World Journal of Emergency Medicine ›› 2021, Vol. 12 ›› Issue (2): 124-130.doi: 10.5847/wjem.j.1920-8642.2021.02.007
• Original Article • Previous Articles Next Articles
Xin Lu1, Wei Han2, Yan-xia Gao3, Shi-gong Guo4, Shi-yuan Yu1, Xue-zhong Yu1, Hua-dong Zhu1, Yi Li1( )
)
Received:2020-02-16
Revised:2020-06-20
Online:2021-04-01
Published:2021-04-01
Contact:
Yi Li
E-mail:billliyi@126.com
Xin Lu, Wei Han, Yan-xia Gao, Shi-gong Guo, Shi-yuan Yu, Xue-zhong Yu, Hua-dong Zhu, Yi Li. Efficacy and safety of corticosteroids in immunocompetent patients with septic shock[J]. World Journal of Emergency Medicine, 2021, 12(2): 124-130.
Add to citation manager EndNote|Ris|BibTeX
URL: http://wjem.com.cn/EN/10.5847/wjem.j.1920-8642.2021.02.007
Table 1
Characteristics of included RCTs comparing corticosteroids versus control in immunocompetent patients with septic shock
| Study | Design and study place | Sample size (corticosteroids /control) | Excluded population (major selection criteria) | Intervention | Outcomesa |
|---|---|---|---|---|---|
| Annane et al[ | Multicenter (19 sites), France | 150/149 | Advanced form of cancer or AIDS infection | IV hydrocortisone 50 mg bolus q6h and po fludrocortisone 50 μg qd versus placebo for seven days | ICU mortality, 28-day mortality, hospital mortality, one-year mortality, seven-day mortality,b shock reversal, and safety outcomes |
| Briegel et al[ (1999) | One center, Germany | 20/20 | End-stage neoplasm, organ transplant recipients | IV hydrocortisone 100 mg loading, followed by 0.18 mg/(kg·h) continuous infusion until shock reversal, then reduced to 0.08 mg/(kg·h) for six days, then tapered off versus placebo (physiologic saline solution) | Shock reversal, 28-day mortality,b ICU mortality, hospital mortality, one-year mortality, seven-day mortality,b and safety outcomes |
| Cicarelli et al[ | One center, Brazil | 14/15 | Immunosuppression therapy, end stage neoplasm with a life expectancy of less than three months | IV dexamethasone 0.2 mg/kg q36h for three doses versus placebo (0.9% physiological saline solution) | Seven-day mortality, 28-day mortality, and shock reversal |
| Doluee et al[ | One center, Iran | 56/52 | Malignancy | IV hydrocortisone 50 mg q6h versus placebo (saline in the same volume) for seven days | Twenty-eight-day mortality |
| Luce et al[ (1988) | One center, USA | 38/37 | Severe immunodeficiency and AIDS | IV methylprednisolone 30 mg/kg q6h for four doses versus mannitol placebo | Incidence of ARDS, hospital mortality, and safety outcomes |
| Lv et al[ (2017) | One center, China | 58/60 | Immunosuppression | IV hydrocortisone 200 mg/d for six days, then tapered off versus placebo (normal saline) | Hospital mortality, 28-day mortality, shock reversal, and length of stay in ICU and hospital |
| Oppert et al[ (2005) | One center, Germany | 18/23 | HIV positive or recipients of organ transplants | IV hydrocortisone 50 mg bolus, followed by 0.18 mg/(kg·h) continuous infusion until shock reversal, then tapered off versus placebo | Time to cessation of vasopressor support, 28-day mortality, and shock reversal |
| Sprung et al[ (2008) | Multicenter (52 sites), Europe and Israel | 251/248 | Immunosuppression | IV hydrocortisone 50 mg q6h for five days, then tapered to 50 mg q12h for three days, then 50 mg QD for three days versus placebo | Mortality in ICU and hospital, 28-day mortality, one-year mortality, shock reversal, length of stay in ICU and hospital, and safety outcomes |
| Wan et al[ (2014) | One center, China | 62/27 | Advanced form of cancer or HIV infection | IV hydrocortisone 50 mg q6h for seven days or five days versus saline | Shock reversal, 28-day mortality, seven-day mortality, length of stay in ICU, and safety outcomes |
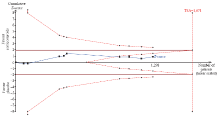
Figure 3.
