12
2023
... More than 4,000 species of snakes are found worldwide, with more than half being colubrid snakes and approximately 20% being venomous snakes (over 800 species), including more than 400 species of cobras and more than 380 species of viper.[1] Nearly 250 species of venomous snakes are of great medical importance.[2] Snakebites are an important public health concern, mainly affecting tropical areas on both sides of the equator, with the highest incidence in Southeast Asia, sub-Saharan Africa, and South America. Approximately 95% of snakebites occur in developing countries. Out of the 4.5-5.4 million cases of snakebites worldwide each year, approximately 20% are “dry bites” without detoxification (i.e., no release of toxins during the bite, no symptoms or signs of poisoning or only minor wound manifestations).[3]Approximately 1.8-2.7 million people are severely poisoned, and nearly 400,000 snakebite victims develop varying degrees of disability.[4-5] Snakebites cause 81,000-138,000 deaths worldwide, with a mortality rate of up to 0.8/100,000.[6] ...
... China has a diverse range of snake species distributed throughout the country, mainly south of the Yangtze River. More than 300 species of snakes have been identified, including more than 100 species of venomous snakes, of which more than 80 species are highly toxic species of the Elapidae and Viperidae families, and a few are venomous species of the family Colubridae.[1] WHO has identified 23 medically important venomous snake species in China.[2] An estimated 250,000-280,000 cases of venomous snakebites occur in China each year, affecting mainly patients aged ≥50 years. Limbs are the most frequently bitten parts of the body, with lower limbs being more commonly affected than upper limbs. Snakebites frequently occur from April to October each year, with the peak incidence occurring from July to September.[1] There are significant differences in the venomous snake spectrum among Chinese provinces and regions, and the level of treatment for snakebites varies greatly from region to region, resulting in high mortality and disability rates. This guideline aims to improve public awareness of snakebites and facilitate standardization of the clinical treatment of snakebites. The overarching objective is to reduce snakebite-associated mortality and disability. ...
... [1] There are significant differences in the venomous snake spectrum among Chinese provinces and regions, and the level of treatment for snakebites varies greatly from region to region, resulting in high mortality and disability rates. This guideline aims to improve public awareness of snakebites and facilitate standardization of the clinical treatment of snakebites. The overarching objective is to reduce snakebite-associated mortality and disability. ...
... Toxins act on the neuromuscular junction of skeletal muscle. The main targets of toxins are acetylcholine receptors on motor nerve endings (presynaptic membrane) and motor endplates (postsynaptic membrane), resulting in flaccid paralysis, which is observed in most Elapidae and a few viper venom bites. β-bungarotoxin, PLA2, and dendrimer toxins act on the presynaptic membrane; α-bungarotoxin, weak toxin, black green ironhead toxin, and PLA2 act on the postsynaptic membrane; acetylcholinesterase acts on the synaptic cleft.[15] Most venomous snakes contain only a single neurotoxin that binds to the presynaptic membrane (such as the Sri Lankan viper, many-banded krait, and Coastal Taipan) or the postsynaptic membrane (such as the king cobra and cobra). Many-banded krait venom contains α-bungarotoxin, β-bungarotoxin, κ-bungarotoxin, and γ-bungarotoxin, which affect both presynaptic and postsynaptic membranes.[1,22] Most toxins have a high affinity for binding to neuromuscular receptors and are not easily dissociated, especially after binding to presynaptic receptors, which impedes clinical recovery. However, the effects of postsynaptic neurotoxins can be rapidly reversed by antivenom serum. [23-24] The three-finger toxin PLA2 and weak toxin of the Bengal cobra have certain effects on the autonomic nervous system.[15] ...
