INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a common condition associated with critical illnesses, which causes substantial mortalities. Approximately 200,000 ARDS cases per year occur in the USA, and the mortality is as high as 36%-44%.[1,2] Sepsis is often the main cause of ARDS, which may lead to multiple organ failure.[3] Lung inflammation, hypoxemia, and non-cardiogenic pulmonary edema formation are characteristic features.[4,5]
Alveolar epithelium is one of the main sites of cell injuries in ARDS. Neutrophils contribute to lung inflammation and play important roles in the pathogenesis and progression of ARDS. The activated neutrophils damage epithelial cells,[6] which causes increased entry of fluid into the alveolar lumens, decreased clearance of fluid from the alveolar airspace, and decreased production of surfactant.[7]
MicroRNAs (miRNAs) are small noncoding ribonucleic acid (RNA) molecules and are recognized as endogenous physiological regulators of gene expression.[8] Given that an individual miRNA could potentially alter complicated cellular processes including cell growth, apoptosis, inflammatory-immune responses, and cell-cell interaction,[9,10] it is not surprising that their wrong settings may be involved in the pathogenesis of ARDS.
Many miRNAs are expressed in the lung. MicroRNA-17 (miR-17), microRNA-92a (miR-92a), and microRNA-127 (miR-127) regulate the lung development.[11,12] Although miRNAs play a dominating role in several physiological functions, they are also involved in the pathogenesis of many diseases. The abnormal expression of miRNAs is associated with cardiac disorders,[13] vascular diseases,[14] cancers,[15] and pulmonary diseases like ARDS.[16]
As an important miRNA, microRNA-145 (miR-145) has been studied in various cancers.[15,17] It could potentially alter complex cellular processes, such as cell growth, cell cycle, apoptosis, and invasion.[18] A previous study reported that the expression of miR-145 was significantly reduced in myocardial ischemia/reperfusion (I/R) injury in the rats,[19] indicating that the abnormal expression of miR-145 was involved in myocardial I/R injury. Hypoxia could promote umbilical cord mesenchymal stem cell (UCMSC) differentiation into alveolar epithelial cells, and this effect was mainly mediated by miR-145.[20] Lipopolysaccharide (LPS)-induced liver inflammation was probably mediated by miR-145 through interleukin-1 receptor-associated kinase 1 (IRAK1) and nuclear factor-kappa B (NF-κB) pathways.[21] Furthermore, miR-145 suppression reversed the LPS-induced inflammatory injury on ATDC5 cells.[22]
However, the role of miR-145 in ARDS has not been investigated. In the present study, we aimed to identify miRNA-145 involved in ARDS by using an animal model of ARDS. In addition, we tried to focus on the relationship between miR-145 and mitochondrial function, which plays a critical role in regulating the cell injury of ARDS.
METHODS
Animals
The study was proved by the Ethics Department of Zhongshan Hospital, Fudan University. A total of 24 male Sprague-Dawley rats, aged 6-8 weeks, purchased from the Animal Center of Fudan University, and bred under pathogen free conditions, were housed separately in a temperature-controlled room with a 12-hour light/12-hour dark cycle. Animals were allowed free access to food and water.
Animal treatment
Rats were randomly assigned into two groups. They were anesthetized with an intraperitoneal injection of avertin (25 mg/kg) and fixed at a 60° angle on a table in a supine position. The oropharynx was lifted with forceps, allowing for the direct visualization of the trachea. LPS at a dose of 0.5 mg/kg (Sigma, USA) was injected into the trachea using an 18G catheter attached to a 1 mL syringe as previously described.[23] Control animals received an equal volume of phosphate-buffered saline (PBS). Rats were sacrificed at 6, 24, and 72 hours after LPS/PBS instillation after the intraperitoneal injection of avertin (25 mg/kg).
RNA isolation and analysis
RNA was isolated from the median and caudal lobe of the right lung using the Qiagen RNeasy Mini Kit following the manufacturer’s instructions. Quantitative polymerase chain reaction (qPCR) was performed for expressions of miRNAs using the primers for miR-145 as follows: 5′-GUCCAGUUUUCCCAGGAAUCCCU-3′.
Determination of the lung water content
The right main bronchus was ligated, and the cranial and accessory lobes of the right lung were excised. After wet weights were measured, the cranial and accessory lobes of the right lung were placed in an oven at 60 °C for 72 hours to allow determination of the wet-to-dry (W/D) weight ratio.
Bronchoalveolar lavage (BAL)
The BAL was performed in the left lung. Totally 2 mL PBS (4 °C) was slowly infused. The fluid was slowly withdrawn and reinfused for another two times. The recovered fluid was collected for further analysis.
Cytokines in bronchoalveolar lavage fluid (BALF)
Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) levels in BALF were measured using rat TNF-α and IL-6 enzyme-linked immunosorbent assay (ELISA)kits (R&D Systems) according to the manufacturer’s recommendations.
Hematoxylin and eosin (H&E) staining
Lung tissues were fixed and processed for H&E staining. Briefly, lung tissues were fixed by 10% PBS-buffered formalin through trachea catheterization at a transpulmonary pressure of 15 cmH2O (1 cmH2O=0.098 kPa), and then overnight at 4 °C with agitation. After paraffin processing, the tissues were cut into semi-thin 4-5 μm thick and stained with H&E for histological analysis.
Transmission electron microscope
The preparation of lung tissues for transmission electron microscopy was made following the procedure described previously.[24] Lung samples were obtained and fixed with 2.5% glutaraldehyde in PBS buffer. Then, lung samples were post-fixed with 1% OsO4 in PBS buffer for 1 hour, followed by dehydration. Tissues were embedded in 50% propylene oxide/50% resin. Sectioning was performed on an ultramicrotome (60 nm thickness). Samples were stained with lead citrate, and examined with an electron microscope (Hitachi H-600, Japan).
Target gene prediction of miRNA
The target genes of prognostic miR-145 were predicted using TargetScan and miRDB analysis tools. The overlapping genes were analyzed. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed for the target genes. The P-value <0.05 and gene count ≥3 were set as the cut-off criteria.
Statistical analysis
The data were expressed as mean±standard deviation (SD). The expression levels of miRNAs in ARDS and control rats were analyzed by Wilcoxon signed-rank test. The P-value <0.05 was considered statistically significant. The statistical analysis was performed using SPSS software and Prism 6.0.
RESULTS
MiR-145 down-regulated in LPS-induced ARDS rat lung
With H&E staining, there were few inflammatory cells or lymphocyte infiltrations, and the structure of alveolar was almost intact in the control group; at 6 hours after LPS injection, the lymphocytes in the alveolar were significantly increased, especially increased around bronchi and lung vessels, and there was protein-like fluid filled in the alveola; at 24 hours after LPS injection, there were more lymphocyte infiltrations, the alveolar structures were disrupted and filled with inflammatory cells, and alveolar interval was much thicker than that in the control group; at 72 hours after LPS injection, the lymphocyte infiltrations still existed, but decreased compared with those at 24 hours (Figures 1A[a-d]). According to the lung injury scoring system,[25] scores significantly increased in LPS instillation lungs, especially at 24 hours and 72 hours after LPS injection (Figure 1B). At 6 hours after LPS injection, the W/D ratio of LPS lungs was about 7.5 compared with 3.0 in the control group; at 24 hours after LPS injection, the W/D ratio of LPS lungs was about 7.0, much higher than that in the control group (P<0.05); at 72 hours after LPS injection, the W/D ratio of LPS lungs was about 4.5, higher than that in the control group (P<0.05) (Figure 1C). The cytokines in BALF were measured at 6 hours (n=4), 24 hours (n=4), and 72 hours (n=4) after LPS instillation. The results showed that TNF-α peaked at 6 hours after LPS and gradually decreased; IL-6 peaked at 24 hours after LPS and gradually decreased to baseline at 72 hours (Figures 1D, E). The miR-145 messenger RNA (mRNA) expression was measured at 6, 24, and 72 hours after LPS instillation using qPCR. The results showed that with LPS instillation, the miR-145 expression was significantly decreased (Figure 1F).
Figure 1.
Figure 1.
Rat lung microRNA-145 expression with LPS instillation. A: H&E staining, ×10; red star: lymphocytes infiltration; blue arrow: protein-like fluid filled in alveola; black arrow: alveolar interval thickened; a: control lung; b: 6 hours after LPS instillation; c: 24 hours after LPS instillation; d: 72 hours after LPS instillation; LPS: lipopolysaccharide; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; **P<0.01.
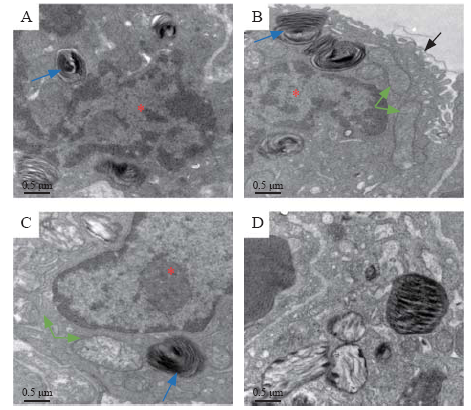
Mitochondrial dysfunction found in the epithelial cells of LPS-induced ARDS rat lung
The ultrastructure of alveolar epithelial cells was detected using a transmission electron microscope. There was a clear nuclear and lamellar bodies in the control lung epithelial cells, and the electron density of mitochondria was homogeneous. At 6 hours after LPS, the villi of epithelial cells turned incomplete, while there were still intact lamellar bodies and mitochondria. At 24 hours after LPS injection, the structures of the lamellar bodies and mitochondrial cristae were in disorder, and the electron density of mitochondria was gradually increased. At 72 hours after LPS injection, there was obvious structure disturbance of mitochondria and lamellar bodies, with the absence of mitochondrial cristae, which was the obvious evidence of mitochondrial injury (Figures 2A-D).
Figure 2.
Figure 2.
TEM structure of alveolar epithelial cell (×20,000). Red star: nuclear of alveolar epithelial cell; blue arrow: lamellar bodies; green arrow: mitochondria; black arrow: villus of alveolar epithelial cell; A: control lung (72 hours after PBS); B: 6 hours after LPS instillation; C: 24 hours after LPS instillation; D: 72 hours after LPS instillation; TEM: transmission electron microscope; PBS: phosphate-buffered saline; LPS: lipopolysaccharide.
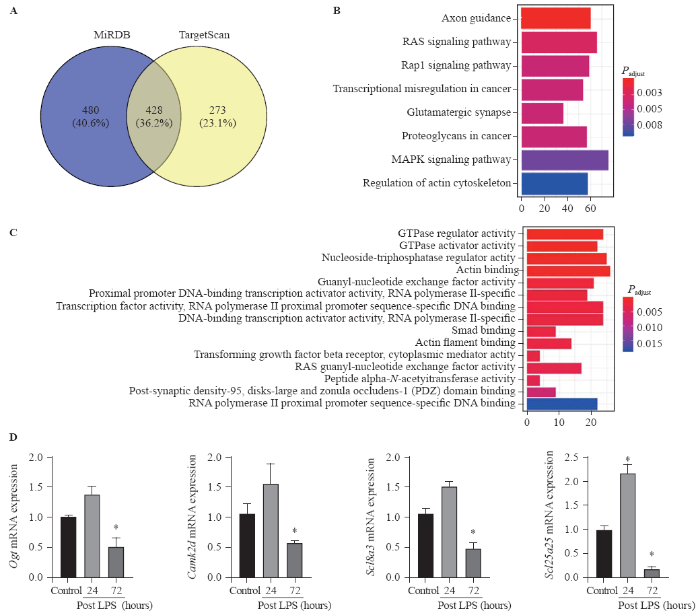
Predicted target genes of miR-145 related to mitochondrial dysfunction
TargetScan and miRDB databases were used to search miR-145 so as to predict the target genes. A total of 428 overlapping genes were identified (Figure 3A). The enrichment analysis was performed to elucidate the biological function of target genes. The KEGG pathways were significantly enriched in mitogen-activated protein kinase (MAPK) signaling pathway and RAS signaling pathway. In addition, the GO biological process was mainly enriched in gene binding, signal transduction, and transcription regulation (Figures 3B, C). Moreover, using the Rat Genome Database at the National Center for Biotechnology Information (NCBI), seven mitochondria-associated genes regulated by miR-145 were identified, including Slc1a2, Cftr, Ogt, Acs14, Camk2d, Slc8a3, and Slc25a25. All seven target genes were verified using qPCR in ARDS lungs. The results showed that Ogt, Camk2d, Slc8a3, and Slc25a25 were slightly up-regulated at 24 hours after LPS injection, while significantly down-regulated at 72 hours after LPS injection (P<0.05) (Figure 3D).
Figure 3.
Figure 3.
Bioinformatics data of microRNA-145 target genes and pathways. Compared with control, *P<0.05; A: target genes of microRNA-145 predicted using TargetScan and miRDB tools; B: KEGG pathway analysis (Y-axis representing the enriched KEGG terms, X-axis representing the amount of the microRNA-145-related mRNAs enriched in KEGG terms); C: GO biological process (Y-axis representing the enriched GO terms, X-axis representing the amount of the microRNA-145-related mRNAs enriched in GO terms); D: Ogt, Camk2d, Scl8a3, and Scl25a25 mRNAs were slightly up-regulated at 24 hours post LPS instillation, while significantly down-regulated at 72 hours post LPS instillation; MAPK: mitogen-activated protein kinase; KEGG: Kyoto Encyclopedia of Genes and Genomes; GO: Gene Ontology; LPS: lipopolysaccharide.
DISCUSSION
ARDS is one of the most critical diseases in intensive care units (ICUs), which seriously affects the prognosis and life quality of critically ill patients. It is characterized by the acute onset of respiratory failure associated with diffuse interstitial pulmonary edema in the absence of left ventricular failure. It has been proved that the degeneration of surfactant is one of the most important causes of ARDS. Reduced secretion of surfactant is associated with worse outcome. A potential role for intact mitochondria in surfactant production and secretion is supported by studies reporting that intramitochondrial delivery of glutathione in rats significantly preserved surfactant producing and secreting functions of type II cells.[26]
LPS could induce inflammatory responses in various diseases, including ARDS.[21,22,23] The intratracheal instillation of LPS is proved to be an excellent in vivo model of lung injury, and it is widely used for investigating ARDS. In our study, the results revealed that LPS up-regulated IL-6 and TNF-α expression, promptly stimulated cytokines responses, and significantly damaged the epithelial barrier. Inflammatory cells infiltrated in the alveolar air space and the para-vascular space, and the separation of alveola was much thickened after LPS instillation, which would finally form the hyaloid membrane in ARDS. With these pathological manifestations, we scored the inflammatory and structure disruption levels based on the previous study,[25] and we concluded that intratracheal instillation of LPS was a convenient and sufficient way to set up an ARDS model in rats.
The miRNAs are small non-coding RNAs that play a crucial role in many disease processes, including malignancy and inflammatory processes. Abnormal expression of miRNAs, such as miR-126-5p, miR-1246, miR-34a, miR-27a, and miR-223, has been found in lung injury.[23,27-30] MiR-145 is an important molecular marker, which has been proven to mediate cell proliferation, cell cycle, apoptosis, and invasion.[15] Researchers also found that miR-145 played a role in cervical epithelial cell barrier.[18] These studies demonstrated that miR-145 was associated with epithelial cell injury in cancer. However, whether miR-145 was involved in regulating LPS-induced ARDS remains unknown.
In our study, we found that the expression of miR-145 decreased in ARDS lungs, which was corresponding to the level of mitochondrial damage observed by transmission electron microscope (TEM) in lung epithelial cells. We found that the electron density of mitochondria began to increase at 24 hours after LPS injection, and there was obvious structure disturbance of mitochondria and lamellar bodies, as well as with the absence of mitochondrial cristae. As described in previous studies, miR-145 played an important role in regulating the mitochondrial apoptotic pathway in tumor cells, partly through its ability to target various anti-apoptotic molecules.[31] Moreover, the abnormal expression of miR-145 was associated with vascular smooth muscle cells’ response to hydrogen peroxide-induced oxidative stress, indicating that miR-145 may participate in the regulation of the oxidative stress-triggered apoptosis and the regulation of the mitochondrial apoptotic pathway. Furthermore, programmed cell death 4 (PDCD4) was identified as a novel target of miR-145 in cardiomyocyte, and the overexpression of PDCD4 could remarkably restore the miR-145-inhibited cardiomyocyte apoptosis and mitochondrial dysfunction after hypoxia injury.[32] However, little is known about whether miR-145 is associated with lung epithelial cell apoptosis or how it interferes with the mitochondrial apoptotic pathway.
Previous studies have reported that inherited mitochondrial polymorphisms, genes, and pathways were associated with epithelial ovarian cancer risk, including TERF and PPARGC1a.[33] Cystic fibrosis transmembrane conductance regulator (CFTR) silencing results in lipid homeostasis disruption and mitochondrial dysfunction in intestinal epithelial cells, and it regulates neuronal apoptosis following cerebral I/R via mitochondrial oxidative stress-dependent pathway.[34,35] Ogt is catalytically active in vivo and supports mitochondrial structure, health, and survival.[36] In our study, we found that Ogt, Camk2d, Slc8a3, and Slc25a25 were significantly down-regulated at 72 hours after LPS injection, which verified the results that Ogt, Camk2d, Slc8a3, and Slc25a25 were target genes of miR-145. Further studies are needed to confirm how miR-145 regulates its target genes, and to confirm the pathways we speculated from the bioinformatics data.
CONCLUSIONS
The current study provided evidence related to the role of miR-145 in mitochondrial function in LPS-induced ARDS. The miR-145 was down-regulated in LPS-induced lung injury, which might affect its downstream genes targeting mitochondrial functions such as Ogt, Camk2d, Slc8a3, and Slc25a25. Bioinformatics data indicated that the regulation of miR-145 may be through MAPK and RAS signaling pathways. These results provide evidence that miR-145 may play a role in inflammatory-related epithelial barrier disruption, and further studies are needed to elucidate the specific mechanisms.
Funding: None.
Ethical approval: All applicable international, national, and institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants.
Conflicts of interest: The authors declare that they have no competing interests.
Contributors: YH and SCM contributed equally to this work. CYT conceived the project, conducted the study, and gave administrative support to this study. SCM and JLW analyzed and interpreted the data. WW, MZ, and SLD contributed to data acquisition. MM helped with data analysis. ZJS and YJX revised the manuscript. CYT, ZJS, and YJX were co-corresponding authors. All authors read and approved the final manuscript.
Reference
Incidence and outcomes of acute lung injury
DOI:10.1056/NEJMoa050333
URL
PMID:16236739
[Cited within: 1]

