INTRODUCTION
The infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is spreading worldwide.[1-5] A pandemic of coronavirus disease (COVID-19) has been declared by the World Health Organization (WHO) despite many restrictive measures that have been implemented globally.[6] As of March 15, 2020, more than 1,500,000 cases have been confirmed, and around 16,000 death occurred.[6] The estimated mortality of COVID-19 is 3%-4% based on the data collected. Although this may be an overestimate as more potential cases with mild symptoms haven’t been identified, the mortality of COVID-19 is considerably higher than that caused by influenza. The early recognition and treatment of patients at a high risk of developing serious illness are critical to improving the outcome of diseases caused by microbial invasion such as sepsis.[7] Some recent studies have reported the epidemiologic and clinical features of patients with COVID-19,[ 1,2,8] but little is known regarding the characteristics of critically ill patients and how to early detect patients who are at a high risk of death. Caring for critically ill patients will be a crucial part of battling COVID-19.
In this retrospective cohort study, we aim to investigate the clinical characteristics and laboratory findings of intensive care unit (ICU) patients with COVID-19 and to identify potential indicators for early recognition of patients with a high risk of death.
METHODS
Participants
This retrospective cohort study recruited consecutive adult patients (aged ≥18 years) admitted between February 8 to February 29, 2020, to the three ICUs of Zhongfaxincheng Campus of Tongji Hospital Affiliated to Huazhong University of Science and Technology in Wuhan, which is one of the designated hospitals to receive patients with confirmed SARS-CoV-2 infection by using a quantitative polymerase chain reaction test of throat swab samples or sputum samples according to the WHO guidance. Those who met the following criteria would be considered to be transferred to the ICU: (1) respiratory rate >30 breaths per minute; (2) SpO2 <93%; (3) PaO2/FiO2 <300 mmHg (1 mmHg=0.133 kPa); (4) in presence with respiratory failure; (5) in presence with shock; (6) with other conditions that need to be monitored in the ICU. These ICUs were in charged by the medical staff from the hospitals affiliated with Peking University. This study was approved by the Ethics Committee of Peking University People’s Hospital and was conducted in accordance with the Declaration of Helsinki.
Data collection
The detailed clinical information of each patient was obtained by physicians using a standard questionnaire after they were admitted to the ICU. Clinical information including demographic data, medical history, comorbidities, symptoms, signs, laboratory findings, chest computed tomographic (CT) scans, and treatments was recorded. We also collected each patient’s clinical characteristics, including the Sequential Organ Failure Assessment (SOFA) score and quick SOFA (qSOFA) score.
To identify useful biomarkers for predicting in-hospital death of these patients, we included laboratory variables measured on day one after ICU admission in the analysis.
Laboratory measurements
The complete blood count was measured by Sysmex XN-9000 automatic hematology analyzer (Sysmex, Japan). The neutrophil-to-lymphocyte ratio (NLR) was calculated by the absolute neutrophil count divided by the absolute lymphocyte count. Myohemoglobin (Myo), creatine kinase muscle brain isoenzyme (CK-MB), and high sensitive troponin I (hs-TNI) were measured by Abbott ARCHITECT i2000SR chemiluminescence immunoanalyzer (Abbott Laboratories, USA). Coagulation parameters were performed by Stago STA-R automatic blood coagulation analyzer (Stago, France). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), direct bilirubin (DB), albumin, blood urea nitrogen (BUN), creatinine, high sensitive C-reactive protein (hs-CRP), and ferritin were measured using Roche Cobas 8000 automatic biochemical analyzer (Roche, Switzerland). Thyroid hormones, cytokines interleukin-2 receptor (IL-2R), IL-1β, IL-6, IL-8, IL-10, tumor necrosis factor-α (TNF-α), and N-terminal pro-brain natriuretic peptide (NT-proBNP) were detected by Roche Cobas e602 electrochemical luminescence analyzer (Roche, Germany). Immunoglobulin A (IgA), IgG, IgM, and complement 3 (C3) and C4 were performed by IMAGE 8000.
Outcomes
The primary outcome was in-hospital mortality. Secondary outcomes included the incidence of acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), heart failure, liver dysfunction, and coagulopathy. ARDS was defined according to the Berlin definition,[9] and AKI was diagnosed based on the Kidney Disease Improving Global Outcomes (KDIGO) criteria.[10] Heart failure was diagnosed according to the 2016 European Society of Cardiology guidelines.[11] Septic shock was defined by persisting hypotension requiring vasopressors to maintain a mean arterial pressure of 65 mmHg or higher and a serum lactate level greater than 2 mmol/L despite adequate volume resuscitation.[12]
Statistical analysis
Continuous variables were presented as median and interquartile range (IQR), and categorical variables were expressed as percentages. Baseline characteristics between the survivor and non-survivor groups were compared with the unpaired Student’s t-test or Mann-Whitney U-test for continuous variables and the Chi-square or Fisher’s exact test for categorical variables. Univariate logistic regression analyses were performed to examine the association between each predictor and in-hospital mortality separately. We also conducted a forward stepwise multivariate logistic regression to determine the independent predictors of ICU mortality. A criterion of P<0.05 for entry and P≥0.05 for removal was imposed in this procedure. Odds ratios (ORs) for continuous variables were described using standardized ORs, which were associated with a one standard deviation change in the variable. The receiver operating characteristic (ROC) curve was used to evaluate the performance of indicators to predict mortality. The curve represented a plot of sensitivity (se) vs. 1-specificity (sp). A ROC curve was also constructed for the combination of NLR and ferritin for predicting in-hospital mortality according to the Mackinnon and Mulligan’s weighted sum rule.[13] The differences between the area under the curve (AUC) (C-index) were tested by Hanley-McNeil methods.[14] A two-sided P-value of less than 0.05 was considered to indicate statistical significance. The analyses were performed with SPSS 25.0 software (SPSS Inc., Chicago, Illinois, USA) and MedCalc 18.11.3 software (MedCalc Software bvba, Ostend, Belgium).