... Neurotoxic snakes such as Bungarus multicinctus mainly cause flaccid and descending paralysis, gradually affecting muscles innervated by cranial nerves as well as cervical flexor muscles, medulla oblongata, respiratory muscles, trunk and limb muscles. Typical descending paralysis first involves the eyelid muscles, manifesting as bilateral ptosis, typically occurring within hours of the bite. Next, the external eye muscles are involved, causing diplopia, fixed pupil dilation, facial paralysis with slurred speech and difficulty opening the mouth. The palate, mandible, tongue, and throat are subsequently involved, resulting in the accumulation of pharyngeal secretions and the loss of the pharyngeal reflex. Then, the paralysis symptoms develop and continue to descend to the neck muscles and medulla oblongata muscles. The involvement of medulla oblongata causes difficulty in swallowing and the loss of airway protection function, leading to a high risk of aspiration or suffocation. Cervical muscle paralysis may manifest as a soft and weak neck. The involvement of respiratory muscles leads to shallow and rapid breathing, decreased ventilation capacity, abnormal abdominal breathing, use of auxiliary muscles, and cyanosis. Once severe dyspnea occurs, it will quickly lead to respiratory arrest. The time elapsed between snakebite and the occurrence of respiratory failure ranges from 30 min to more than 24 h, with an average of 6-12 h. Finally, the limb muscles are involved, manifesting as weakness. The proximal muscles are involved first, followed by the distal muscles. In severe cases, complete paralysis of the limbs may occur, with attenuation or disappearance of deep tendon reflexes. The recovery of neurological function usually occurs in the reverse sequence. Distal muscle strength first recovers, followed by a gradual recovery of proximal muscle strength, and finally upper eyelid ptosis and ophthalmoplegia.[1,27,31] ...
... There are many methods for determining the severity of snakebites, and each evaluation method has advantages and disadvantages. This guideline recommends two evaluation methods for clinical reference. A simple method for assessing clinical severity is presented in Table 1.[1] This method is easy to remember and practice, suitable for clinical judgment by emergency physicians, but the content is relatively rough. ...
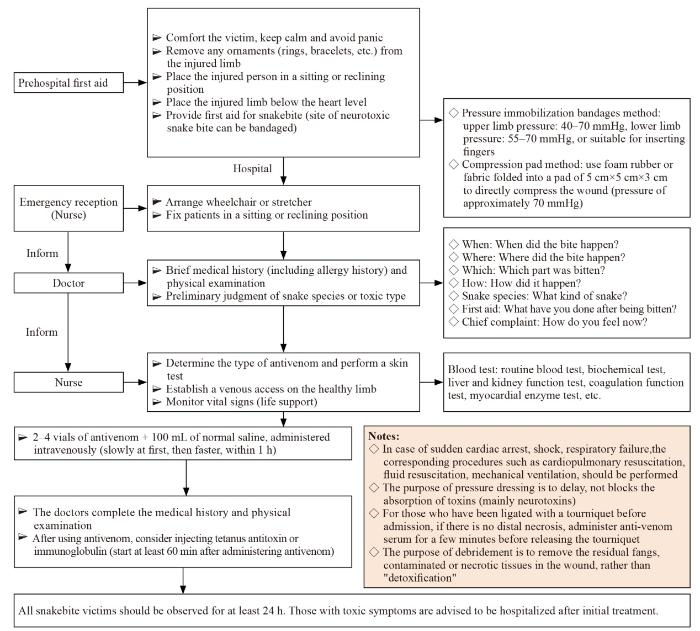
... On-site first aid aims to ensure the safety and physical integrity of snakebite victims, delay toxin absorption, prevent complications, and ensure immediate transport of the victim to a medical center capable of treating snakebites to minimize additional harm. In order not to delay first aid, refer to the protocol for snakebites (Figure 1). The following first aid methods can be used:[1,48⇓-50] ...
... Most of the conscious patients with ACS only present with pain and sensory abnormalities; manifestations of arterial ischemia, such as pallor, numbness, and pulselessness, are rare or occur only in the later stages of severe ACS. Therefore, it is not possible to determine compartment syndrome on the basis of “soft signs”, such as local swelling and hardening, disproportionate pain, and stretch pain. The indications for decompression of the SVCS include at least the following four criteria:[1] (1) coagulopathy is corrected or significantly improved; (2) clinical indications consistent with ACS; (3) pressure difference (△P = diastolic pressure - intra-fascial pressure) ≤30 mmHg or absolute pressure in the fascia compartment >40 mmHg; and (4) presence of signs of neurological and/or vascular damage and blood flow impairment. △P>30 mmHg can be used as an exclusion criterion for SVCS.[91] ...