BACKGROUND: Acute lung injury is a critical illness syndrome consisting of acute hypoxemic respiratory failure with bilateral pulmonary infiltrates that are not attributed to left atrial hypertension. Despite recent advances in our understanding of the mechanism and treatment of acute lung injury, its incidence and outcomes in the United States have been unclear. METHODS: We conducted a prospective, population-based, cohort study in 21 hospitals in and around King County, Washington, from April 1999 through July 2000, using a validated screening protocol to identify patients who met the consensus criteria for acute lung injury. RESULTS: A total of 1113 King County residents undergoing mechanical ventilation met the criteria for acute lung injury and were 15 years of age or older. On the basis of this figure, the crude incidence of acute lung injury was 78.9 per 100,000 person-years and the age-adjusted incidence was 86.2 per 100,000 person-years. The in-hospital mortality rate was 38.5 percent. The incidence of acute lung injury increased with age from 16 per 100,000 person-years for those 15 through 19 years of age to 306 per 100,000 person-years for those 75 through 84 years of age. Mortality increased with age from 24 percent for patients 15 through 19 years of age to 60 percent for patients 85 years of age or older (P<0.001). We estimate that each year in the United States there are 190,600 cases of acute lung injury, which are associated with 74,500 deaths and 3.6 million hospital days. CONCLUSIONS: Acute lung injury has a substantial impact on public health, with an incidence in the United States that is considerably higher than previous reports have suggested.
Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review
DOI:10.1164/rccm.200805-722OC
URL
PMID:19011152
[Cited within: 1]

