World Journal of Emergency Medicine ›› 2013, Vol. 4 ›› Issue (2): 138-143.doi: 10.5847/wjem.j.issn.1920-8642.2013.02.010
• Original Articles • Previous Articles Next Articles
Qiang Su, Lang Li( ), Yang-chun Liu, You Zhou, Wei-ming Wen
), Yang-chun Liu, You Zhou, Wei-ming Wen
Received:2013-01-06
Accepted:2013-05-06
Online:2013-06-15
Published:2013-06-15
Contact:
Lang Li
E-mail:drlilang@163.com
Qiang Su, Lang Li, Yang-chun Liu, You Zhou, Wei-ming Wen. Effect of metoprolol on myocardial apoptosis after coronary microembolization in rats[J]. World Journal of Emergency Medicine, 2013, 4(2): 138-143.
Add to citation manager EndNote|Ris|BibTeX
URL: http://wjem.com.cn//EN/10.5847/wjem.j.issn.1920-8642.2013.02.010
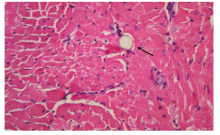
Figure 1.
Histopathology of post-CME myocardial microinfarcts. HE staining of tissue samples from the coronary microembolization group revealed myocardial microinfarcts with a low level of inflammatory cell infiltration. However, a portion of cardiomyocyte nuclei is not visible. The arrow indicates the presence of a 42 μm microsphere following the experimental protocol (HE staining, original magnification×400).
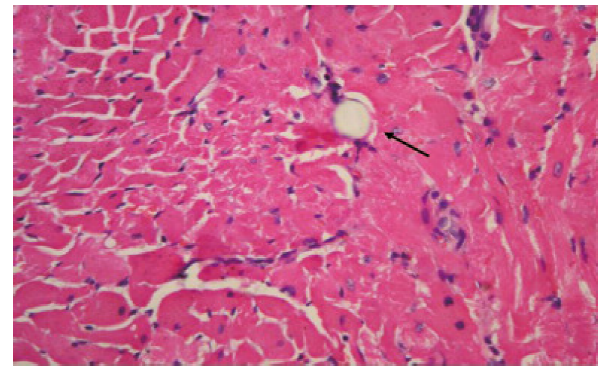
Figure 2.
Histopathology of myocardial microinfarcts. Hematoxylin basic fuchsin picric acid (HBFP) staining of tissue samples revealed no infarct in the control group. Microinfarcts in the tissue samples from the CME and metoprolol groups are indicated by arrows. Normal myocardial cytoplasm is stained yellow, the nuclei are stained blue, and the ischemic myocardium is stained red (HBFP staining,original magnification×100).
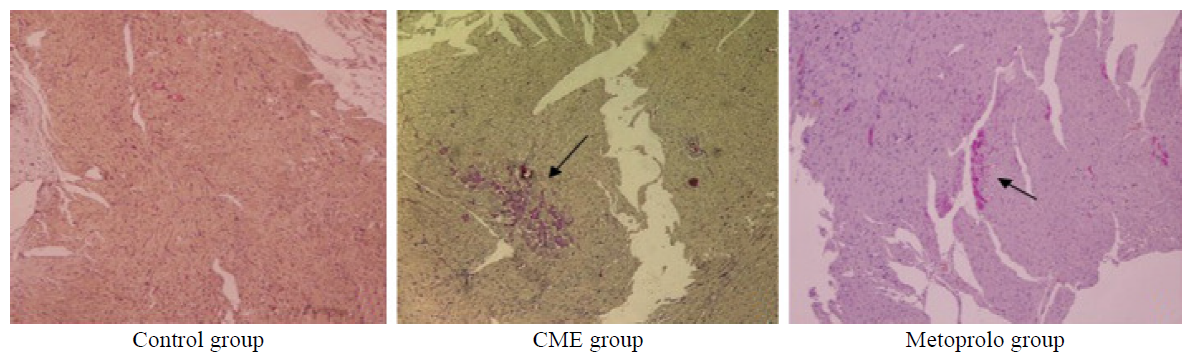
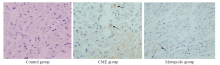
Figure 3.