... After using sufficient amounts of antivenom, providing positional drainage can facilitate the reabsorption of fluid in the tissue space of the swollen area, thereby reducing local pressure, relieving swelling, and alleviating swelling-related pain. The swelling of the affected limb can be reduced by elevating it not lower than the sternal angle.[1,111] ...
... Various hemotoxic and cytotoxic snakebites can produce local tension blisters or blood blisters. High-tension bullae are at risk of rupture or tearing and secondary infection, delaying wound healing. A sterile syringe can be used to aspirate the blister fluid in a low-lying position, or a microincision can be made to promote the outflow of the blister fluid. After the blister fluid is released, it can be lightly compressed with sterile gauze to avoid the recurrence of the blister. In cases of suspected infection of the blister fluid, it should be sent for culture to facilitate the timely detection of pathogenic bacteria and the use of appropriate antibiotics.[1] ...
... Traditional Chinese medicine has a long history of treating venomous snakebites. The oral and external administration of Chinese medicine, as well as acupuncture and cupping, could improve local and systemic symptoms and enhance therapeutic effects.[146] According to traditional Chinese medicine theory, the basic pathogenesis of snakebite toxicity is the lack of discharge of snake venom and the internalization of the toxin.[147] Snakebites are divided into three types: “wind toxins”, “fire toxins”, and “wind-fire toxins”. According to the syndrome type and clinical manifestations, the treatment of snakebites is based on the identification and application of methods for clearing away heat and detoxification, dispelling wind and opening up orifices, cooling the blood and stopping hemorrhage, and promoting diuresis and catharsis.[148] Alkaloids, flavonoids, phenols, and other active ingredients in some proprietary traditional Chinese medicine snakebite detoxification preparations have a certain inhibitory effect on enzyme snake venom components such as PLA2 and protein hydrolase.[149-150] Some traditional Chinese medicines have anti-inflammatory and antioxidant effects, which can help reduce swelling.[1] For ulcers caused by snakebites, in addition to sufficient antivenom and local debridement, traditional Chinese medicine that promote pus removal, tissue regeneration, and wound healing can be administered as adjunctive treatments.[151,152]A large-sample meta-analysis indicated that traditional Chinese medicines used for the treatment of pit viper bites can improve local symptoms and reduce swelling,[153] but more robust studies are required to confirm the reliability of the conclusions. ...
... Maintaining a clean environment, including cleaning up the dead leaves and weeds surrounding the house, is important. On encountering a snake, one should avoid provoking it. Usually, a snake’s attack range is approximately half of its body length, averaging 0.30-0.60 m. A few venomous snakes, such as Chinese cobras, spraying cobras, and cervical groove snakes, can spray venom several times up to a distance of 1.0-2.5 m.[1] ...
2
... More than 4,000 species of snakes are found worldwide, with more than half being colubrid snakes and approximately 20% being venomous snakes (over 800 species), including more than 400 species of cobras and more than 380 species of viper.[1] Nearly 250 species of venomous snakes are of great medical importance.[2] Snakebites are an important public health concern, mainly affecting tropical areas on both sides of the equator, with the highest incidence in Southeast Asia, sub-Saharan Africa, and South America. Approximately 95% of snakebites occur in developing countries. Out of the 4.5-5.4 million cases of snakebites worldwide each year, approximately 20% are “dry bites” without detoxification (i.e., no release of toxins during the bite, no symptoms or signs of poisoning or only minor wound manifestations).[3]Approximately 1.8-2.7 million people are severely poisoned, and nearly 400,000 snakebite victims develop varying degrees of disability.[4-5] Snakebites cause 81,000-138,000 deaths worldwide, with a mortality rate of up to 0.8/100,000.[6] ...