RATIONALE: It is commonly stated that mortality from acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) is decreasing. OBJECTIVES: To systematically review the literature assessing ARDS mortality over time and to determine patient- and study-level factors independently associated with mortality. METHODS: We searched multiple databases (MEDLINE, EMBASE, CINAHL, Cochrane CENTRAL) for prospective observational studies or randomized controlled trials (RCTs) published during the period 1984 to 2006 that enrolled 50 or more patients with ALI/ARDS and reported mortality. We pooled mortality estimates using random-effects meta-analysis and examined mortality trends before and after 1994 (when a consensus definition of ALI/ARDS was published) and factors associated with mortality using meta-regression models. MEASUREMENTS AND MAIN RESULTS: Of 4,966 studies, 89 met inclusion criteria (53 observational, 36 RCTs). There was a total of 18,900 patients (mean age 51.6 years; 39% female). Overall pooled weighted mortality was 44.3% (95% confidence interval [CI], 41.8-46.9). Mortality decreased with time in observational studies conducted before 1994; no temporal associations with mortality were demonstrated in RCTs (any time) or observational studies (after 1994). Pooled mortality from 1994 to 2006 was 44.0% (95% CI, 40.1-47.5) for observational studies, and 36.2% (95% CI, 32.1-40.5) for RCTs. Meta-regression identified study type (observational versus RCT, odds ratio, 1.36; 95% CI, 1.08-1.73) and patient age (odds ratio per additional 10 yr, 1.27; 95% CI, 1.07-1.50) as the only factors associated with mortality. CONCLUSIONS: A decrease in ARDS mortality was only seen in observational studies from 1984 to 1993. Mortality did not decrease between 1994 (when a consensus definition was published) and 2006, and is lower in RCTs than observational studies.
Acute respiratory distress syndrome
URL PMID:28792873 [Cited within: 1]
The acute respiratory distress syndrome: pathogenesis and treatment
URL PMID:20936936 [Cited within: 1]
Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome
DOI:10.1177/0885066606287045
URL
PMID:16672636
[Cited within: 1]