Trial sequential analysis of all trials for short-term mortality. TSA: trial sequential analysis. The required information size was 1,671 patients. The incidence in the control arm of 45.1% with a relative risk reduction of 15.0% produced an incidence of 38.3% in the corticosteroid group. The TSA-adjusted 95% confidence interval for a relative risk of 0.95 was 0.83 to 1.09 and the cumulative Z-curves crossed futility area.
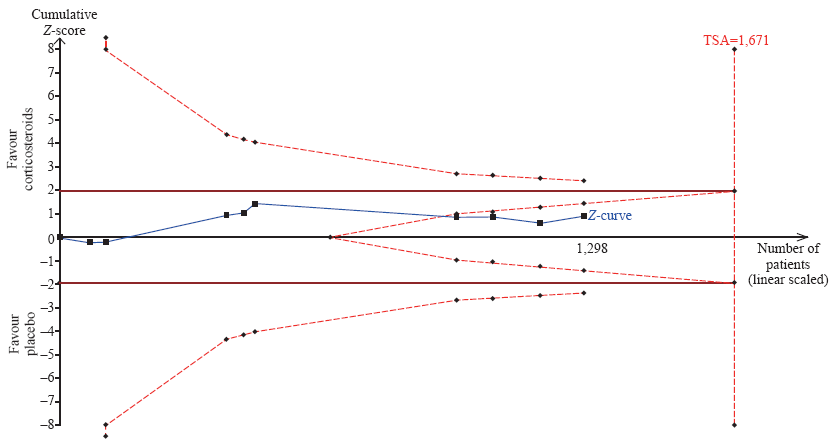
Table 2
Summary of findings for all included RCTs (grading of recommendations assessment, development, and evaluation)
| Outcomes | Anticipated absolute effects a (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with control | Risk with corticosteroids | ||||
| Short-term mortality | 452 per 1,000 | 429 per 1,000 (384-479) | RR 0.95 (0.85-1.06) | 1,298 (nine RCTs) | ⊕⊕⊕? moderateb |
| Long-term mortality | 606 per 1,000 | 582 per 1,000 (528-649) | RR 0.96 (0.87-1.07) | 816 (three RCTs) | ⊕⊕⊕⊕ high |
| Seven-day mortality | 412 per 1,000 | 280 per 1,000 (210-371) | RR 0.68 (0.51-0.90) | 457 (four RCTs) | ⊕⊕?? lowb, c |
| Time to shock reversal | Ranging from 75.81 to 91.2 hours | MD -21.56 (-32.95 to -10.16) | 263 (four RCTs) | ⊕⊕⊕? moderateb | |
| Shock reversal | 648 per 1,000 | 700-1,000 (642-765) | RR 1.08 (0.99-1.18) | 997 (five RCTs) | ⊕⊕⊕? moderateb |
| Infection | 257 per 1,000 | 280 per 1,000 (224-352) | RR 1.09 (0.87-1.37) | 894 (four RCTs) | ⊕⊕⊕? moderatec |
| Gastrointestinal bleeding | 88 per 1,000 | 100 per 1,000 (69-143) | RR 1.14 (0.79-1.63) | 927 (four RCTs) | ⊕⊕?? lowb, c |
| Hyperglycemia | 657 per 1,000 | 749 per 1,000 (676-834) | RR 1.14 (1.03-1.27) | 539 (two RCTs) | ⊕⊕⊕? moderateb |
| 1 |
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M , et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016; 315(8):801-10.
doi: 10.1001/jama.2016.0287 pmid: 26903338 |
| 2 |
Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS , et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016; 315(8):775-87.
doi: 10.1001/jama.2016.0289 pmid: 26903336 |
| 3 | Liu SH, Liang HY, Li HY, Ding XF, Sun TW, Wang J . Effect of low high-density lipoprotein levels on mortality of septic patients: a systematic review and meta-analysis of cohort studies. World[J] Emerg Med. 2020; 11(2):109-16. |
| 4 | Wittebole X, Scicluna BP . Classification of patients with septic shock: are we there yet?[J] Crit Care. 2018; 47:320-21. |
| 5 |
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R , et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017; 43(3):304-77.