... China has a diverse range of snake species distributed throughout the country, mainly south of the Yangtze River. More than 300 species of snakes have been identified, including more than 100 species of venomous snakes, of which more than 80 species are highly toxic species of the Elapidae and Viperidae families, and a few are venomous species of the family Colubridae.[1] WHO has identified 23 medically important venomous snake species in China.[2] An estimated 250,000-280,000 cases of venomous snakebites occur in China each year, affecting mainly patients aged ≥50 years. Limbs are the most frequently bitten parts of the body, with lower limbs being more commonly affected than upper limbs. Snakebites frequently occur from April to October each year, with the peak incidence occurring from July to September.[1] There are significant differences in the venomous snake spectrum among Chinese provinces and regions, and the level of treatment for snakebites varies greatly from region to region, resulting in high mortality and disability rates. This guideline aims to improve public awareness of snakebites and facilitate standardization of the clinical treatment of snakebites. The overarching objective is to reduce snakebite-associated mortality and disability. ...
Current knowledge on snake dry bites
2
2020
... More than 4,000 species of snakes are found worldwide, with more than half being colubrid snakes and approximately 20% being venomous snakes (over 800 species), including more than 400 species of cobras and more than 380 species of viper.[1] Nearly 250 species of venomous snakes are of great medical importance.[2] Snakebites are an important public health concern, mainly affecting tropical areas on both sides of the equator, with the highest incidence in Southeast Asia, sub-Saharan Africa, and South America. Approximately 95% of snakebites occur in developing countries. Out of the 4.5-5.4 million cases of snakebites worldwide each year, approximately 20% are “dry bites” without detoxification (i.e., no release of toxins during the bite, no symptoms or signs of poisoning or only minor wound manifestations).[3]Approximately 1.8-2.7 million people are severely poisoned, and nearly 400,000 snakebite victims develop varying degrees of disability.[4-5] Snakebites cause 81,000-138,000 deaths worldwide, with a mortality rate of up to 0.8/100,000.[6] ...
... The clinical manifestations of venomous snakebites vary depending on the snake species or toxin content. Approximately 20% (1.75%-50%) of venomous snakebites are “dry bites”.[3] The severity of toxic manifestations depends on the amount of venom released by the snake and the time elapsed between the bite and medical treatment.[29] The main clinical manifestations are the neurotoxic triad (bilateral ptosis, descending paralysis, dyspnea/acute respiratory failure), the hematotoxic triad (consumptive coagulopathy, local bleeding, and systemic bleeding), and the cytotoxic triad (severe pain, progressive swelling, and tissue damage). Some Colubridae are known to produce varying degrees of toxic effects.[30] Most victims present with mild pain, tooth marks or lacerations, local mild reactive edema, or small amounts of bleeding, which usually resolve within 24-36 h. A minority of cases exhibit severe symptoms. ...
Clinical aspects of snakebite envenoming and its treatment in low-resource settings
1
2023
... More than 4,000 species of snakes are found worldwide, with more than half being colubrid snakes and approximately 20% being venomous snakes (over 800 species), including more than 400 species of cobras and more than 380 species of viper.[1] Nearly 250 species of venomous snakes are of great medical importance.[2] Snakebites are an important public health concern, mainly affecting tropical areas on both sides of the equator, with the highest incidence in Southeast Asia, sub-Saharan Africa, and South America. Approximately 95% of snakebites occur in developing countries. Out of the 4.5-5.4 million cases of snakebites worldwide each year, approximately 20% are “dry bites” without detoxification (i.e., no release of toxins during the bite, no symptoms or signs of poisoning or only minor wound manifestations).[3]Approximately 1.8-2.7 million people are severely poisoned, and nearly 400,000 snakebite victims develop varying degrees of disability.[4-5] Snakebites cause 81,000-138,000 deaths worldwide, with a mortality rate of up to 0.8/100,000.[6] ...
Snake-bite envenoming: a priority neglected tropical disease
1
2017
... More than 4,000 species of snakes are found worldwide, with more than half being colubrid snakes and approximately 20% being venomous snakes (over 800 species), including more than 400 species of cobras and more than 380 species of viper.[1] Nearly 250 species of venomous snakes are of great medical importance.[2] Snakebites are an important public health concern, mainly affecting tropical areas on both sides of the equator, with the highest incidence in Southeast Asia, sub-Saharan Africa, and South America. Approximately 95% of snakebites occur in developing countries. Out of the 4.5-5.4 million cases of snakebites worldwide each year, approximately 20% are “dry bites” without detoxification (i.e., no release of toxins during the bite, no symptoms or signs of poisoning or only minor wound manifestations).[3]Approximately 1.8-2.7 million people are severely poisoned, and nearly 400,000 snakebite victims develop varying degrees of disability.[4-5] Snakebites cause 81,000-138,000 deaths worldwide, with a mortality rate of up to 0.8/100,000.[6] ...