Acute lung injury and the acute respiratory distress syndrome are common syndromes with a high mortality rate that affect both medical and surgical patients. Better understanding of the pathophysiology of acute lung injury and the acute respiratory distress syndrome and advances in supportive care and mechanical ventilation have led to improved clinical outcomes since the syndrome was first described in 1967. Although several promising pharmacological therapies, including surfactant, nitric oxide, glucocorticoids and lysofylline, have been studied in patients with acute lung injury and the acute respiratory distress syndrome, none of these pharmacological treatments reduced mortality. This article provides an overview of pharmacological therapies of acute lung injury and the acute respiratory distress syndrome tested in clinical trials and current recommendations for their use as well as a discussion of potential future pharmacological therapies including beta(2)-adrenergic agonist therapy, keratinocyte growth factor, and activated protein C.
Contribution of neutrophils to acute lung injury
URL PMID:21046059 [Cited within: 1]
Phenotypic heterogeneity in lung capillary and extra-alveolar endothelial cells. Increased extra-alveolar endothelial permeability is sufficient to decrease compliance
URL PMID:17950075 [Cited within: 1]
MicroRNAs: genomics, biogenesis, mechanism, and function
DOI:10.1016/s0092-8674(04)00045-5
URL
PMID:14744438
[Cited within: 1]

MicroRNAs (miRNAs) are endogenous approximately 22 nt RNAs that can play important regulatory roles in animals and plants by targeting mRNAs for cleavage or translational repression. Although they escaped notice until relatively recently, miRNAs comprise one of the more abundant classes of gene regulatory molecules in multicellular organisms and likely influence the output of many protein-coding genes.
MicroRNA control of cell-cell signaling during development and disease
DOI:10.4161/cc.6447
URL
PMID:18677099
[Cited within: 1]

MicroRNAs (miRNAs) are critical post-transcriptional regulators that may collectively control a majority of animal genes. With thousands of miRNAs identified, a pressing challenge is now to understand their specific biological activities. Many predicted miRNA:target interactions only subtly alter gene activity. It has consequently not been trivial to deduce how miRNAs are relevant to phenotype, and by extension, relevant to disease. We note that the major signal transduction cascades that control animal development are highly dose-sensitive and frequently altered in human disorders. On this basis, we hypothesize that developmental cell signaling pathways represent prime candidates for mediating some of the major phenotypic consequences of miRNA deregulation, especially under gain-of-function conditions. This perspective reviews the evidence for miRNA targeting of the major signaling pathways, and discusses its implications for how aberrant miRNA activity might underlie human disease and cancer.
MicroRNAs modulate hematopoietic lineage differentiation
DOI:10.1126/science.1091903
URL
PMID:14657504
[Cited within: 1]