Photomicrographs showing effect of metoprolol on apoptosis following CME. TUNEL assay revealed the myocardial apoptosis in the control, CME, and metoprolol groups. Normal cell nuclei are stained pale blue while the apoptotic cardiomyocyte nuclei (arrows) are stained brown (original magnification×400).
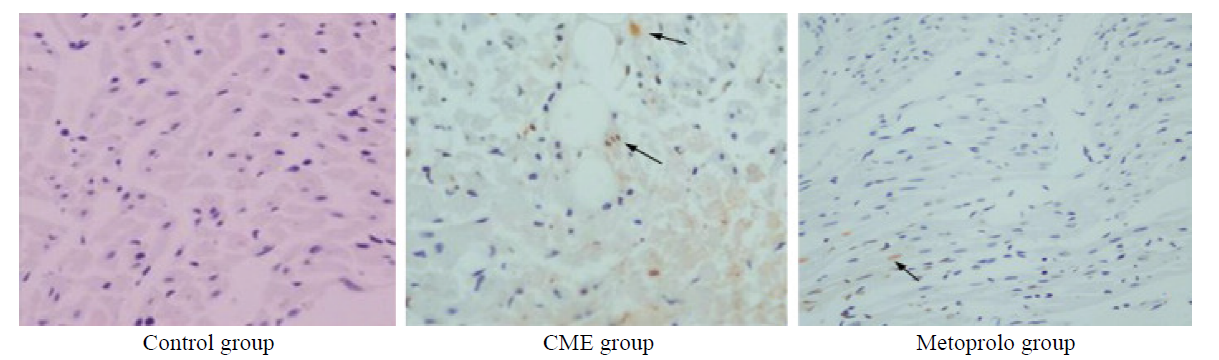
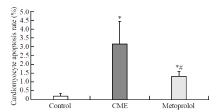
Figure 4.
Graph showing effect of metoprolol on apoptosis following CME. Myocardial apoptosis after CME (TUNEL assay) was detected primarily in the myocardial microinfarction foci and the peripheral zones. In control animals, myocardial apoptosis was occasionally found in the subendocardium and papillary muscles. The data (n=10) are expressed as means ± SD.Compared with the control group, *P<0.05;compared with the CME group, #P<0.05.
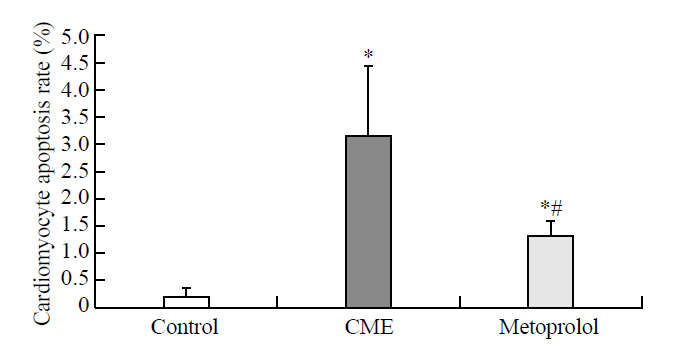

Figure 5.