doi: 10.1007/s00134-017-4683-6 pmid: 28101605 |
| 6 |
Vincent JL . How I treat septic shock. Intensive Care Med. 2018; 44(12):2242-4.
doi: 10.1007/s00134-018-5401-8 pmid: 30315330 |
| 7 |
Group CS . The effectiveness of hydrocortisone in the management of severe infections. JAMA. 1963; 183:462-5.
doi: 10.1001/jama.1963.63700060029012 |
| 8 | Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R , et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl[J] Med. 2018; 378(9):797-808. |
| 9 | Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S , et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl[J] Med. 2018; 378(9):809-18. |
| 10 | Zhou X, Hu C, Yao L, Fan Z, Sun L, Wang Y , et al. Effect of adjunctive corticosteroids on clinical outcomes in adult patients with septic shock: a meta-analysis of randomized controlled trials and trial sequential analysis.[J] Crit Care. 2018; 48:296-306. |
| 11 | Xu R, Wang Q, Huang Y, Wu L, Liu Q, Hu W , et al. Do low-dose corticosteroids improve survival or shock reversal from septic shock in adults? Meta-analysis with trial sequential analysis.[J] Int Med Res. 2018; 46(7):2513-24. |
| 12 |
Rygard SL, Butler E, Granholm A, Moller MH, Cohen J, Finfer S , et al. Low-dose corticosteroids for adult patients with septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2018; 44(7):1003-16.
doi: 10.1007/s00134-018-5197-6 pmid: 29761216 |
| 13 |
Rochwerg B, Oczkowski SJ, Siemieniuk RAC, Agoritsas T, Belley-Cote E, D'Aragon F, et al. Corticosteroids in sepsis: an updated systematic review and meta-analysis. Crit Care Med. 2018; 46(9):1411-20.
doi: 10.1097/CCM.0000000000003262 pmid: 29979221 |
| 14 | Lyu QQ, Chen QH, Zheng RQ, Yu JQ, Gu XH . Effect of low-dose hydrocortisone therapy in adult patients with septic shock: a meta-analysis with trial sequential analysis of randomized controlled trials.[J] Intensive Care Med. 2020; 35(10):971-83. |
| 15 |
Zhu Y, Wen Y, Jiang Q, Guo N, Cai Y, Shen X . The effectiveness and safety of corticosteroids therapy in adult critical ill patients with septic shock: a meta-analysis of randomized controlled trials. Shock. 2019; 52(2):198-207.
doi: 10.1097/SHK.0000000000001202 pmid: 29889815 |
| 16 |
Lian XJ, Huang DZ, Cao YS, Wei YX, Lian ZZ, Qin TH , et al. Reevaluating the role of corticosteroids in septic shock: an updated meta-analysis of randomized controlled trials. Biomed Res Int. 2019; 2019:3175047.
doi: 10.1155/2019/3175047 pmid: 31281831 |
| 17 |
Fang F, Zhang Y, Tang J, Lunsford LD, Li T, Tang R , et al. Association of corticosteroid treatment with outcomes in adult patients with sepsis: a systematic review and meta-analysis. JAMA Intern Med. 2019; 179(2):213-23.
doi: 10.1001/jamainternmed.2018.5849 pmid: 30575845 |
| 18 | Ni YN, Liu YM, Wang YW, Liang BM, Liang ZA . Can corticosteroids reduce the mortality of patients with severe sepsis? A systematic review and meta-analysis. Am[J] Emerg Med. 2019; 37(9):1657-64. |
| 19 | Wu J, Huang M, Wang Q, Ma Y, Jiang L . Effects and safety of separate low-dose hydrocortisone use in patients with septic shock: a meta-analysis. Hong Kong[J] Emerg Med. 2020; 27(1):39-50. |
| 20 | Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y , et al. Corticosteroids for treating sepsis in children and adults. Cochrane Database Syst Rev. 2019; 12(12):CD002243. |
| 21 |
Lin LL, Gu HY, Luo J, Wang L, Zhang C, Niu YM , et al. Impact and beneficial critical points of clinical outcome in corticosteroid management of adult patients with sepsis: meta-analysis and grade assessment. Front Pharmacol. 2019; 10:1101.
