World Journal of Emergency Medicine ›› 2020, Vol. 11 ›› Issue (4): 246-254.doi: 10.5847/wjem.j.1920-8642.2020.04.007
• Original Article • Previous Articles Next Articles
Rui Zhang1,2,3,4, Meng-yang Xue1,2,3,4, Bao-shan Liu1,2,3,4, Wen-jun Wang1,2,3,4, Xin-hui Fan1,2,3,4, Bo-yuan Zheng1,2,3,4, Qiu-huan Yuan1,2,3,4, Feng Xu1,2,3,4, Jia-li Wang1,2,3,4( ), Yu-guo Chen1,2,3,4(
), Yu-guo Chen1,2,3,4( )
)
Received:2019-10-26
Accepted:2020-04-06
Online:2020-10-01
Published:2020-10-01
Contact:
Jia-li Wang,Yu-guo Chen
E-mail:wangjiali_2000@126.com;chen919085@sdu.edu.cn
Rui Zhang, Meng-yang Xue, Bao-shan Liu, Wen-jun Wang, Xin-hui Fan, Bo-yuan Zheng, Qiu-huan Yuan, Feng Xu, Jia-li Wang, Yu-guo Chen. Aldehyde dehydrogenase 2 preserves mitochondrial morphology and attenuates hypoxia/reoxygenation-induced cardiomyocyte injury[J]. World Journal of Emergency Medicine, 2020, 11(4): 246-254.
Add to citation manager EndNote|Ris|BibTeX
URL: http://wjem.com.cn//EN/10.5847/wjem.j.1920-8642.2020.04.007

Figure 1.
ALDH2 activation inhibited OGD/R-induced cell apoptosis. A: representative photographs and quantitative data of apoptotic cardiomyocytes in the control group, the OGD/R (10 hours/1 hour) group, the OGD/R (12 hours/1 hour) group, and the OGD/R (14 hours/1 hour) group by FITC-Annexin V/PI staining using flow cytometry; B: representative photographs and quantitative data of apoptotic cardiomyocytes in the control group, the OGD/R (14 hours/1 