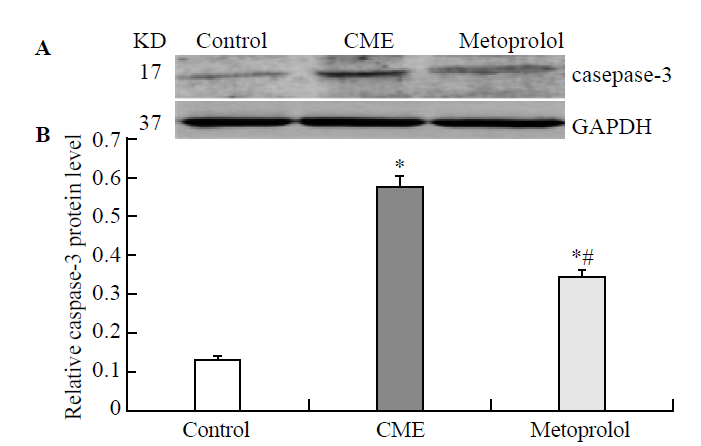
Effect of metoprolol on caspase-3 expression following CME. A: The relative expression levels of activated caspase-3 protein were determined by using western blot; the IA values were normalized to GAPDH expression levels. Lanes 1, 2, and 3 in the representative gel show caspase-3 expression in the control, CME (after 6 hours), and metoprolol groups, respectively. B: The data obtained (n=10) were expressed and compared between the groups as means ± SD values, herein presented graphically. Compared with the control group, *P<0.05; compared with the CME group, #P<0.05.
| 1 |
Heusch G, Kleinbongard P, Böse D, Levkau B, Haude M, Schulz R, et al. Coronary microembolization: from bedside to bench and back to bedside. Circulation 2009; 120:1822-1836.
doi: 10.1161/CIRCULATIONAHA.109.888784 pmid: 19884481 |
| 2 |
Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol 2000; 36:1202-1209.
pmid: 11028471 |
| 3 |
Kretzschmar D, Jung C, Otto S, Utschig S, Hartmann M, Lehmann T, et al. Detection of coronary microembolization by Doppler ultrasound in patients with stable angina pectoris during percutaneous coronary interventions under an adjunctive antithrombotic therapy with abciximab: design and rationale of the High Intensity Transient Signals ReoPro (HITS-RP) study. Cardiovasc Ultrasound 2012; 10:21.
doi: 10.1186/1476-7120-10-21 pmid: 22613136 |
| 4 |
Dörge H, Neumann T, Behrends M, Skyschally A, Schulz R, Kasper C, et al. Perfusion-contraction mismatch with coronary microvascular obstruction: role of inflammation. Am J Physiol Heart Circ Physiol 2000; 279:H2587-2592.
pmid: 11087208 |
| 5 |
Carlsson M, Martin AJ, Ursell PC, Saloner D, Saeed M. Magnetic resonance imaging quantification of left ventricular dysfunction following coronary microembolization. Magn Reson Med 2009; 61:595-602.
doi: 10.1002/mrm.21869 pmid: 19097239 |
| 6 |
Waksman R, Douglas JS, Scott NA, Ghazzal ZM, Yee-Peterson J, King SB, 3rd, et al. Distal embolization is common after directional atherectomy in coronary arteries and saphenous vein grafts. Am Heart J 1995; 129:430-435.
doi: 10.1016/0002-8703(95)90263-5 pmid: 7872166 |
| 7 |
Grube E, Schofer J Jü, Webb J, Schuler G, Colombo A, Sievert H, et al. Evaluation of a balloon occlusion and aspiration system for protection from distal embolization during stenting in saphenous vein grafts. Am J Cardiol 2002; 89:941-945.
pmid: 11950432 |
| 8 |
Lu Y, Li L, Zhao X, Huang W, Wen W. Beta blocker metoprolol protects against contractile dysfunction in rats after coronary microembolization by regulating expression of myocardial inflammatory cytokines. Life Sci 2011; 88:1009-1015.
doi: 10.1016/j.lfs.2011.03.012 pmid: 21443890 |
| 9 |
Wang FW, Osman A, Otero J, Stouffer GA, Waxman S, Afzal A, et al. Distal myocardial protection during percutaneous coronary intervention with an intracoronary beta-blocker. Circulation 2003; 107:2914-2919.