doi: 10.3389/fphar.2019.01101 pmid: 31607929 |
| 22 | Lu X, Wang X, Gao Y, Yu S, Zhao L, Zhang Z , et al. Efficacy and safety of corticosteroids for septic shock in immunocompromised patients: a cohort study from MIMIC. Am J Emerg Med. 2020; S0735- 6757(20) 30075-9. |
| 23 | Thorlund K, Engstrom J, Wetterslev J, Brok J, Imberger G. User manual for trial sequential analysis(TSA). Copenhagen Trial Unit , Centre for Clinical Intervention Research, Copenhagen, Denmark. 2011; 1-115. Available at www.ctu.dk/tsa. |
| 24 | Lv QQ, Gu XH, Chen QH, Yu JQ, Zheng RQ . Early initiation of low-dose hydrocortisone treatment for septic shock in adults: a randomized clinical trial. Am[J] Emerg Med. 2017; 35(12):1810-14. |
| 25 | Wan X, Su M, Yue J, Huang Y, Zhu W, Wan L . Clinical trial of low-dose corticosteroid treatment for surgical postoperative patients with septic shock. Journal of Kunming Medical University. 2014; 35:92-9. |
| 26 | Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K , et al. Hydrocortisone therapy for patients with septic shock. N Engl[J] Med. 2008; 358(2):111-24. |
| 27 |
Cicarelli DD, Vieira JE, Benseñor FE . Early dexamethasone treatment for septic shock patients: a prospective randomized clinical trial. Sao Paulo Med J. 2007; 125(4):237-41.
doi: 10.1590/s1516-31802007000400009 pmid: 17992396 |
| 28 |
Oppert M, Schindler R, Husung C, Offermann K, Gräf KJ, Boenisch O , et al. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med. 2005; 33(11):2457-64.
doi: 10.1097/01.ccm.0000186370.78639.23 pmid: 16276166 |
| 29 |
Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM , et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002; 288(7):862-71.
doi: 10.1001/jama.288.7.862 pmid: 12186604 |
| 30 |
Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G , et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med. 1999; 27(4):723-32.
doi: 10.1097/00003246-199904000-00025 pmid: 10321661 |
| 31 |
Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF . Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988; 138(1):62-8.
doi: 10.1164/ajrccm/138.1.62 pmid: 3202402 |
| 32 |
Doluee MT, Salehi M, Gharaee AM, Jalalyazdi M, Reihani H . The effect of physiologic dose of intravenous hydrocortisone in patients with refractory septic shock: a randomized control trial. Journal of Emergency Practice and Trauma. 2018; 4(1):29-33.
doi: 10.15171/jept.2017.25 |
| 33 |
Marik PE . The role of glucocorticoids as adjunctive treatment for sepsis in the modern era. Lancet Respir Med. 2018; 6(10):793-800.
doi: 10.1016/S2213-2600(18)30265-0 pmid: 30006071 |
| 34 |
Skrupky LP, Kerby PW, Hotchkiss RS . Advances in the management of sepsis and the understanding of key immunologic defects. Anesthesiology. 2011; 115(6):1349-62.
doi: 10.1097/ALN.0b013e31823422e8 |
| 35 |
Cecconi M, Evans L, Levy M, Rhodes A . Sepsis and septic shock. Lancet. 2018; 392(10141):75-87.
doi: 10.1016/S0140-6736(18)30696-2 pmid: 29937192 |
| 36 |
MacDonald RD . Articles that may change your practice: steroids and septic shock. Air Med J. 2018; 37(6):343-4.