MicroRNAs (miRNAs) are an abundant class of approximately 22-nucleotide regulatory RNAs found in plants and animals. Some miRNAs of plants, Caenorhabditis elegans, and Drosophila play important gene-regulatory roles during development by pairing to target mRNAs to specify posttranscriptional repression of these messages. We identify three miRNAs that are specifically expressed in hematopoietic cells and show that their expression is dynamically regulated during early hematopoiesis and lineage commitment. One of these miRNAs, miR-181, was preferentially expressed in the B-lymphoid cells of mouse bone marrow, and its ectopic expression in hematopoietic stem/progenitor cells led to an increased fraction of B-lineage cells in both tissue-culture differentiation assays and adult mice. Our results indicate that microRNAs are components of the molecular circuitry that controls mouse hematopoiesis and suggest that other microRNAs have similar regulatory roles during other facets of vertebrate development.
MicroRNA-127 modulates fetal lung development
URL PMID:19439715 [Cited within: 1]
MiR-17 family of microRNAs controls FGF10-mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E-Cadherin distribution
DOI:10.1016/j.ydbio.2009.06.020
URL
PMID:19559694
[Cited within: 1]

The miR-17 family of microRNAs has recently been recognized for its importance during lung development. The transgenic overexpression of the entire miR-17-92 cluster in the lung epithelium led to elevated cellular proliferation and inhibition of differentiation, while targeted deletion of miR-17-92 and miR-106b-25 clusters showed embryonic or early post-natal lethality. Herein we demonstrate that miR-17 and its paralogs, miR-20a, and miR-106b, are highly expressed during the pseudoglandular stage and identify their critical functional role during embryonic lung development. Simultaneous downregulation of these three miRNAs in explants of isolated lung epithelium altered FGF10 induced budding morphogenesis, an effect that was rescued by synthetic miR-17. E-Cadherin levels were reduced, and its distribution was altered by miR-17, miR-20a and miR-106b downregulation, while conversely, beta-catenin activity was augmented, and expression of its downstream targets, including Bmp4 as well as Fgfr2b, increased. Finally, we identified Stat3 and Mapk14 as key direct targets of miR-17, miR-20a, and miR-106b and showed that simultaneous overexpression of Stat3 and Mapk14 mimics the alteration of E-Cadherin distribution observed after miR-17, miR-20a, and miR-106b downregulation. We conclude that the mir-17 family of miRNA modulates FGF10-FGFR2b downstream signaling by specifically targeting Stat3 and Mapk14, hence regulating E-Cadherin expression, which in turn modulates epithelial bud morphogenesis in response to FGF10 signaling.
MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment
DOI:10.1172/JCI62876
URL
PMID:23281405
[Cited within: 1]

The management of cardiovascular risk through lifestyle modification and pharmacotherapy is paramount to the prevention of cardiovascular disease. Epidemiological studies have identified obesity, dyslipidemia, diabetes, and hypertension as interrelated factors that negatively affect cardiovascular health. Recently, genetic and pharmacological evidence in model systems has implicated microRNAs as dynamic modifiers of disease pathogenesis. An expanded understanding of the function of microRNAs in gene regulatory networks associated with cardiovascular risk will enable identification of novel genetic mechanisms of disease and inform the development of innovative therapeutic strategies.
MicroRNAs in vascular and metabolic disease
URL PMID:22302757 [Cited within: 1]
MicroRNA-145 inhibits proliferation and promotes apoptosis of HepG2 cells by targeting ROCK1 through the ROCK1/NF-κB signaling pathway
URL PMID:31002128 [Cited within: 3]
Characteristics of microRNAs and their potential relevance for the diagnosis and therapy of the acute respiratory distress syndrome: from bench to bedside
DOI:10.1016/j.trsl.2015.11.004
URL
PMID:26687392
[Cited within: 1]

Acute respiratory distress syndrome (ARDS) is a complex disease associated with high morbidity and mortality. Biomarkers and specific pharmacologic treatment of the syndrome are lacking. MicroRNAs (miRNAs) are small ( approximately 19-22 nucleotides) noncoding RNA molecules whose function is the regulation of gene expression. Their uncommon biochemical characteristics (eg, their resistance to degradation because of extreme temperature and pH fluctuations, freeze-thaw cycles, long storage times in frozen conditions, and RNAse digestion) and their presence in a wide range of different biological fluids and the relatively low number of individual miRNAs make these molecules good biomarkers in different clinical conditions. In addition, miRNAs are suitable therapeutic targets as their expression can be modulated by different available strategies. The aim of the present review is to offer clinicians a global perspective of miRNA, covering their structure and nomenclature, biogenesis, effects on gene expression, regulation of expression, and features as disease biomarkers and therapeutic targets, with special attention to ARDS. Because of the early stage of research on miRNAs applied to ARDS, attention has been focused on how knowledge sourced from basic and translational research could inspire future clinical studies.
SNAI2 modulates colorectal cancer 5-fluorouracil sensitivity through miR145 repression
DOI:10.1158/1535-7163.MCT-14-0207
URL
PMID:25249558
[Cited within: 1]

Epithelial-to-mesenchymal transition (EMT) has been associated with poor treatment outcomes in various malignancies and is inversely associated with miRNA145 expression. Therefore, we hypothesized that SNAI2 (Slug) may mediate 5-fluorouracil (5FU) chemotherapy resistance through inhibition of miR145 in colorectal cancer and thus represents a novel therapeutic target to enhance current colorectal cancer treatment strategies. Compared with parental DLD1 colon cancer cells, 5FU-resistant (5FUr) DLD1 cells demonstrated features of EMT, including >2-fold enhanced invasion (P < 0.001) and migration, suppressed E-cadherin expression, and 2-fold increased SNAI2 expression. DLD1 and HCT116 cells with stable expression of SNAI2 (DLD1/SNAI2; HCT116/SNAI2) also demonstrated EMT features such as the decreased E-cadherin as well as significantly decreased miR145 expression, as compared with control empty vector cells. On the basis of an miR145 luciferase promoter assay, we demonstrated that SNAI2 repressed activity of the miR145 promoter in the DLD1 and HCT116 cells. In addition, the ectopic expressing SNAI2 cell lines demonstrated decreased 5FU sensitivity, and, conversely, miR145 replacement significantly enhanced 5FU sensitivity. In the parental SW620 colon cancer cell line with high SNAI2 and low miR145 levels, inhibition of SNAI2 directly with short hairpin sequence for SNAI2 and miR145 replacement therapy both decreased vimentin expression and increased in vitro 5FU sensitivity. In pretreatment rectal cancer patient biopsy samples, low miR145 expression levels correlated with poor response to neoadjuvant 5FU-based chemoradiation. These results suggested that the SNAI2:miR145 pathway may represent a novel clinical therapeutic target in colorectal cancer and may serve as a response predictor to chemoradiation therapy.
MiR-143 and miR-145 disrupt the cervical epithelial barrier through dysregulation of cell adhesion, apoptosis and proliferation
DOI:10.1038/s41598-017-03217-7
URL
PMID:28596604
[Cited within: 2]