doi: 10.1161/01.CIR.0000072787.25131.03 pmid: 12771007 |
| 10 |
Uretsky BF, Birnbaum Y, Osman A, Gupta R, Paniagua O, Chamoun A, et al. Distal myocardial protection with intracoronary beta blocker when added to a Gp IIb/IIIa platelet receptor blocker during percutaneous coronary intervention improves clinical outcome. Catheter Cardiovasc Interv 2008; 72:488-497.
doi: 10.1002/ccd.21677 pmid: 18814223 |
| 11 |
Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation 2004; 110:1083-1090.
doi: 10.1161/01.CIR.0000139844.15045.F9 pmid: 15313944 |
| 12 |
Seqqat R, Guo X, Rafiq K, Kolpakov MA, Guo J, Koch WJ, et al. Beta1-adrenergic receptors promote focal adhesion signaling downregulation and myocyte apoptosis in acute volume overload. J Mol Cell Cardiol 2012; 53:240-249.
doi: 10.1016/j.yjmcc.2012.05.004 pmid: 22609523 |
| 13 |
Thielmann M, Dörge H, Martin C, Belosjorow S, Schwanke U, van De Sand A, et al. Myocardial dysfunction with coronary microembolization: signal transduction through a sequence of nitric oxide, tumor necrosis factor-alpha, and sphingosine. Circ Res 2002; 90:807-813.
doi: 10.1161/01.res.0000014451.75415.36 pmid: 11964374 |
| 14 |
Li L, Li DH, Qu N, Wen WM, Huang WQ. The role of ERK1/2 signaling pathway in coronary microembolization-induced rat myocardial inflammation and injury. Cardiology 2010; 117:207-215.
doi: 10.1159/000321713 pmid: 21150201 |
| 15 |
Kalenikova EI, Gorodetskaya EA, Zacharova NV, Shechter AB, Medvedev OS. Perindopril effects on angiotensin I elimination in lung after experimental myocardial injury induced by intracoronary microembolization in rats. J Cardiovasc Pharmacol 1998; 32:608-615.
pmid: 9781929 |
| 16 |
Zhang QY, Ge JB, Chen JZ, Zhu JH, Zhang LH, Lau CP, et al. Mast cell contributes to cardiomyocyte apoptosis after coronary microembolization. J Histochem Cytochem 2006; 54:515-523.
doi: 10.1369/jhc.5A6804.2005 pmid: 16344327 |
| 17 |
Grund F, Sommerschild HT, Lyberg T, Kirkeboen KA, Ilebekk A. Microembolization in pigs: effects on coronary blood flow and myocardial ischemic tolerance. Am J Physiol 1999; 277:H533-542.
doi: 10.1152/ajpheart.1999.277.2.H533 pmid: 10444478 |
| 18 |
Skyschally A, Schulz R, Erbel R, Heusch G. Reduced coronary and inotropic reserves with coronary microembolization. Am J Physiol Heart Circ Physiol 2002; 282:H611-614.
doi: 10.1152/ajpheart.00797.2001 pmid: 11788409 |
| 19 |
Miura M, Thomas R, Ganz W, Sokol T, Shell WE, Toshimitsu T, et al. The effect of delay in propranolol administration on reduction of myocardial infarct size after experimental coronary artery occlusion in dogs. Circulation 1979; 59:1148-1157.
pmid: 436207 |
| 20 |
Holleyman CR, Larson DF. Apoptosis in the ischemic reperfused myocardium. Perfusion 2001; 16:491-502.
doi: 10.1177/026765910101600609 pmid: 11761089 |
| 21 |
Li X, Luo R, Jiang R, Meng X, Wu X, Zhang S, et al. The role of the Hsp90/Akt pathway in myocardial calpain-induced caspase-3 activation and apoptosis during sepsis. BMC Cardiovasc Disord 2013; 13:8.
pmid: 23425388 |
| 22 |
Wu L, Xi Z, Guo R, Liu S, Yang S, Liu D, et al. Exogenous ARC down-regulates caspase-3 expression and inhibits apoptosis of broiler chicken cardiomyocytes exposed to hydrogen peroxide. Avian Pathol 2013; 42:32-37.