doi: 10.1016/j.amj.2018.08.001 pmid: 30424846 |
| [1] | Ren-qi Yao, Chao Ren, Di Ren, Jin-xiu Li, Ying Li, Xue-yan Liu, Lei Huang, Yong Liu, Mian Peng, Yong-wen Feng, Yong-ming Yao. Development of septic shock and prognostic assessment in critically ill patients with coronavirus disease outside Wuhan, China [J]. World Journal of Emergency Medicine, 2021, 12(4): 293-298. |
| [2] | Yu-qing Cui, Xian-fei Ding, Huo-yan Liang, Dong Wang, Xiao-juan Zhang, Li-feng Li, Quan-cheng Kan, Le-xin Wang, Tong-wen Sun. Efficacy and safety of low-dose corticosteroids for acute respiratory distress syndrome: A systematic review and meta-analysis [J]. World Journal of Emergency Medicine, 2021, 12(3): 207-213. |
| [3] | Shao-hua Liu, Huo-yan Liang, Hong-yi Li, Xian-fei Ding, Tong-wen Sun, Jing Wang. Effect of low high-density lipoprotein levels on mortality of septic patients: A systematic review and meta-analysis of cohort studies [J]. World Journal of Emergency Medicine, 2020, 11(2): 109-116. |
| [4] | Shi-yuan Yu, Yan-xia Gao, Joseph Walline, Xin Lu, Li-na Zhao, Yuan-xu Huang, Jiang Tao, An-yong Yu, Na Ta, Ren-ju Xiao, Yi Li. Role of penehyclidine in acute organophosphorus pesticide poisoning [J]. World Journal of Emergency Medicine, 2020, 11(1): 37-47. |
| [5] | Yan Ma, Xiang-you Yu, Yi Wang. Dose-related effects of dexmedetomidine on immunomodulation and mortality to septic shock in rats [J]. World Journal of Emergency Medicine, 2018, 9(1): 56-63. |
| [6] | Gan-nan Wang, Xu-feng Chen, Li Qiao, Yong Mei, Jin-ru Lv, Xi-hua Huang, Bin Shen, Jin-song Zhang. Comparison of extracorporeal and conventional cardiopulmonary resuscitation: A meta-analysis of 2 260 patients with cardiac arrest [J]. World Journal of Emergency Medicine, 2017, 8(1): 5-11. |
| [7] | Li-ping Chen, Jun-hui Chen, Ying Chen, Chao Wu, Xiao-hong Yang. Efficacy and safety of glucocorticoids in the treatment of community-acquired pneumonia: A meta-analysis of randomized controlled trials [J]. World Journal of Emergency Medicine, 2015, 6(3): 172-178. |
| [8] | Mohsen Ebrahimi, Abbas Heydari, Reza Mazlom, Amir Mirhaghi. The reliability of the Australasian Triage Scale: a meta-analysis [J]. World Journal of Emergency Medicine, 2015, 6(2): 94-99. |
| [9] | Liang-shan Peng, Juan Li, Gao-sheng Zhou, Lie-hua Deng, Hua-guo Yao. Relationships between genetic polymorphisms of triggering receptor expressed on myeloid cells-1 and septic shock in a Chinese Han population [J]. World Journal of Emergency Medicine, 2015, 6(2): 123-130. |
| [10] | Xu-rui Luo, Hui-li Zhang, Geng-jin Chen, Wen-shu Ding, Liang Huang. Active compression-decompression cardiopulmonary resuscitation (CPR) versus standard CPR for cardiac arrest patients: a meta-analysis [J]. World Journal of Emergency Medicine, 2013, 4(4): 266-272. |
| [11] | Xiao-ping Wang, Qing-ming Lin, Shen Zhao, Shi-rong Lin, Feng Chen. Therapeutic benefits of mild hypothermia in patients successfully resuscitated from cardiac arrest:A meta-analysis [J]. World Journal of Emergency Medicine, 2013, 4(4): 260-265. |
| [12] | Yuan-hua Lu, Ling Liu, Xiao-hua Qiu, Qin Yu, Yi Yang, Hai-bo Qiu. Effect of early goal directed therapy on tissue perfusion in patients with septic shock [J]. World Journal of Emergency Medicine, 2013, 4(2): 117-122. |
| [13] | Xue-zhong Xing, Yong Gao, Hai-jun Wang, Quan-hui Yang, Chu-lin Huang, Shi-ning Qu, Hao Zhang, Hao Wang, Qing-ling Xiao, Ke-lin Sun. Risk factors and prognosis of critically ill cancer patients with postoperative acute respiratory insufficiency [J]. World Journal of Emergency Medicine, 2013, 4(1): 43-47. |
| [14] | Hong-sheng Ren, Shi-xue Gao, Chun-ting Wang, Yu-feng Chu, Jin-jiao Jiang, Ji-cheng Zhang, Mei Meng, Guo-qian Qi, Min Ding. Effects of high-volume hemofiltration on alveolar-arterial oxygen exchange in patients with refractory septic shock [J]. World Journal of Emergency Medicine, 2011, 2(2): 127-131. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