RESULTS
Baseline characteristics
A total of 121 ICU patients were screened for eligibility during the study period. The median age was 66 (56-72) years, and 57.0% were male. Their SOFA and qSOFA scores were 1 (1-4) and 1 (1-1), respectively. A total of 35 (28.9%) patients died during their hospital stay, and 86 patients were discharged after recovery.
Compared with survivors, non-survivors were older and had worse organ function indicted by different parameters and higher Acute Physiology and Chronic Health Evaluation II (APACHE II), SOFA, and qSOFA score. More patients developed ARDS (97.1% vs. 5.8%), AKI (54.3% vs. 1.2%), septic shock (57.1% vs. 0%), heart failure (57.1% vs. 11.6%), liver dysfunction (20.0% vs. 3.5%), and coagulopathy (77.1% vs. 19.8%) in non-survivors than in survivors.
Of six different cytokines or their receptors, the level of circulating IL-2R was significantly higher in non-survivors than in survivors, while the differences in the levels of IL-6, IL-8, IL-10, and TNF-α between groups reached marginal statistic significance. There was no significant difference in the levels of IgA, IgG, IgM, C3, and C4 between survivors and non-survivors.
Predictors of in-hospital mortality
Univariate logistic regression analysis demonstrated that those who were older, and had higher SOFA and qSOFA scores had significantly greater hazard of in-hospital death. To detect potential biomarkers for the prediction of fatal outcomes of patients with COVID-19, all laboratory variables were also examined by univariate logistic regression analysis. We found a total of 28 variables were associated with in-hospital mortality (Table 1).
Table 1 Performance of variables for predicting in-hospital mortality in COVID-19 patients*
| Variables | AUC | 95% CI | Cut-off value | Sensitivity (%) | Specificity (%) | P |
|---|---|---|---|---|---|---|
| SOFA | 0.960 | 0.908-0.987 | 2 | 94.29 | 93.02 | <0.001 |
| Free triiodothyronine | 0.863 | 0.761-0.932 | 3.25 pmol/L | 94.44 | 75.93 | <0.001 |
| Neutrophil-to-lymphocyte ratio | 0.857 | 0.782-0.914 | 8.48 | 85.71 | 79.07 | <0.001 |
| Procalcitonin | 0.850 | 0.771-0.910 | 0.38 ng/mL | 84.85 | 76.54 | <0.001 |
| Neutrophil count | 0.842 | 0.764-0.902 | 5.71×109/L | 82.86 | 77.91 | <0.001 |
| Prothrombin time | 0.828 | 0.748-0.891 | 15.2 seconds | 71.43 | 85.88 | <0.001 |
| White blood cell count | 0.827 | 0.747-0.889 | 7.04×109/L | 85.71 | 70.93 | <0.001 |
| Ferritin | 0.827 | 0.744-0.892 | 862.7 μg/L | 94.12 | 58.44 | <0.001 |
| Direct bilirubin | 0.826 | 0.746-0.889 | 5.2 μmol/L | 80.00 | 74.12 | <0.001 |
| High sensitive C-reactive protein | 0.824 | 0.742-0.889 | 131.1 mg/L | 68.57 | 85.00 | <0.001 |
| Albumin | 0.811 | 0.730-0.877 | 31.9 g/L | 94.29 | 61.63 | <0.001 |
| High sensitive troponin I | 0.808 | 0.722-0.877 | 33.4 ng/mL | 73.53 | 86.84 | <0.001 |
| Total bilirubin | 0.794 | 0.711-0.862 | 10.1 μmol/L | 85.71 | 61.63 | <0.001 |
| Lactate dehydrogenase | 0.794 | 0.710-0.862 | 425 U/L | 77.14 | 76.74 | <0.001 |
| NT-proBNP | 0.793 | 0.704-0.864 | 247 pg/mL | 96.97 | 56.58 | <0.001 |
| Creatine kinase muscle brain isoenzyme | 0.793 | 0.695-0.870 | 2.2 ng/mL | 70.37 | 87.69 | <0.001 |
| Fibrin degradation products | 0.792 | 0.701-0.866 | 8.9 μg/mL | 81.25 | 69.01 | <0.001 |
| D-dimer | 0.786 | 0.702-0.856 | 2.21 μg/mL | 80.00 | 68.24 | <0.001 |
| Aspartate aminotransferase | 0.763 | 0.677-0.836 | 33 U/L | 88.57 | 60.47 | <0.001 |
| Blood urea nitrogen | 0.761 | 0.675-0.834 | 5.2 μmol/L | 91.43 | 56.98 | <0.001 |
| Lymphocyte count | 0.734 | 0.646-0.811 | 0.65×109/L | 68.57 | 74.42 | <0.001 |
| qSOFA | 0.734 | 0.646-0.810 | 0 | 97.14 | 32.56 | <0.001 |
| Myohemoglobin | 0.726 | 0.622-0.814 | 87 ng/mL | 77.78 | 60.94 | <0.001 |
| Interleukin-2 receptor | 0.720 | 0.627-0.802 | 1,215 U/mL | 73.53 | 64.47 | <0.001 |
| Glucose | 0.717 | 0.626-0.797 | 6.39 mmol/L | 77.14 | 65.43 | <0.001 |
| AT3 | 0.710 | 0.611-0.796 | 78% | 45.16 | 87.14 | <0.001 |
| Free thyroxine | 0.693 | 0.573-0.796 | 19.35 pmol/L | 83.33 | 55.56 | 0.003 |
| Alanine aminotransferase | 0.677 | 0.586-0.759 | 50 U/L | 42.86 | 88.37 | 0.001 |