Molecular mechanisms regulating preterm birth (PTB)-associated cervical remodeling remain unclear. Prior work demonstrated an altered miRNA profile, with significant increases in miR-143 and miR-145, in cervical cells of women destined to have a PTB. The study objective was to determine the effect of miR-143 and miR-145 on the cervical epithelial barrier and to elucidate the mechanisms by which these miRNAs modify cervical epithelial cell function. Ectocervical and endocervical cells transfected with miR-negative control, miR-143 or miR-145 were used in cell permeability and flow cytometry assays for apoptosis and proliferation. miR-143 and miR-145 target genes associated with cell adhesion, apoptosis and proliferation were measured. Epithelial cell permeability was increased in miR-143 and miR-145 transfected cervical epithelial cells. Cell adhesion genes, JAM-A and FSCN1, were downregulated with overexpression of miR-143 and miR-145. miR-143 and miR-145 transfection decreased cervical cell number by increasing apoptosis and decreasing cell proliferation through initiation of cell cycle arrest. Apoptosis genes, BCL2 and BIRC5, and proliferation genes, CDK1 and CCND2, were repressed by miR-143 and miR-145. These findings suggest that miR-143 and miR-145 play a significant role in cervical epithelial barrier breakdown through diverse mechanisms and could contribute to premature cervical remodeling associated with PTB.
Long non-coding RNA MALAT1 functions as a mediator in cardioprotective effects of fentanyl in myocardial ischemia-reperfusion injury
DOI:10.1002/cbin.10701
URL
PMID:27862640
[Cited within: 1]

Long non-coding (lncRNA) MALAT1 can be increased by hypoxia or ischemic limbs. Also, downregulation of MALAT1 contributes to reduction of cardiomyocyte apoptosis. However, the functional involvement of MALAT1 in myocardial ischemia-reperfusion (I/R) injury has not been defined. This study investigated the functional involvement of lncRNA-MALAT1 in cardioprotective effects of fentanyl. HL-1, a cardiac muscle cell line from the AT-1 mouse atrial cardiomyocyte tumor lineage was pre-treated with fentanyl and generated cell model of hypoxia-reoxygenation (H/R). Relative expression of MALAT1, miR-145, and Bnip3 mRNA in cells was determined by quantitative real-time PCR. Cardiomyocyte H/R injury was indicated by lactate dehydrogenase (LDH) release and cell apoptosis. The results showed that fentanyl abrogates expression of responsive gene for H/R and induces downregulation of MALAT1 and Bnip3 and upregulation of miR-145. We found that miR-145/Bnip3 pathway was negatively regulated by MALAT1 in H/R-HL-1 cell with or without fentanyl treatment. Moreover, both MALAT1 overexpression and miR-145 knockdown reverse cardioprotective effects of fentanyl, as indicated by increase in LDH release and cell apoptosis. The reversal effect of MALAT1 for fentanyl is confirmed in cardiac ischemia/reperfusion (I/R) mice. In summary, lncRNA-MALAT1 is sensitive to H/R injury and abrogates cardioprotective effects of Fentanyl by negatively regulating miR-145/Bnip3 pathway.
Hypoxia promotes the skewed differentiation of umbilical cord mesenchymal stem cells toward type II alveolar epithelial cells by regulating microRNA-145
URL PMID:28789953 [Cited within: 1]
Emodin weakens liver inflammatory injury triggered by lipopolysaccharide through elevating microRNA-145 in vitro and in vivo
DOI:10.1080/21691401.2019.1614015
URL
PMID:31079494
[Cited within: 2]

Hepatitis is a severe liver disease with worldwide distribution. This study explored the anti-inflammatory influence of Emodin on LPS-triggered liver injury in vitro and in vivo. In vitro, we discovered that LPS treatment triggered L-02 cell and primary hepatocyte inflammatory injury. Emodin weakened the LPS-triggered cell inflammatory injury and NF-kappaB pathway activation. Emodin alleviated LPS-stimulated decrease of miR-145 expression. Moreover, miR-145 participated in the regulation of pro-inflammatory factors expression and negatively regulated IRAK1. Besides, IRAK1 exert regulatory roles in the activation of NF-kappaB pathway. In vivo, we found that Emodin pre-treatment weakened the LPS-triggered increases of pro-inflammatory factors expression, up-regulations of AST and ALT level, liver cell apoptosis, reduction of miR-145 and enhancement of IRAK1. Our research verified that Emodin weakened LPS-triggered liver cell inflammatory injury in vitro and in vivo might be achieved by elevating miR-145, decreasing IRAK1 and then suppressing NF-kappaB pathway.
Salidroside protects ATDC5 cells against lipopolysaccharide-induced injury through up-regulation of microRNA-145 in osteoarthritis
DOI:10.1016/j.intimp.2018.12.041
URL
PMID:30586667
[Cited within: 2]

BACKGROUND: Osteoarthritis (OA) is a kind of degenerative disease characterized by the degeneration of the articular cartilage. Salidroside (SAL) is an active component of Rhodiola rosea L., which exhibits diverse pharmacological effects in different diseases. However, the effects of SAL on OA remain largely unclear. The study aimed to investigate the roles of SAL in lipopolysaccharides (LPS)-induced inflammatory injury in murine ATDC5 chondrocyte cells. METHODS: LPS induced ATDC5 cell injury model was constructed by determining cell viability, apoptosis, apoptosis-associated factors as well as inflammatory cytokines expressions and concentrations. Then, the various concentrations of SAL were used to treat ATDC5 cells, and the effect of SAL on LPS-induce inflammatory injury was detected. After treatment with SAL, the expression level of miR-145 was measured by qRT-PCR. Subsequently, miR-145 inhibitor and corresponding control were transfected into ATDC5 cells to explore the influences of miR-145 in LPS-induce inflammatory injury. Besides, the key signaling pathways of NF-kappaB and p38MAPK were analyzed by using western blot. RESULTS: LPS inhibited cell viability, induced apoptosis, activated cleaved-caspase-3/-9 expression, as well as increased IL-6, MCP-1 and TNF-alpha expressions and secretions in ATDC5 cells. SAL significantly alleviated LPS-induced inflammatory injury. Meanwhile, the expression of miR-145 was up-regulated by SAL. The protective effect of SAL on LPS-induced injury was obviously reversed by miR-145 inhibition. Furthermore, SAL inactivated NF-kappaB and p38MAPK signaling pathways by regulating miR-145. CONCLUSIONS: These findings suggested that SAL could protect ATDC5 cells against LPS-induced injury via up-regulation of miR-145 in ATDC5 chondrocyte cells.
MicroRNA-27a alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis through modulating TLR4/MyD88/NF-κB pathway
DOI:10.1080/15384101.2018.1509635
URL
PMID:30231673
[Cited within: 3]