Global mortality of snakebite envenoming between 1990 and 2019
1
2022
... More than 4,000 species of snakes are found worldwide, with more than half being colubrid snakes and approximately 20% being venomous snakes (over 800 species), including more than 400 species of cobras and more than 380 species of viper.[1] Nearly 250 species of venomous snakes are of great medical importance.[2] Snakebites are an important public health concern, mainly affecting tropical areas on both sides of the equator, with the highest incidence in Southeast Asia, sub-Saharan Africa, and South America. Approximately 95% of snakebites occur in developing countries. Out of the 4.5-5.4 million cases of snakebites worldwide each year, approximately 20% are “dry bites” without detoxification (i.e., no release of toxins during the bite, no symptoms or signs of poisoning or only minor wound manifestations).[3]Approximately 1.8-2.7 million people are severely poisoned, and nearly 400,000 snakebite victims develop varying degrees of disability.[4-5] Snakebites cause 81,000-138,000 deaths worldwide, with a mortality rate of up to 0.8/100,000.[6] ...
Manson’s tropical diseases
2
1914
... Snake venom is a complex high-efficiency toxin mixture characterized by its pale yellow or milky white, translucent, and viscous liquid appearance. It is mainly composed of proteins (such as enzymes, polypeptides, and glycoproteins) and non-protein ingredients (such as lipids, metal ions, and biogenic amines). Proteins account for 90%-95% of the dry weight of snake venom.[7-8] Each venomous snake contains a diverse array of toxins, ranging from 20 to over 100 different components, and the types of toxins vary within and between species,[9-10] depending on the region, season, and age of the snake.[11-12] The toxins of the Cobra family are dominated by three-finger toxins and phospholipase A2 (PLA2), while those of the Viper family are dominated by snake venom metalloproteinases, PLA2, and snake venom serine proteases.[13] Snake venom proteins can produce various toxic effects depending on their targets.[9,14-15] For example, PLA2 mainly acts on various receptors in the plasma membrane and axonal membrane of muscle cells.[16] Snake venom metalloproteinases have multiple targets, the most notable being type IV collagen and coagulation factors.[17] Snake venom serine proteases mainly affect coagulation factors.[9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
... Most acute adverse reactions occur within 1-2 h after the initiation of antivenom treatment. Close monitoring for adverse reactions, focusing on observing the aggravation and amelioration of toxic symptoms, signs, and laboratory indices, is necessary to facilitate the timely administration of additional treatment or calibration of medication. Depending on the severity of envenomation, patients should be monitored at least 2, 6, 12, and 24 h after the first use of antivenom. Blood routine, coagulation function, (cardiac) muscle enzymes, and other laboratory tests should be repeated at 6, 12, and 24 h.[74] After a sufficient neutralizing dose of antivenom is used, the median recovery time for coagulation dysfunction is 6 h.[7,23,77] Therefore, indications for additional medication include persistence of toxic symptoms 6 h after the first dose of antivenom, re-aggravation of coagulation dysfunction after transient recovery, recurrence of bleeding 1-2 h after cessation of bleeding, and continued deterioration of neurological or cardiovascular function for 1 h after the first dose of antivenom. ...