| Creatine kinase | 0.664 | 0.569-0.750 | 207 U/L | 53.57 | 84.88 | 0.012 |
| Platelet count | 0.650 | 0.558-0.734 | 239×109/L | 85.71 | 44.19 | 0.008 |
| Age | 0.626 | 0.534-0.712 | 56 | 88.57 | 31.40 | 0.021 |
*: ranked by area under the curve (AUC); CI: confidence interval; SOFA: Sequential Organ Failure Assessment; qSOFA: quick SOFA; NT-proBNP: N-terminal pro-B-type natriuretic peptide; AT3: antithrombin 3.
To evaluate the performance of these laboratory variables for the prediction of in-hospital mortality, ROC curves were constructed. In addition to the SOFA score, which had the highest discriminability to predict in-hospital death, we found 12 variables with the potential ability to early detect patients at a high risk of fatality, as suggested by AUC greater than 0.80. The D-dimer, which was reported as a risk factor of death in a previous study,[15] had a relatively lower ability to identify patients with a high risk of fatality than those variables, including other coagulation parameters prothrombin time (PT) and fibrinogen degradation product (FDP).
When each of these variables was included in a stepwise multiple logistic model in which in-hospital mortality was the dependent variable, all variables could independently predict the primary outcome.
Values of neutrophil/lymphocyte, triiodothyronine (T3), and ferritin in predicting in-hospital mortality
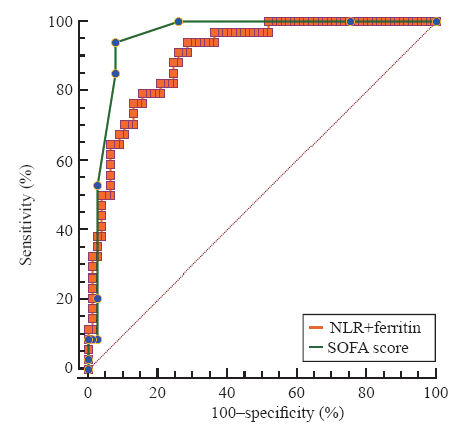
Among these 12 variables, some of them are unspecific markers of inflammation such as white blood cells, neutrophils, hs-CRP, and procalcitonin, while others represent the dysfunction of different organs, including direct bilirubin, albumin, PT, hs-TNI, and international normalized ratio of PT (INR-PT). We further evaluated biomarkers, including NLR, free triiodothyronine (FT3), and ferritin. The AUCs for NLR, FT3, and ferritin were 0.857, 0.863, and 0.827, and the optimal cut-off values were 8.48, 3.25 pmol/L, and 863 μg/L, respectively. The AUCs of these three biomarkers were lower than that of SOFA score. However, the combination of NLR and ferritin had a comparable AUC with SOFA score to predict the in-hospital mortality (0.901 vs. 0.955, P=0.085) (Figure 1).
Figure 1.
Figure 1.
Receiver operating characteristic curve for the combination of neutrophil-to-lymphocyte ratio (NLR) and ferritin as compared with Sequential Organ Failure Assessment (SOFA) score in predicting in-hospital mortality in COVID-19 patients.
DISCUSSION
In this retrospective cohort study, we found that the infection of SARS-CoV-2 not only caused lung injury but also targeted other organs to induce multiple-organ dysfunction, including ARDS, AKI, and septic shock. These acute organ dysfunctions combined with older age were associated with fatal outcomes of these patients. In addition, this study investigated in detail the laboratory parameters and found biomarkers that can be used to identify COVID-19 patients at a high risk of death.
A previous study reported that COVID-19 patients with worse outcome tended to be older, had more underlying medical conditions, and were more likely to have organ dysfunction than those survived.[16] A later study, which confirmed previous findings, further demonstrated that age, D-dimer, and SOFA scores were independently associated with in-hospital mortality in critically ill patients with COVID-19.[15] Our current study extended these findings by investigating more biomarkers and their performance to predict a fatal outcome. We found biomarkers with moderate-to-high predictive performance ability for poor outcomes, including NLR, FT3, and ferritin. The calculation of SOFA scores, which is comprised of six variables, can be time-consuming. This might limit its application in clinical practice, especially when facing the heavy burdens on the health care system during the pandemic. Therefore, the current study provides useful information to quickly evaluate the patients at a high risk of death. Importantly, the combination of NLR and ferritin is as powerful as the SOFA score for the prediction of in-hospital mortality.