hour) group, and the OGD/R (14 hours/1 hour) group+Alda-1 group by TUNEL staining; scale bar: 50 μM; data are means±standard error of mean (SEM); FITC-Annexin V/PI: fluorescein isothiocyanate (FITC)-Annexin V/propidium iodide (PI); TUNEL: terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling; DAPI: 4’,6-diamidino-2-phenylindole; *P<0.05 vs. control group; #P<0.05 vs. OGD/R (14 hours/1 hour) group.
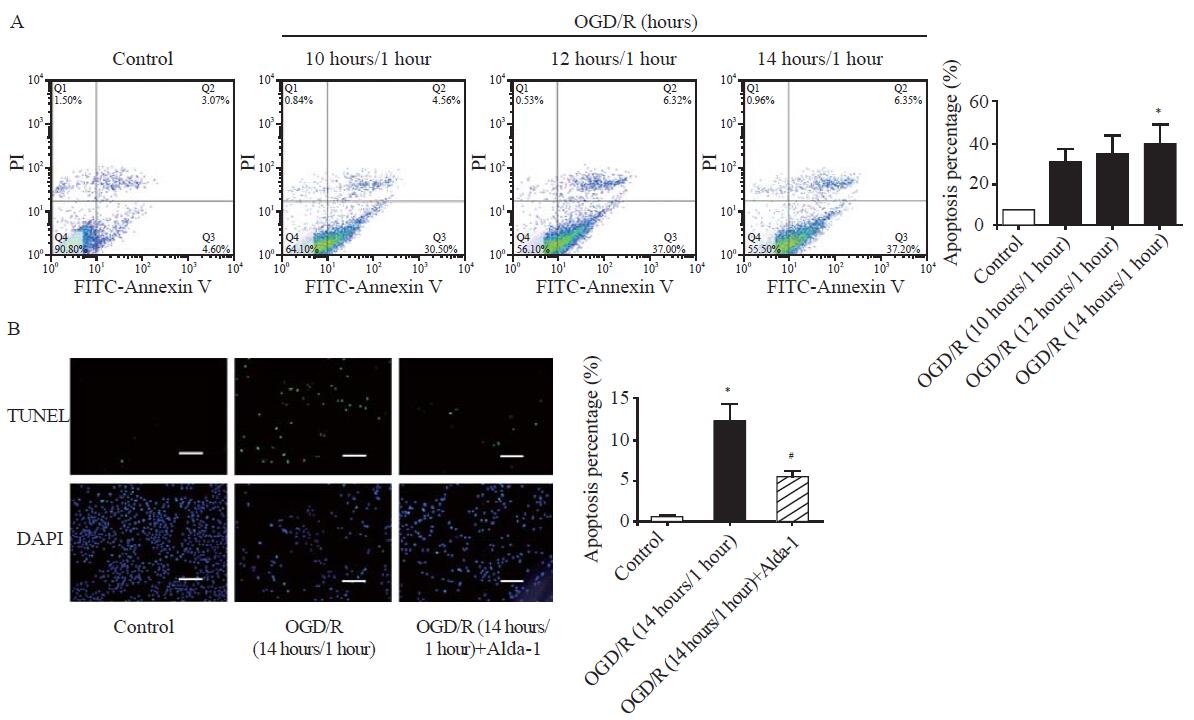
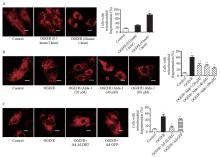
Figure 2.
Both ALDH2 activation and overexpression inhibited OGD/R-induced mitochondrial fission. A: representative photographs and the percentage of cells with mitochondrial fragmentation in the control group, the OGD/R (0.5 hours/1 hour) group, and the OGD/R (4 hours/1 hour) group by mitochondrial staining; scale bar: 25 μM; B: representative photographs and the percentage of cells with mitochondrial fragmentation in the control group, the OGD/R (4 hours/1 hour) group, and the OGD/R (4 hours/1 hour)+Alda-1 group in the concentration range of 20-80 μM by mitochondrial staining; scale bar: 25 μM; C: representative photographs and the percentage of cells with mitochondrial fragmentation in the control group, the OGD/R group, the OGD/R+Ad-ALDH2 group, and the OGD/R+Ad-GFP group; scale bar: 25 μM; Data are means±standard error of mean (SEM). *P<0.05 vs. control group; #P<0.05 vs. OGD/R group.
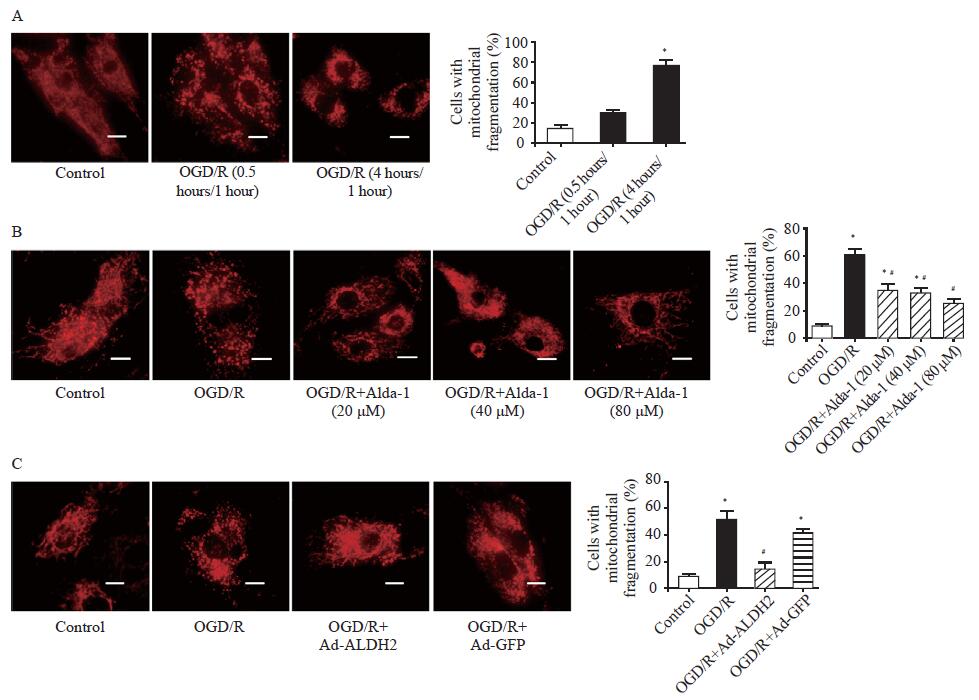

Figure 3.