doi: 10.1080/03079457.2012.757289 pmid: 23391179 |
| [1] | Hui Fu, Qiao-sheng Wang, Qiong Luo, Si Tan, Hua Su, Shi-lin Tang, Zheng-liang Zhao, Li-ping Huang. Simvastatin inhibits apoptosis of endothelial cells induced by sepsis through upregulating the expression of Bcl-2 and downregulating Bax [J]. World Journal of Emergency Medicine, 2014, 5(4): 291-297. |
| [2] | Jian Lu, Yi Shen, Hui-yin Qian, Li-jun Liu, Bao-chun Zhou, Yan Xiao, Jin-ning Mao, Guo-yin An, Ming-zhong Rui, Tao Wang, Chang-lai Zhu. Effects of mild hypothermia on the ROS and expression of caspase-3 mRNA and LC3 of hippocampus nerve cells in rats after cardiopulmonary resuscitation [J]. World Journal of Emergency Medicine, 2014, 5(4): 298-305. |
| [3] | Guo-ming Zhang, Yu Wang, Tian-de Li, Xiao-yan Li, Shao-ping Su, Yuan-yuan Sun, Xiu-hua Liu. Post-conditioning with gradually increased reperfusion provides better cardioprotection in rats [J]. World Journal of Emergency Medicine, 2014, 5(2): 128-134. |
| [4] | Pei-ren Shan, Wei-wei Xu, Zhou-qing Huang, Jun Pu, Wei-jian Huang. Protective role of retinoid X receptor in H9c2 cardiomyocytes from hypoxia/reoxygenation injury in rats [J]. World Journal of Emergency Medicine, 2014, 5(2): 122-127. |
| [5] | Yan-jun Qin, Xin-liang Zhang, Yue-qing Yu, Xiao-hua Bian, Shi-min Dong. Cardioprotective effect of erythropoietin on sepsis-induced myocardial injury in rats [J]. World Journal of Emergency Medicine, 2013, 4(3): 215-223. |
| [6] | Zhi-jian Zhang, Li-bo Peng, Ya-juan Luo, Cong-yang Zhou. Prospective experimental studies on the renal protective effect of ulinastatin after paraquat poisoning [J]. World Journal of Emergency Medicine, 2012, 3(4): 299-304. |
| [7] | Ying-zhen Wang, Shi-wen Wang, You-cheng Zhang, Zhi-jiang Sun. Protective effect of exogenous IGF-I on the intestinal mucosal barrier in rats with severe acute pancreatitis [J]. World Journal of Emergency Medicine, 2012, 3(3): 213-220. |
| [8] | Shi-hui Zhou, Yan-fei Sun, Gang Wang. Effects of hyperbaric oxygen on intestinal mucosa apoptosis caused by ischemia-reperfusion injury in rats [J]. World Journal of Emergency Medicine, 2012, 3(2): 135-140. |
| [9] | Ping Yan, Shou-quan Chen, Zhang-ping Li, Jie Zhang, Ji-ke Xue, Wan-tie Wang, Wei-jia Huang, Jun-yan Cheng, Hui-ping Li. Effect of exogenous phosphocreatine on cardiomycytic apoptosis and expression of Bcl-2 and Bax after cardiopulmonary resuscitation in rats [J]. World Journal of Emergency Medicine, 2011, 2(4): 291-295. |
| [10] | Jian-min Li, Pan Zhang, Ya-ning Zhao, Chang-xiang Chen, Shu-xing Li. Protective effects of edaravone on diffuse brain injury in rats [J]. World Journal of Emergency Medicine, 2011, 2(3): 222-227. |
| [11] | Ying Wang, Zhi-yang Sun, Kui-ming Zhang, Guo-qiang Xu, Guang Li. Bcl-2 in suppressing neuronal apoptosis after spinal cord injury [J]. World Journal of Emergency Medicine, 2011, 2(1): 38-44. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