The NLR is an easily accessed indicator that combines both the changes in neutrophils and lymphocytes. NLR has been gaining growing attraction in many fields of medicine in the past decade.[17] It is more sensitive than neutrophils and lymphocytes alone to detect inflammation and predict the outcome of different diseases.[18,19,20] Under physiologic stress, its level can be increased by endogenous cortisol and catecholamines. Our study demonstrates that NLR has a potential ability to identify COVID-19 patients at a high risk of death, indicating that a higher inflammation and physiologic stress existed in non-survival patients with COVID-19.
Ferritin is a regulator of iron homeostasis.[21] Growing evidence showed that it can be served as a biomarker of inflammation.[22] Moreover, ferritin also has a pathogenic role in inflammatory diseases by directly modulating lymphocyte function.[23] During the acute phase of infection, macrophages and other cells secrete ferritin to suppress overactive inflammatory responses that cause so-called hyperferritinemia syndrome.[23,24] This process is believed to contribute to cytokine storm.[23] Our study found that the level of circulating ferritin was significantly higher in non-survivors than in survivors, and can be used as a useful biomarker to predict worse outcomes of patients with COVID-19. As a coincidence, a very recent study found that ferritin was a predictor of poor outcomes in patients with influenza infection.[25] In addition, some effective treatments of inflammatory diseases have been found to benefit from targeting ferritin.[23] The potential therapeutic target of ferritin is worthy of further study.
Changes in circulating hormone levels are a common phenomenon during critical illness.[26] Thyroid hormones play a key role in the maintenance of body growth by modulating metabolism and the immune system. Our previous study[27] and others demonstrated that reduced thyroid hormones were associated with the severity of the diseases and the outcomes of critically ill patients. These alterations of thyroid hormone levels are referred to as “euthyroid sick syndrome” or “nonthyroidal illness syndrome (NTIS)”.[28] This study found FT3 was significantly lower in non-survivors than in survivors, indicating that it reflected the severity of COVID-19. Indeed, FT3 is associated with the SOFA score and has a moderate-to-excellent ability to predict in-hospital mortality of patients with COVID-19. The reduced circulating FT3 is a predictor of in-hospital mortality, indicating that the infection of SARS-CoV-2 may suppress the production of FT3 or promote the clearance of it. The underlying mechanism of this is however unclear and needs to be investigated by further studies.
Cytokines play important roles in regulating the immune response to microbial infection. However, the unregulated release of cytokines, so-called cytokine storm, can lead to multiple organ injury and cause a fatal outcome.[29] Although the cytokine storm has drawn broad attention regarding its pathogenic role in COVID-19,[30] little is known about the alteration in the level of different cytokines during the process of this disease. A recent study reported that the level of pro-inflammatory cytokine IL-6 was significantly higher in non-survivors than in survivors. Patients with elevated IL-6 had a higher risk of death.[15] However, the performance of IL-6 for the prediction of fatality was not evaluated in this study. Moreover, other major pro- and anti-inflammatory cytokines were not investigated. The current study evaluated six different cytokines or their receptors and found that IL-2R was significantly higher in non-survivors than in survivors. The current study found, besides IL-6, pro-inflammatory cytokines and their receptors (IL-8, TNF-α, IL-2) as well as anti-inflammatory cytokines (IL-10) were also higher in non-survivor, indicating that a higher degree of both pro- and anti-inflammatory occurred in severer ill patients with SARS-CoV-2 infection. These cytokines and their signal pathways may serve as the potential therapeutic target for COVID-19, which requires further studies to explore.
This study has some limitations. First, this was a single-center study with 121 ICU patients recruited. The relationship between these indicators and in-hospital mortality may be changed if more patients from different centers were included. Second, we only collected the laboratory indicators at admission to ICU, and the dynamics of these indicators were unknown. However, only patients with life-threatening conditions can be transferred to the ICU, and therefore laboratory findings at day one only reflected the severity of these ICU patients at an early stage of the disease. Third, not every variable was available for all patients in our study. Thus the direct comparison of discriminability between some variables was not conducted as the number of available cases was limited. Fourth, some patients may have bacterial infection during the treatment which might affect the level of these biomarkers.
CONCLUSIONS
In this retrospective cohort study, we find three biomarkers to predict in-hospital death of patients with COVID-19, and provide important information for the risk stratification of these patients in future clinical practice.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Jennifer Shing for English editing for this paper.
Funding: This work was supported by the National Key Research and Development Project of the Ministry of Science and Technology, China (2018YFC1313700) and “Gaoyuan” Project of Pudong Health and Family Planning Commission (PWYgy2018-6).
Ethical approval: This study was approved by the Ethics Committee of Peking University People’s Hospital.
Conflicts of interest: The authors declare that they have no competing interests.
Contributors: WG and LYR contributed equally to this work. FLW and TBW conceived, designed the study, analyzed the data and wrote the paper. WG, WBG, LYR, JHZ, ZD, and QGG contributed to data acquisition and analysis.