ALDH2 suppressed Drp1 phosphorylation at Ser616 but not affected the expression levels of mitochondrial dynamics-regulating proteins. A: representative immunoblots and quantification of the expression levels of Fis1, Mfn1, Mfn2, and Opa1 in the control group and the OGD/R group; B: representative immunoblots and quantification of levels of total Drp1, phosphorylated Drp1 (p-Drp1) at Ser616, total AMPK, and phosphorylated AMPK (p-AMPK) at Thr172 in the control group, the OGD/R group, the OGD/R+Ad-ALDH2 group, and the OGD/R+Ad-GFP group; data are mean±standard error of mean (SEM); *P<0.05 vs. control group; #P<0.05 vs. OGD/R group.

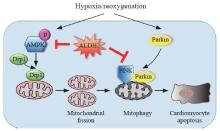
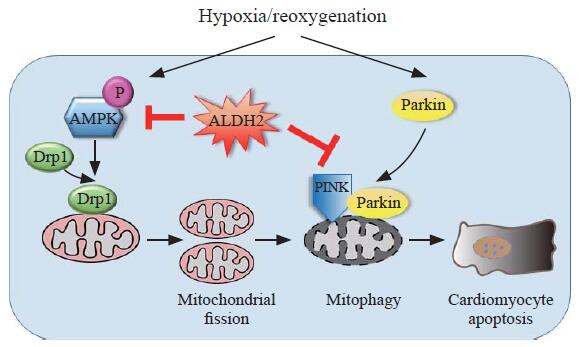
Figure 4.
Schematic illustration of the protective effects of ALDH2 on H/R-induced cardiomyocyte damage. Under H/R, on the one hand, AMPK and Drp1 are activated by phosphorylation which triggers excessive mitochondrial fission, leading to apoptosis; on the other hand, demonstrated by our previous study,[13] PINK/Parkin-dependent mitophagy is activated, contributing to apoptosis; through suppressing either AMPK/Drp1 pathway or PINK/Parkin pathway, ALDH2 could exert cardioprotection effects.
| 1 |
Vogel B, Claessen BE, Arnold SV, Chan D, Cohen DJ, Giannitsis E, et al. ST-segment elevation myocardial infarction. Nat Rev Dis Primers. 2019; 5(1):39.
doi: 10.1038/s41572-019-0090-3 pmid: 31171787 |
| 2 |
Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013; 123(1):92-100.
doi: 10.1172/JCI62874 |
| 3 |
Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007; 357(11):1121-35.
doi: 10.1056/NEJMra071667 pmid: 17855673 |
| 4 |
Dorn GW, 2nd. Mitochondrial dynamics in heart disease. Biochim Biophys Acta. 2013; 1833(1):233-41.
doi: 10.1016/j.bbamcr.2012.03.008 pmid: 22450031 |
| 5 |
Archer SL. Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. N Engl J Med. 2013; 369(23):2236-51.
doi: 10.1056/NEJMra1215233 pmid: 24304053 |
| 6 |
Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010; 121(18):2012-22.
doi: 10.1161/CIRCULATIONAHA.109.906610 pmid: 20421521 |
| 7 |
Dorn GW, 2nd. Evolving concepts of mitochondrial dynamics. Annu Rev Physiol. 2019; 81:1-17.
doi: 10.1146/annurev-physiol-020518-114358 pmid: 30256725 |
| 8 |
Li Y, Liu X. Novel insights into the role of mitochondrial fusion and fission in cardiomyocyte apoptosis induced by ischemia/reperfusion. J Cell Physiol. 2018; 233(8):5589-97.
doi: 10.1002/jcp.26522 pmid: 29528108 |
| 9 |
Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008; 321(5895):1493-5.
doi: 10.1126/science.1158554 pmid: 18787169 |
| 10 |
Guo JM, Liu AJ, Zang P, Dong WZ, Ying L, Wang W, et al. ALDH2 protects against stroke by clearing 4-HNE. Cell Res. 2013; 23(7):915-30.