Pharmacokinetics of snake venom
1
2018
... Snake venom is a complex high-efficiency toxin mixture characterized by its pale yellow or milky white, translucent, and viscous liquid appearance. It is mainly composed of proteins (such as enzymes, polypeptides, and glycoproteins) and non-protein ingredients (such as lipids, metal ions, and biogenic amines). Proteins account for 90%-95% of the dry weight of snake venom.[7-8] Each venomous snake contains a diverse array of toxins, ranging from 20 to over 100 different components, and the types of toxins vary within and between species,[9-10] depending on the region, season, and age of the snake.[11-12] The toxins of the Cobra family are dominated by three-finger toxins and phospholipase A2 (PLA2), while those of the Viper family are dominated by snake venom metalloproteinases, PLA2, and snake venom serine proteases.[13] Snake venom proteins can produce various toxic effects depending on their targets.[9,14-15] For example, PLA2 mainly acts on various receptors in the plasma membrane and axonal membrane of muscle cells.[16] Snake venom metalloproteinases have multiple targets, the most notable being type IV collagen and coagulation factors.[17] Snake venom serine proteases mainly affect coagulation factors.[9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
The chemistry of snake venom and its medicinal potential
3
2022
... Snake venom is a complex high-efficiency toxin mixture characterized by its pale yellow or milky white, translucent, and viscous liquid appearance. It is mainly composed of proteins (such as enzymes, polypeptides, and glycoproteins) and non-protein ingredients (such as lipids, metal ions, and biogenic amines). Proteins account for 90%-95% of the dry weight of snake venom.[7-8] Each venomous snake contains a diverse array of toxins, ranging from 20 to over 100 different components, and the types of toxins vary within and between species,[9-10] depending on the region, season, and age of the snake.[11-12] The toxins of the Cobra family are dominated by three-finger toxins and phospholipase A2 (PLA2), while those of the Viper family are dominated by snake venom metalloproteinases, PLA2, and snake venom serine proteases.[13] Snake venom proteins can produce various toxic effects depending on their targets.[9,14-15] For example, PLA2 mainly acts on various receptors in the plasma membrane and axonal membrane of muscle cells.[16] Snake venom metalloproteinases have multiple targets, the most notable being type IV collagen and coagulation factors.[17] Snake venom serine proteases mainly affect coagulation factors.[9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
... [9,14-15] For example, PLA2 mainly acts on various receptors in the plasma membrane and axonal membrane of muscle cells.[16] Snake venom metalloproteinases have multiple targets, the most notable being type IV collagen and coagulation factors.[17] Snake venom serine proteases mainly affect coagulation factors.[9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
... [9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
Causes and consequences of snake venom variation
2
2020
... Snake venom is a complex high-efficiency toxin mixture characterized by its pale yellow or milky white, translucent, and viscous liquid appearance. It is mainly composed of proteins (such as enzymes, polypeptides, and glycoproteins) and non-protein ingredients (such as lipids, metal ions, and biogenic amines). Proteins account for 90%-95% of the dry weight of snake venom.[7-8] Each venomous snake contains a diverse array of toxins, ranging from 20 to over 100 different components, and the types of toxins vary within and between species,[9-10] depending on the region, season, and age of the snake.[11-12] The toxins of the Cobra family are dominated by three-finger toxins and phospholipase A2 (PLA2), while those of the Viper family are dominated by snake venom metalloproteinases, PLA2, and snake venom serine proteases.[13] Snake venom proteins can produce various toxic effects depending on their targets.[9,14-15] For example, PLA2 mainly acts on various receptors in the plasma membrane and axonal membrane of muscle cells.[16] Snake venom metalloproteinases have multiple targets, the most notable being type IV collagen and coagulation factors.[17] Snake venom serine proteases mainly affect coagulation factors.[9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
... Homogeneity specificity refers to the use of specific antivenoms for specific venomous snakes, such as using corresponding antivenoms for Bungarus multicinctus, Naja atra, Agkistrodon acutus, and Pallas’s pit viper. Each venomous snake contains different types and levels of toxins, and there are cross-reactions and differences in content between different venomous snakes. The antivenom produced by a particular species of venom-immunized animals also contains multiple antibody components specific to different toxins. The specific antibodies in different varieties of antivenom vary, and there are also cross-reactions between antibody components.[10] Therefore, it is recommended to use a combination of the same or similar toxic antivenoms for bites from venomous snakes for which specific antivenom is not available. For bites from Trimeresurus stejnegeri or Protobothrops acutus, priority should be given to using antivenom against Pallas’ pit viper, followed by antivenom against Agkistrodon acutus or a combination of these. For bites from viper, antivenom against Pallas’ pit viper plus antivenom against Agkistrodon acutus should be used. For bites from Ophiophagus hannah, antivenom against Bungarus multicinctus plus antivenom against Naja atra should be used. For bites from Bungarus multicinctus, antivenom against Bungarus multicinctus plus antivenom against Naja atra should be used. For bites from Naja atra, antivenom against Naja atra should be used. For bites from sea snakes, antivenom against Bungarus multicinctus plus antivenom against Naja atra should be used. ...