Acute lung injury (ALI) is a critical clinical condition with a high mortality rate, characterized with excessive uncontrolled inflammation and apoptosis. Recently, microRNAs (miRNAs) have been found to play crucial roles in the amelioration of various inflammation-induced diseases, including ALI. However, it remains unknown the biological function and regulatory mechanisms of miRNAs in the regulation of in fl ammation and apoptosis in ALI. The aim of this study is to identify and evaluate the potential role of miRNAs in ALI and reveal the underlying molecular mechanisms of their effects. Here, we analyzed microRNA expression profiles in lung tissues from LPS-challenged mice using miRNA microarray. Because microRNA-27a (miR-27a) was one of the miRNAs being most significantly downregulated, which has an important role in regulation of inflammation, we investigated its function. Overexpression of miR-27a by agomir-27a improved lung injury, as evidenced by the reduced histopathological changes, lung wet/dry (W/D) ratio, lung microvascular permeability and apoptosis in the lung tissues, as well as ameliorative survival of ALI mice. This was accompanied by the alleviating of inflammation, such as the reduced total BALF cell and neutrophil counts, decreased levels of tumor necrosis factor alpha (TNF-alpha), interleukin-1 (IL-6) interleukin-1beta (IL-1beta) and myeloperoxidase (MPO) activity in BAL fluid. Toll-like receptor 4 (TLR4), an important regulator of the nuclear factor kappa-B (NF-kappaB) signaling pathway, was identified as a novel target of miR-27a in RAW264.7 cells. Furthermore, our results showed that LPS stimulation increased the expression of MyD88 and NF-kappaB p65 (p-p65), but inhibited the expression of inhibitor of nuclear factor-kappaB-alpha (IkappaB-alpha), suggesting the activation of NF-kappaB signaling pathway. Further investigations revealed that agomir-miR-27a reversed the promoting effect of LPS on NF-kappaB signaling pathway. The results here suggested that miR-27a alleviates LPS-induced ALI in mice via reducing in fl ammation and apoptosis through blocking TLR4/MyD88/NF-kappaB activation.
Processing tissue and cells for transmission electron microscopy in diagnostic pathology and research
URL PMID:17947985 [Cited within: 1]
An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals
DOI:10.1165/rcmb.2009-0210ST
URL
PMID:21531958
[Cited within: 2]

Acute lung injury (ALI) is well defined in humans, but there is no agreement as to the main features of acute lung injury in animal models. A Committee was organized to determine the main features that characterize ALI in animal models and to identify the most relevant methods to assess these features. We used a Delphi approach in which a series of questionnaires were distributed to a panel of experts in experimental lung injury. The Committee concluded that the main features of experimental ALI include histological evidence of tissue injury, alteration of the alveolar capillary barrier, presence of an inflammatory response, and evidence of physiological dysfunction; they recommended that, to determine if ALI has occurred, at least three of these four main features of ALI should be present. The Committee also identified key
Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats
URL
PMID:10924012
[Cited within: 1]

BACKGROUND: Chronic alcohol abuse increases the incidence and severity of acute lung injury in critically ill patients. Previously we determined that ethanol ingestion in rats dramatically decreased alveolar epithelial cellular levels of glutathione and surfactant synthesis and secretion in vitro. Previous studies in alcoholic liver disease suggest that mitochondrial glutathione levels, and not cellular levels per se, are involved in the pathogenesis of ethanol-mediated hepatotoxicity. Therefore, we hypothesized that alveolar epithelial mitochondrial glutathione depletion mediates the observed defects in surfactant synthesis and secretion in ethanol-fed rats. METHODS: Male Sprague-Dawley rats were fed the Lieber-DeCarli liquid diet with or without ethanol (36% of total calories) for 6 weeks. In some experiments, ethanol-fed rats were then switched to the control diet for 1 week, with or without glutathione supplementation with either N-acetylcysteine (NAC) or procysteine (PRO). Alveolar epithelial type II cells were then isolated and glutathione levels (cytosolic and mitochondrial) and surfactant production (synthesis and secretion) were determined. RESULTS: Ethanol ingestion decreased (p < 0.05) mitochondrial and cytosolic levels of glutathione, and surfactant synthesis and secretion in isolated type II cells when compared to cells from control-fed rats. NAC treatment restored (p < 0.05) cytosolic but not mitochondrial glutathione levels (p > 0.05), and had no effect (p > 0.05) on surfactant synthesis and secretion in type II cells isolated from ethanol-fed rats. In contrast, PRO treatment restored (p < 0.05) cytosolic and mitochondrial glutathione levels, and normalized (p < 0.05) surfactant synthesis and secretion in type II cells isolated from ethanol-fed rats. CONCLUSIONS: These results suggest that mitochondrial, and not simply cytosolic, replacement of glutathione is necessary to improve surfactant function in critically ill patients with a history of alcohol abuse.
Down-regulation of microRNA-126-5p contributes to overexpression of VEGFA in lipopolysaccharide-induced acute lung injury
URL PMID:27146208 [Cited within: 1]
p53 and miR-34a feedback promotes lung epithelial injury and pulmonary fibrosis
DOI:10.1016/j.ajpath.2016.12.020
URL
PMID:28273432