Reference
A novel coronavirus from patients with pneumonia in China, 2019
DOI:10.1056/NEJMoa2001017
URL
PMID:31978945
[Cited within: 2]

In December 2019, a cluster of patients with pneumonia of unknown cause was linked to a seafood wholesale market in Wuhan, China. A previously unknown betacoronavirus was discovered through the use of unbiased sequencing in samples from patients with pneumonia. Human airway epithelial cells were used to isolate a novel coronavirus, named 2019-nCoV, which formed a clade within the subgenus sarbecovirus, Orthocoronavirinae subfamily. Different from both MERS-CoV and SARS-CoV, 2019-nCoV is the seventh member of the family of coronaviruses that infect humans. Enhanced surveillance and further investigation are ongoing. (Funded by the National Key Research and Development Program of China and the National Major Project for Control and Prevention of Infectious Disease in China.).
Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China
DOI:10.1001/jama.2020.1585
URL
PMID:32031570
[Cited within: 1]

Importance: In December 2019, novel coronavirus (2019-nCoV)-infected pneumonia (NCIP) occurred in Wuhan, China. The number of cases has increased rapidly but information on the clinical characteristics of affected patients is limited. Objective: To describe the epidemiological and clinical characteristics of NCIP. Design, Setting, and Participants: Retrospective, single-center case series of the 138 consecutive hospitalized patients with confirmed NCIP at Zhongnan Hospital of Wuhan University in Wuhan, China, from January 1 to January 28, 2020; final date of follow-up was February 3, 2020. Exposures: Documented NCIP. Main Outcomes and Measures: Epidemiological, demographic, clinical, laboratory, radiological, and treatment data were collected and analyzed. Outcomes of critically ill patients and noncritically ill patients were compared. Presumed hospital-related transmission was suspected if a cluster of health professionals or hospitalized patients in the same wards became infected and a possible source of infection could be tracked. Results: Of 138 hospitalized patients with NCIP, the median age was 56 years (interquartile range, 42-68; range, 22-92 years) and 75 (54.3%) were men. Hospital-associated transmission was suspected as the presumed mechanism of infection for affected health professionals (40 [29%]) and hospitalized patients (17 [12.3%]). Common symptoms included fever (136 [98.6%]), fatigue (96 [69.6%]), and dry cough (82 [59.4%]). Lymphopenia (lymphocyte count, 0.8 x 109/L [interquartile range {IQR}, 0.6-1.1]) occurred in 97 patients (70.3%), prolonged prothrombin time (13.0 seconds [IQR, 12.3-13.7]) in 80 patients (58%), and elevated lactate dehydrogenase (261 U/L [IQR, 182-403]) in 55 patients (39.9%). Chest computed tomographic scans showed bilateral patchy shadows or ground glass opacity in the lungs of all patients. Most patients received antiviral therapy (oseltamivir, 124 [89.9%]), and many received antibacterial therapy (moxifloxacin, 89 [64.4%]; ceftriaxone, 34 [24.6%]; azithromycin, 25 [18.1%]) and glucocorticoid therapy (62 [44.9%]). Thirty-six patients (26.1%) were transferred to the intensive care unit (ICU) because of complications, including acute respiratory distress syndrome (22 [61.1%]), arrhythmia (16 [44.4%]), and shock (11 [30.6%]). The median time from first symptom to dyspnea was 5.0 days, to hospital admission was 7.0 days, and to ARDS was 8.0 days. Patients treated in the ICU (n = 36), compared with patients not treated in the ICU (n = 102), were older (median age, 66 years vs 51 years), were more likely to have underlying comorbidities (26 [72.2%] vs 38 [37.3%]), and were more likely to have dyspnea (23 [63.9%] vs 20 [19.6%]), and anorexia (24 [66.7%] vs 31 [30.4%]). Of the 36 cases in the ICU, 4 (11.1%) received high-flow oxygen therapy, 15 (41.7%) received noninvasive ventilation, and 17 (47.2%) received invasive ventilation (4 were switched to extracorporeal membrane oxygenation). As of February 3, 47 patients (34.1%) were discharged and 6 died (overall mortality, 4.3%), but the remaining patients are still hospitalized. Among those discharged alive (n = 47), the median hospital stay was 10 days (IQR, 7.0-14.0). Conclusions and Relevance: In this single-center case series of 138 hospitalized patients with confirmed NCIP in Wuhan, China, presumed hospital-related transmission of 2019-nCoV was suspected in 41% of patients, 26% of patients received ICU care, and mortality was 4.3%.
Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China
DOI:10.1001/jama.2020.1623 URL PMID:32031568
First case of 2019 novel coronavirus in the United States
DOI:10.1056/NEJMoa2001191
URL
PMID:32004427

An outbreak of novel coronavirus (2019-nCoV) that began in Wuhan, China, has spread rapidly, with cases now confirmed in multiple countries. We report the first case of 2019-nCoV infection confirmed in the United States and describe the identification, diagnosis, clinical course, and management of the case, including the patient's initial mild symptoms at presentation with progression to pneumonia on day 9 of illness. This case highlights the importance of close coordination between clinicians and public health authorities at the local, state, and federal levels, as well as the need for rapid dissemination of clinical information related to the care of patients with this emerging infection.