doi: 10.1038/cr.2013.69 |
| 11 |
Ding J, Zhang Q, Luo Q, Ying Y, Liu Y, Li Y, et al. Alda-1 attenuates lung ischemia-reperfusion injury by reducing 4-hydroxy-2-nonenal in alveolar epithelial cells. Crit Care Med. 2016; 44(7):e544-52.
doi: 10.1097/CCM.0000000000001563 pmid: 26757166 |
| 12 |
Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J. 2011; 32(8):1025-38.
doi: 10.1093/eurheartj/ehq253 |
| 13 |
Ji W, Wei S, Hao P, Xing J, Yuan Q, Wang J, et al. Aldehyde dehydrogenase 2 has cardioprotective effects on myocardial ischaemia/reperfusion injury via suppressing mitophagy. Front Pharmacol. 2016; 7:101.
doi: 10.3389/fphar.2016.00101 pmid: 27148058 |
| 14 |
Toyama EQ, Herzig S, Courchet J, Lewis TL, Loson OC, Hellberg K, et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science. 2016; 351(6270):275-81.
doi: 10.1126/science.aab4138 pmid: 26816379 |
| 15 |
Liu B, Zhang R, Wei S, Yuan Q, Xue M, Hao P, et al. ALDH2 protects against alcoholic cardiomyopathy through a mechanism involving the p38 MAPK/CREB pathway and local renin-angiotensin system inhibition in cardiomyocytes. Int J Cardiol. 2018; 257:150-59.
doi: 10.1016/j.ijcard.2017.11.094 pmid: 29506687 |
| 16 |
Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009; 119(5):1275-85.
doi: 10.1172/JCI37829 pmid: 19349686 |
| 17 |
Wang K, Long B, Zhou LY, Liu F, Zhou QY, Liu CY, et al. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat Commun. 2014; 5:3596.
doi: 10.1038/ncomms4596 pmid: 24710105 |
| 18 |
Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, et al. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013; 2(5):e000461.
doi: 10.1161/JAHA.113.000461 pmid: 24103571 |
| 19 |
Maneechote C, Palee S, Chattipakorn SC, Chattipakorn N. Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J Cell Mol Med. 2017; 21(11):2643-53.
doi: 10.1111/jcmm.13330 pmid: 28941171 |
| 20 |
Braun T, Bober E, Singh S, Agarwal DP, Goedde HW. Evidence for a signal peptide at the amino-terminal end of human mitochondrial aldehyde dehydrogenase. FEBS Lett. 1987; 215(2):233-6.
doi: 10.1016/0014-5793(87)80152-7 pmid: 3582651 |
| 21 |
Tilokani L, Nagashima S, Paupe V, Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 2018; 62(3):341-60.
doi: 10.1042/EBC20170104 pmid: 30030364 |
| 22 |
Kraus F, Ryan MT. The constriction and scission machineries involved in mitochondrial fission. J Cell Sci. 2017; 130(18):2953-60.
doi: 10.1242/jcs.199562 pmid: 28842472 |
| 23 |
Ong SB, Hausenloy DJ. Mitochondrial morphology and cardiovascular disease. Cardiovasc Res. 2010; 88(1):16-29.
doi: 10.1093/cvr/cvq237 pmid: 20631158 |
| 24 |
Long B, Wang K, Li N, Murtaza I, Xiao JY, Fan YY, et al. miR-761 regulates the mitochondrial network by targeting mitochondrial fission factor. Free Radic Biol Med. 2013; 65:371-79.
doi: 10.1016/j.freeradbiomed.2013.07.009 pmid: 23867156 |
| 25 |
Wang K, Zhang DL, Long B, An T, Zhang J, Zhou LY, et al. NFAT4-dependent miR-324-5p regulates mitochondrial morphology and cardiomyocyte cell death by targeting Mtfr1. Cell Death Dis. 2015; 6:e2007.
doi: 10.1038/cddis.2015.348 pmid: 26633713 |
| 26 |
Wang K, Gan TY, Li N, Liu CY, Zhou LY, Gao JN, et al. Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ. 2017; 24(6):1111-20.