Idiopathic pulmonary fibrosis (IPF) is a progressive and fatal interstitial lung disease. The pathogenesis of interstitial lung diseases, including its most common form, IPF, remains poorly understood. Alveolar epithelial cell (AEC) apoptosis, proliferation, and accumulation of myofibroblasts and extracellular matrix deposition results in progressive loss of lung function in IPF. We found induction of tumor suppressor protein, p53, and apoptosis with suppression of urokinase-type plasminogen activator (uPA) and the uPA receptor in AECs from the lungs of IPF patients, and in mice with bleomycin, cigarette smoke, silica, or sepsis-induced lung injury. Treatment with the caveolin-1 scaffolding domain peptide (CSP) reversed these effects. Consistent with induction of p53, AECs from IPF lungs or mice with diverse types of lung injuries showed increased p53 acetylation and miR-34a expression with reduction in Sirt1. This was significantly reduced after treatment of wild-type mice with CSP, and uPA-deficient mice were unresponsive. Bleomycin failed to induce miR-34a in p53- or plasminogen activator inhibitor-1 (PAI-1)-deficient mice. CSP-mediated inhibition of miR-34a restored Sirt1, suppressed p53 acetylation and apoptosis in injured AECs, and prevented pulmonary fibrosis (PF). AEC-specific suppression of miR-34a inhibited bleomycin-induced p53, PAI-1, and apoptosis and prevented PF, whereas overexpression of precursor-miR-34a increased p53, PAI-1, and apoptosis in AECs of mice unexposed to bleomycin. Our study validates p53-miR-34a feedback as a potential therapeutic target in PF.
Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice
MicroRNA-1246 mediates lipopolysaccharide-induced pulmonary endothelial cell apoptosis and acute lung injury by targeting angiotensin-converting enzyme 2
URL PMID:28386354 [Cited within: 1]
Quercetin induces the apoptosis of human ovarian carcinoma cells by upregulating the expression of microRNA-145
DOI:10.3892/mmr.2015.3679
URL
PMID:25937243
[Cited within: 1]

Ovarian cancer is one of the most malignant types of cancer of the female human reproductive track, posing a severe threat to the health of the female population. Numerous previous studies have demonstrated that microRNA (miR)-145 is downregulated in ovarian cancer, and that quercetin can inhibit the growth of cancer cells via regulating the expression of miRs. Therefore, the present study investigated the effect of quercetin on the expression of miR-145 in SKOV-3 and A2780 human ovarian cancer cell lines. The results revealed that the expression levels of cleaved caspase-3 in the SKOV-3 and A2780 cells were significantly increased following treatment to induce overexpression of miR-145 compared with treatment with quercetin alone (P<0.01). However, the expression of cleaved caspase-3 in the anti-miR-145 (miR-145 inhibitor) group of cells was markedly decreased compared with that in the miR-145 overexpression group (P<0.01). Taken together, the results suggested that treatment with quercetin induced the apoptosis of human ovarian carcinoma cells through activation of the extrinsic death receptor mediated and intrinsic mitochondrial apoptotic pathways.
Overexpression of microRNA-145 protects against rat myocardial infarction through targeting PDCD4
URL
PMID:29218098
[Cited within: 1]

Myocardial infarction (MI) is a common cardiovascular disease with high mortality. The aim of the present study was to determine the biological role of miR-145 in MI rats and hypoxia-injured cardiomyocytes and to elucidate the potential mechanism. MI rats were induced by left anterior descending artery (LAD) ligation. qRT-PCR and western blot analysis were performed to determine the mRNA and protein levels, respectively. Compared with sham group, miR-145 levels in MI group were significantly decreased. We observed that lentivirus-mediated overexpression of miR-145 significantly improves cardiac function, reduces infarcted tissue size and prevents post-infarction induced apoptosis in rats after MI. Furthermore, PDCD4 was identified as a novel target of miR-145 in cardiomyocytes, and overexpression of PDCD4 could remarkably restore the miR-145-inhibited cardiomyocytes apoptosis and mitochondrial dysfunction after hypoxia injury. Therefore, our study indicated that miR-145/PDCD4 axis might be potential therapeutic targets for the treatment of MI, and its cardioprotective effect may be attributed to a reduction of mitochondria-mediated apoptosis.
Inherited variants in mitochondrial biogenesis genes may influence epithelial ovarian cancer risk
DOI:10.1158/1055-9965.EPI-10-1224
URL
PMID:21447778
[Cited within: 1]

BACKGROUND: Mitochondria contribute to oxidative stress, a phenomenon implicated in ovarian carcinogenesis. We hypothesized that inherited variants in mitochondrial-related genes influence epithelial ovarian cancer (EOC) susceptibility. METHODS: Through a multicenter study of 1,815 Caucasian EOC cases and 1,900 controls, we investigated associations between EOC risk and 128 single nucleotide polymorphisms (SNPs) from 22 genes/regions within the mitochondrial genome (mtDNA) and 2,839 nuclear-encoded SNPs localized to 138 genes involved in mitochondrial biogenesis (BIO, n = 35), steroid hormone metabolism (HOR, n = 13), and oxidative phosphorylation (OXP, n = 90) pathways. Unconditional logistic regression was used to estimate OR and 95% CI between genotype and case status. Overall significance of each gene and pathway was evaluated by using Fisher's method to combine SNP-level evidence. At the SNP level, we investigated whether lifetime ovulation, hormone replacement therapy (HRT), and cigarette smoking were confounders or modifiers of associations. RESULTS: Interindividual variation involving BIO was most strongly associated with EOC risk (empirical P = 0.050), especially for NRF1, MTERF, PPARGC1A, ESRRA, and CAMK2D. Several SNP-level associations strengthened after adjustment for nongenetic factors, particularly for MTERF. Statistical interactions with cigarette smoking and HRT use were observed with MTERF and CAMK2D SNPs, respectively. Overall variation within mtDNA, HOR, and OXP was not statistically significant (empirical P > 0.10). CONCLUSION: We provide novel evidence to suggest that variants in mitochondrial biogenesis genes may influence EOC susceptibility. IMPACT: A deeper understanding of the complex mechanisms implicated in mitochondrial biogenesis and oxidative stress may aid in developing strategies to reduce morbidity and mortality from EOC.
CFTR prevents neuronal apoptosis following cerebral ischemia reperfusion via regulating mitochondrial oxidative stress
CFTR deletion confers mitochondrial dysfunction and disrupts lipid homeostasis in intestinal epithelial cells
DOI:10.3390/nu10070836 URL [Cited within: 1]
Mitochondrial O-GlcNAc transferase (mOGT) regulates mitochondrial structure, function, and survival in HeLa cells
URL PMID:28100784 [Cited within: 1]