Coronavirus disease 2019 (COVID-19) and prosthetic heart valve: An additional coagulative challenge
DOI:10.5847/wjem.j.1920-8642.2020.04.009 URL PMID:33014223 [Cited within: 1]
Coronavirus disease 2019 (COVID-19) situation report - 55
March 15,
The surviving sepsis campaign bundle: 2018 update
URL PMID:29675566 [Cited within: 1]
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China
DOI:10.1016/S0140-6736(20)30183-5
URL
PMID:31986264
[Cited within: 1]

BACKGROUND: A recent cluster of pneumonia cases in Wuhan, China, was caused by a novel betacoronavirus, the 2019 novel coronavirus (2019-nCoV). We report the epidemiological, clinical, laboratory, and radiological characteristics and treatment and clinical outcomes of these patients. METHODS: All patients with suspected 2019-nCoV were admitted to a designated hospital in Wuhan. We prospectively collected and analysed data on patients with laboratory-confirmed 2019-nCoV infection by real-time RT-PCR and next-generation sequencing. Data were obtained with standardised data collection forms shared by WHO and the International Severe Acute Respiratory and Emerging Infection Consortium from electronic medical records. Researchers also directly communicated with patients or their families to ascertain epidemiological and symptom data. Outcomes were also compared between patients who had been admitted to the intensive care unit (ICU) and those who had not. FINDINGS: By Jan 2, 2020, 41 admitted hospital patients had been identified as having laboratory-confirmed 2019-nCoV infection. Most of the infected patients were men (30 [73%] of 41); less than half had underlying diseases (13 [32%]), including diabetes (eight [20%]), hypertension (six [15%]), and cardiovascular disease (six [15%]). Median age was 49.0 years (IQR 41.0-58.0). 27 (66%) of 41 patients had been exposed to Huanan seafood market. One family cluster was found. Common symptoms at onset of illness were fever (40 [98%] of 41 patients), cough (31 [76%]), and myalgia or fatigue (18 [44%]); less common symptoms were sputum production (11 [28%] of 39), headache (three [8%] of 38), haemoptysis (two [5%] of 39), and diarrhoea (one [3%] of 38). Dyspnoea developed in 22 (55%) of 40 patients (median time from illness onset to dyspnoea 8.0 days [IQR 5.0-13.0]). 26 (63%) of 41 patients had lymphopenia. All 41 patients had pneumonia with abnormal findings on chest CT. Complications included acute respiratory distress syndrome (12 [29%]), RNAaemia (six [15%]), acute cardiac injury (five [12%]) and secondary infection (four [10%]). 13 (32%) patients were admitted to an ICU and six (15%) died. Compared with non-ICU patients, ICU patients had higher plasma levels of IL2, IL7, IL10, GSCF, IP10, MCP1, MIP1A, and TNFalpha. INTERPRETATION: The 2019-nCoV infection caused clusters of severe respiratory illness similar to severe acute respiratory syndrome coronavirus and was associated with ICU admission and high mortality. Major gaps in our knowledge of the origin, epidemiology, duration of human transmission, and clinical spectrum of disease need fulfilment by future studies. FUNDING: Ministry of Science and Technology, Chinese Academy of Medical Sciences, National Natural Science Foundation of China, and Beijing Municipal Science and Technology Commission.
Acute respiratory distress syndrome
DOI:10.1001/jama.2012.5669
URL
PMID:22797452
[Cited within: 1]

The acute respiratory distress syndrome (ARDS) was defined in 1994 by the American-European Consensus Conference (AECC); since then, issues regarding the reliability and validity of this definition have emerged. Using a consensus process, a panel of experts convened in 2011 (an initiative of the European Society of Intensive Care Medicine endorsed by the American Thoracic Society and the Society of Critical Care Medicine) developed the Berlin Definition, focusing on feasibility, reliability, validity, and objective evaluation of its performance. A draft definition proposed 3 mutually exclusive categories of ARDS based on degree of hypoxemia: mild (200 mm Hg < PaO2/FIO2 /=10 cm H2O), and corrected expired volume per minute (>/=10 L/min). The draft Berlin Definition was empirically evaluated using patient-level meta-analysis of 4188 patients with ARDS from 4 multicenter clinical data sets and 269 patients with ARDS from 3 single-center data sets containing physiologic information. The 4 ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition. Using the Berlin Definition, stages of mild, moderate, and severe ARDS were associated with increased mortality (27%; 95% CI, 24%-30%; 32%; 95% CI, 29%-34%; and 45%; 95% CI, 42%-48%, respectively; P < .001) and increased median duration of mechanical ventilation in survivors (5 days; interquartile [IQR], 2-11; 7 days; IQR, 4-14; and 9 days; IQR, 5-17, respectively; P < .001). Compared with the AECC definition, the final Berlin Definition had better predictive validity for mortality, with an area under the receiver operating curve of 0.577 (95% CI, 0.561-0.593) vs 0.536 (95% CI, 0.520-0.553; P < .001). This updated and revised Berlin Definition for ARDS addresses a number of the limitations of the AECC definition. The approach of combining consensus discussions with empirical evaluation may serve as a model to create more accurate, evidence-based, critical illness syndrome definitions and to better inform clinical care, research, and health services planning.