Critical care toxicology: Diagnosis and management of the critically poisoned patient
1
2017
... Snake venom is a complex high-efficiency toxin mixture characterized by its pale yellow or milky white, translucent, and viscous liquid appearance. It is mainly composed of proteins (such as enzymes, polypeptides, and glycoproteins) and non-protein ingredients (such as lipids, metal ions, and biogenic amines). Proteins account for 90%-95% of the dry weight of snake venom.[7-8] Each venomous snake contains a diverse array of toxins, ranging from 20 to over 100 different components, and the types of toxins vary within and between species,[9-10] depending on the region, season, and age of the snake.[11-12] The toxins of the Cobra family are dominated by three-finger toxins and phospholipase A2 (PLA2), while those of the Viper family are dominated by snake venom metalloproteinases, PLA2, and snake venom serine proteases.[13] Snake venom proteins can produce various toxic effects depending on their targets.[9,14-15] For example, PLA2 mainly acts on various receptors in the plasma membrane and axonal membrane of muscle cells.[16] Snake venom metalloproteinases have multiple targets, the most notable being type IV collagen and coagulation factors.[17] Snake venom serine proteases mainly affect coagulation factors.[9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
Snake venomics: fundamentals, recent updates, and a look to the next decade
2
2022
... Snake venom is a complex high-efficiency toxin mixture characterized by its pale yellow or milky white, translucent, and viscous liquid appearance. It is mainly composed of proteins (such as enzymes, polypeptides, and glycoproteins) and non-protein ingredients (such as lipids, metal ions, and biogenic amines). Proteins account for 90%-95% of the dry weight of snake venom.[7-8] Each venomous snake contains a diverse array of toxins, ranging from 20 to over 100 different components, and the types of toxins vary within and between species,[9-10] depending on the region, season, and age of the snake.[11-12] The toxins of the Cobra family are dominated by three-finger toxins and phospholipase A2 (PLA2), while those of the Viper family are dominated by snake venom metalloproteinases, PLA2, and snake venom serine proteases.[13] Snake venom proteins can produce various toxic effects depending on their targets.[9,14-15] For example, PLA2 mainly acts on various receptors in the plasma membrane and axonal membrane of muscle cells.[16] Snake venom metalloproteinases have multiple targets, the most notable being type IV collagen and coagulation factors.[17] Snake venom serine proteases mainly affect coagulation factors.[9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
... Cytotoxicity is an important effect of the family of low-molecular-weight non-enzymatic three-finger toxins widely present in the venom of the cobra family and a few Viperidae snakes. The Chinese cobra has the highest cytotoxic content among Asian cobras, accounting for approximately 70% of the total toxins.[12] Cytotoxins affect cell membrane structure and membrane-binding proteins, activating apoptosis and necrotic cell death pathways, which are mostly non-specific effects, resulting in local tissue and skin damage. Cytotoxins in the cobra venom can also depolarize neurons and myocardium, leading to heart failure. ...