doi: 10.1038/cdd.2017.61 pmid: 28498369 |
| 27 |
Zhao L, Zhuang J, Wang Y, Zhou D, Zhao D, Zhu S, et al. Propofol ameliorates H9c2 cells apoptosis induced by oxygen-glucose deprivation and reperfusion injury via inhibiting high levels of mitochondrial fusion and fission. Front Pharmacol. 2019; 10:61.
doi: 10.3389/fphar.2019.00061 pmid: 30809145 |
| 28 |
Cellier L, Tamareille S, Kalakech H, Guillou S, Lenaers G, Prunier F, et al. Remote ischemic conditioning influences mitochondrial dynamics. Shock. 2016; 45(2):192-7.
doi: 10.1097/SHK.0000000000000500 pmid: 26555744 |
| 29 |
Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, et al. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J. 2014; 28(1):316-26.
doi: 10.1096/fj.12-226225 |
| 30 |
Veeranki S, Tyagi SC. Mdivi-1 induced acute changes in the angiogenic profile after ischemia-reperfusion injury in female mice. Physiol Rep. 2017; 5(11):e13298.
doi: 10.14814/phy2.13298 pmid: 28576854 |
| 31 |
Zepeda R, Kuzmicic J, Parra V, Troncoso R, Pennanen C, Riquelme JA, et al. Drp1 loss-of-function reduces cardiomyocyte oxygen dependence protecting the heart from ischemia-reperfusion injury. J Cardiovasc Pharmacol. 2014; 63(6):477-87.
doi: 10.1097/FJC.0000000000000071 pmid: 24477044 |
| 32 |
Din S, Mason M, Volkers M, Johnson B, Cottage CT, Wang Z, et al. Pim-1 preserves mitochondrial morphology by inhibiting dynamin-related protein 1 translocation. Proc Natl Acad Sci U S A. 2013; 110(15):5969-74.
doi: 10.1073/pnas.1213294110 pmid: 23530233 |
| 33 |
Qi D, Young LH. AMPK: energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab. 2015; 26(8):422-9.
doi: 10.1016/j.tem.2015.05.010 pmid: 26160707 |
| 34 |
Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011; 331(6016):456-61.
doi: 10.1126/science.1199113 |
| 35 |
Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011; 13(2):132-41.
doi: 10.1038/ncb2152 pmid: 21258367 |
| 36 |
Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol. 2006; 574(Pt 1):95-112.
doi: 10.1113/jphysiol.2006.109389 pmid: 16690706 |
| 37 |
Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, et al. Characterization of 5’AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta. 1996; 1301(1-2):67-75.
doi: 10.1016/0005-2760(96)00013-6 pmid: 8652652 |
| 38 |
Jaswal JS, Gandhi M, Finegan BA, Dyck JR, Clanachan AS. Inhibition of p38 MAPK and AMPK restores adenosine-induced cardioprotection in hearts stressed by antecedent ischemia by altering glucose utilization. Am J Physiol Heart Circ Physiol. 2007; 293(2):H1107-14.
doi: 10.1152/ajpheart.00455.2007 pmid: 17496214 |
| 39 |
Capano M, Crompton M. Bax translocates to mitochondria of heart cells during simulated ischaemia: involvement of AMP-activated and p38 mitogen-activated protein kinases. Biochem J. 2006; 395(1):57-64.
doi: 10.1042/BJ20051654 pmid: 16321138 |
| 40 |
Bairwa SC, Parajuli N, Dyck JR. The role of AMPK in cardiomyocyte health and survival. Biochim Biophys Acta. 2016; 1862(12):2199-210.
doi: 10.1016/j.bbadis.2016.07.001 pmid: 27412473 |
| 41 |
Ge W, Guo R, Ren J. AMP-dependent kinase and autophagic flux are involved in aldehyde dehydrogenase-2-induced protection against cardiac toxicity of ethanol. Free Radic Biol Med. 2011; 51(9):1736-48.
doi: 10.1016/j.freeradbiomed.2011.08.002 pmid: 21871561 |
| 42 |
Li C, Sun W, Gu C, Yang Z, Quan N, Yang J, et al. Targeting ALDH2 for therapeutic interventions in chronic pain-related myocardial ischemic susceptibility. Theranostics. 2018; 8(4):1027-41.
doi: 10.7150/thno.22414 pmid: 29463997 |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