KDIGO clinical practice guidelines for acute kidney injury
URL PMID:22890468 [Cited within: 1]
2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC
Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3)
DOI:10.1001/jama.2016.0288
URL
PMID:26903335
[Cited within: 1]

IMPORTANCE: The Third International Consensus Definitions Task Force defined sepsis as
Combining cognitive testing and informant report to increase accuracy in screening for dementia
URL PMID:9812113 [Cited within: 1]
A method of comparing the areas under ROC curves derived from same cases
URL PMID:6878708 [Cited within: 1]
Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study
URL PMID:32171076 [Cited within: 3]
Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study
URL PMID:32105632 [Cited within: 1]
The neutrophil-to-lymphocyte ratio: a narrative review
DOI:10.3332/ecancer.2016.702
URL
PMID:28105073
[Cited within: 1]

Cellular-mediated inflammatory response, lymphocytes, neutrophils, and monocytes are increasingly being recognised as having an important role in tumorigenesis and carcinogenesis. In this context, studies have suggested that the neutrophil-to-lymphocyte ratio (NLR) can be used as an independent prognostic factor in a variety of cancers. Particularly in breast cancer, several studies have shown that a high NLR is associated with shorter survival. Because the NLR can be easily determined from the full blood count, it could potentially provide a simple and inexpensive test cancer prognosis. This review addresses the possibilities and limitations of using the NLR as a clinical tool for risk stratification helpful for individual treatment of breast cancer patients. The potential underlying phenomena and some perspectives are discussed.
Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis
DOI:10.1093/jnci/dju124
URL
PMID:24875653
[Cited within: 1]

BACKGROUND: Inflammation may play an important role in cancer progression, and a high neutrophil-to-lymphocyte ratio (NLR) has been reported to be a poor prognostic indicator in several malignancies. Here we quantify the prognostic impact of this biomarker and assess its consistency in solid tumors. METHODS: A systematic review of electronic databases was conducted to identify publications exploring the association of blood NLR and clinical outcome in solid tumors. Overall survival (OS) was the primary outcome, and cancer-specific survival (CSS), progression-free survival (PFS), and disease-free survival (DFS) were secondary outcomes. Data from studies reporting a hazard ratio and 95% confidence interval (CI) or a P value were pooled in a meta-analysis. Pooled hazard ratios were computed and weighted using generic inverse-variance and random-effect modeling. All statistical tests were two-sided. RESULTS: One hundred studies comprising 40559 patients were included in the analysis, 57 of them published in 2012 or later. Median cutoff for NLR was 4. Overall, NLR greater than the cutoff was associated with a hazard ratio for OS of 1.81 (95% CI = 1.67 to 1.97; P < .001), an effect observed in all disease subgroups, sites, and stages. Hazard ratios for NLR greater than the cutoff for CSS, PFS, and DFS were 1.61, 1.63, and 2.27, respectively (all P < .001). CONCLUSIONS: A high NLR is associated with an adverse OS in many solid tumors. The NLR is a readily available and inexpensive biomarker, and its addition to established prognostic scores for clinical decision making warrants further investigation.
Neutrophil to lymphocyte ratio and cardiovascular diseases: a review
URL PMID:23259445 [Cited within: 1]
Neutrophil-to-lymphocyte ratio as a prognostic marker in critically-ill septic patients
DOI:10.1016/j.ajem.2016.10.055
URL
PMID:27806894
[Cited within: 1]

BACKGROUND: We evaluated the associations between the neutrophil-to-lymphocyte ratio (NLR) or changes in NLR and outcomes in septic patients. METHODS: Patients who met the criteria for severe sepsis or septic shock were categorized into five groups according to the quintile of initial NLR value. We defined two risk groups according to NLR value and changes in NLR during the first two days (defined as the persistently low NLR group and the persistently high NLR group). The primary outcome was 28-day mortality. RESULTS: A total of 1395 patients were included. The median initial NLR values from Quintile 1 to Quintile 5 were as follows: 0.2 (IQR [interquartile range], 0.1-0.7), 3.4 (IQR, 2.6-4.7), 8.6 (IQR, 7.1-9.9), 15.4 (IQR, 13.3-17.8), and 31.0 (IQR, 24.6-46.8), respectively. The 28-day mortality values for the same groups were as follows: 24.4%, 12.2%, 11.1%, 11.8%, and 16.1% (P<.01). Cox regression analysis showed that inclusion in Quintile 1 or Quintile 5 was a significant risk factor predicting 28-day mortality compared to Quintile 3 (adjusted hazard ratio [HR]: 1.79 (95% confidence interval [CI], 1.15-2.78) in Quintile 1; 1.67 (95% CI, 1.04-2.66) in Quintile 5). The analysis indicated that persistently low NLR (adjusted HR: 2.25, 95% CI, 1.63-3.11) and persistently high NLR (adjusted HR: 2.65, 95% CI, 1.64-4.29) were significant risk factors. CONCLUSIONS: In summary, the initial NLR measured at ED admission was independently associated with 28-day mortality in patients with severe sepsis and septic shock. In addition, change in NLR may prove to be a valuable prognostic marker.
