attributable to sepsis remain stubbornly high, new treatment algorithms have led to a reduction in overall mortality. Thus, it is important for anesthesiologists and critical care practitioners to be aware of these new therapeutic regimens. The goal of this review is to include practical points on important advances in the treatment of sepsis and provide a vision of future immunotherapeutic approaches.
Sepsis and septic shock
DOI:10.1016/S0140-6736(18)30696-2
URL
PMID:29937192
[Cited within: 1]

Sepsis is a common condition that is associated with unacceptably high mortality and, for many of those who survive, long-term morbidity. Increased awareness of the condition resulting from ongoing campaigns and the evidence arising from research in the past 10 years have increased understanding of this problem among clinicians and lay people, and have led to improved outcomes. The World Health Assembly and WHO made sepsis a global health priority in 2017 and have adopted a resolution to improve the prevention, diagnosis, and management of sepsis. In 2016, a new definition of sepsis (Sepsis-3) was developed. Sepsis is now defined as infection with organ dysfunction. This definition codifies organ dysfunction using the Sequential Organ Failure Assessment score. Ongoing research aims to improve definition of patient populations to allow for individualised management strategies matched to a patient's molecular and biochemical profile. The search continues for improved diagnostic techniques that can facilitate this aim, and for a pharmacological agent that can improve outcomes by modifying the disease process. While waiting for this goal to be achieved, improved basic care driven by education and quality-improvement programmes offers the best hope of increasing favourable outcomes.
Articles that may change your practice: steroids and septic shock
DOI:10.1016/j.amj.2018.08.001 URL PMID:30424846 [Cited within: 1]