Ferritin for the clinician
URL PMID:18835072 [Cited within: 1]
Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells
DOI:10.1039/c3mt00347g URL PMID:24549403 [Cited within: 1]
The hyperferritinemic syndrome: macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome
Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway
DOI:10.1182/blood-2009-11-253815
URL
PMID:20472835
[Cited within: 1]

The serum ferritin concentration is a clinical parameter measured widely for the differential diagnosis of anemia. Its levels increase with elevations of tissue iron stores and with inflammation, but studies on cellular sources of serum ferritin as well as its subunit composition, degree of iron loading and glycosylation have given rise to conflicting results. To gain further understanding of serum ferritin, we have used traditional and modern methodologies to characterize mouse serum ferritin. We find that both splenic macrophages and proximal tubule cells of the kidney are possible cellular sources for serum ferritin and that serum ferritin is secreted by cells rather than being the product of a cytosolic leak from damaged cells. Mouse serum ferritin is composed mostly of L-subunits, whereas it contains few H-subunits and iron content is low. L-subunits of serum ferritin are frequently truncated at the C-terminus, giving rise to a characteristic 17-kD band that has been previously observed in lysosomal ferritin. Taken together with the fact that mouse serum ferritin is not detectably glycosylated, we propose that mouse serum ferritin is secreted through the nonclassical lysosomal secretory pathway.
Elevation of serum ferritin levels for predicting a poor outcome in hospitalized patients with influenza infection
The neuroendocrine response to stress is a dynamic process
URL PMID:11800514 [Cited within: 1]
Relationship between thyroid function and ICU mortality: a prospective observation study
DOI:10.1186/cc11151
URL
PMID:22257427
[Cited within: 1]

INTRODUCTION: Although nonthyroidal illness syndrome is considered to be associated with adverse outcome in ICU patients, the performance of thyroid hormone levels in predicting clinical outcome in ICU patients is unimpressive. This study was conducted to assess the prognostic value of the complete thyroid indicators (free triiodothyronine (FT3), total triiodothyronine; free thyroxine, total thyroxine, thyroid-stimulating hormone and reverse triiodothyronine) in unselected ICU patients. METHODS: A total of 480 consecutive patients without known thyroid diseases were screened for eligibility and followed up during their ICU stay. We collected each patient's baseline characteristics, including the Acute Physiology and Chronic Health Evaluation II (APACHE II) score and thyroid hormone, N-terminal pro-brain natriuretic peptide (NT-proBNP) and C-reactive protein (CRP) levels. The primary outcome was ICU mortality. Potential predictors were analyzed for possible association with outcomes. We also evaluated the ability of thyroid hormones together with APACHE II score to predict ICU mortality by calculation of net reclassification improvement (NRI) and integrated discrimination improvement (IDI) indices. RESULTS: Among the thyroid hormone indicators, FT3 had the greatest power to predict ICU mortality, as suggested by the largest area under the curve (AUC) of 0.762+/-0.028. The AUC for FT3 level was less than that for APACHE II score (0.829+/-0.022) but greater than that for NT-proBNP level (0.724+/-0.030) or CRP level (0.689+/-0.030). Multiple regression analysis revealed that FT3 level (standardized beta=-0.600, P=0.001), APACHE II score (standardized beta=0.912, P<0.001), NT-proBNP level (standardized beta=0.459, P=0.017) and CRP level (standardized beta=0.367, P=0.030) could independently predict primary outcome. The addition of FT3 level to APACHE II score gave an NRI of 54.29% (P<0.001) and an IDI of 36.54% (P<0.001). The level of FT3 was significantly correlated with NT-proBNP levels (r=-0.344, P<0.001) and CRP levels (r=-0.408, P<0.001). CONCLUSION: In unselected ICU patients, FT3 was the most powerful and only independent predictor of ICU mortality among the complete indicators. The addition of FT3 level to the APACHE II score could significantly improve the ability to predict ICU mortality.
Mechanisms behind the non-thyroidal illness syndrome: an update
URL PMID:20016054 [Cited within: 1]
Into the eye of the cytokine storm
DOI:10.1128/MMBR.05015-11
URL
PMID:22390970
[Cited within: 1]

The cytokine storm has captured the attention of the public and the scientific community alike, and while the general notion of an excessive or uncontrolled release of proinflammatory cytokines is well known, the concept of a cytokine storm and the biological consequences of cytokine overproduction are not clearly defined. Cytokine storms are associated with a wide variety of infectious and noninfectious diseases. The term was popularized largely in the context of avian H5N1 influenza virus infection, bringing the term into popular media. In this review, we focus on the cytokine storm in the context of virus infection, and we highlight how high-throughput genomic methods are revealing the importance of the kinetics of cytokine gene expression and the remarkable degree of redundancy and overlap in cytokine signaling. We also address evidence for and against the role of the cytokine storm in the pathology of clinical and infectious disease and discuss why it has been so difficult to use knowledge of the cytokine storm and immunomodulatory therapies to improve the clinical outcomes for patients with severe acute infections.
COVID-19: consider cytokine storm syndromes and immunosuppression
DOI:10.1016/S0140-6736(20)30628-0 URL PMID:32192578 [Cited within: 1]