Snakebite envenoming
4
2017
... Snake venom is a complex high-efficiency toxin mixture characterized by its pale yellow or milky white, translucent, and viscous liquid appearance. It is mainly composed of proteins (such as enzymes, polypeptides, and glycoproteins) and non-protein ingredients (such as lipids, metal ions, and biogenic amines). Proteins account for 90%-95% of the dry weight of snake venom.[7-8] Each venomous snake contains a diverse array of toxins, ranging from 20 to over 100 different components, and the types of toxins vary within and between species,[9-10] depending on the region, season, and age of the snake.[11-12] The toxins of the Cobra family are dominated by three-finger toxins and phospholipase A2 (PLA2), while those of the Viper family are dominated by snake venom metalloproteinases, PLA2, and snake venom serine proteases.[13] Snake venom proteins can produce various toxic effects depending on their targets.[9,14-15] For example, PLA2 mainly acts on various receptors in the plasma membrane and axonal membrane of muscle cells.[16] Snake venom metalloproteinases have multiple targets, the most notable being type IV collagen and coagulation factors.[17] Snake venom serine proteases mainly affect coagulation factors.[9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
... Snakebite is a time-critical emergency. The antivenom binds to snake venom to exert its anti-toxic effect, and the time of initiation of antivenom treatment is directly related to the prognosis. It is critical to shorten the time from the bite of a venomous snake to the use of antivenom.[54⇓-56] This can reverse VICC, hypotension, and postsynaptic neurotoxicity. Early medication can prevent or limit presynaptic neurotoxicity, rhabdomyolysis, and local tissue necrosis.[13] Delayed use of antivenoms increases the risk of death.[57] Antivenoms are antidotes. Early and sufficient antivenom can effectively prevent subsequent damage from snake venom. The sooner it is used, the less damage the venom causes to the tissue and the better the prognosis.[58] As long as it is confirmed or highly suspected that there is a venomous snakebite with progressive intoxication or abnormal laboratory results, antivenom should be administered immediately without waiting for the onset of typical toxic manifestations. If the amount of antivenom is insufficient, the unneutralized toxins in the tissue can still produce toxic manifestations nearly 200 h or longer after the bite.[59-60] Therefore, if antivenom is not used in sufficient amounts in the early stage, as long as the toxic damage continues, antivenom should still be considered several days or even longer after the bite. Antivenom has been shown to be effective even 17 d after the bite of a venomous snake.[61] ...
... Multiple aerobic and anaerobic Gram-positive or Gram-negative pathogenic microorganisms are found in viper oral flora cultures.[112-113] The reported incidence of secondary bacterial infection in venomous snakebite wounds (10.3%-47.5%)[114⇓-116] is much higher than that in non-venomous snakebite wounds.[114] The infection rate in Chinese cobra bite wounds can be as high as 80.9%.[117] However, there is no need for routine infection prevention after snakebites. Once infected, approximately 68% of wounds require surgical intervention.[117] Anti-infection treatment is only suitable for patients with localized or suspected infections (such as abscess formation, increased wound secretion or odor, cellulitis, or positive microbial culture of secretions) or patients with localized tissue necrosis/gangrene.[13] Patients with confirmed infections should be administered anti-infection treatment promptly to prevent or ameliorate local tissue necrosis, systemic infection, and sepsis.[118] Patients with high fibrinogen, elevated alanine aminotransferase or C-reactive protein levels, and severe poisoning are at an increased risk of wound infection.[119] Common pathogens include Morganella, Enterococcus, Bacteroides fragilis, Escherichia coli, and Staphylococcus aureus. Empirical anti-infection treatment options include amoxicillin/clavulanic acid, fluoroquinolones, cefazolin, third-generation antibiotics against spore-forming bacteria, and aminoglycosides.[120⇓-122] Antimicrobial agents should be used based on clinical findings and drug sensitivity results. ...
... If bitten by a snake, measures such as cauterization, incision, and sucking should be avoided at the scene to prevent potential injuries.[13] The patient should be promptly taken to a hospital capable of treating snakebites. ...
A current perspective on snake venom composition and constituent protein families
1
2023
... Snake venom is a complex high-efficiency toxin mixture characterized by its pale yellow or milky white, translucent, and viscous liquid appearance. It is mainly composed of proteins (such as enzymes, polypeptides, and glycoproteins) and non-protein ingredients (such as lipids, metal ions, and biogenic amines). Proteins account for 90%-95% of the dry weight of snake venom.[7-8] Each venomous snake contains a diverse array of toxins, ranging from 20 to over 100 different components, and the types of toxins vary within and between species,[9-10] depending on the region, season, and age of the snake.[11-12] The toxins of the Cobra family are dominated by three-finger toxins and phospholipase A2 (PLA2), while those of the Viper family are dominated by snake venom metalloproteinases, PLA2, and snake venom serine proteases.[13] Snake venom proteins can produce various toxic effects depending on their targets.[9,14-15] For example, PLA2 mainly acts on various receptors in the plasma membrane and axonal membrane of muscle cells.[16] Snake venom metalloproteinases have multiple targets, the most notable being type IV collagen and coagulation factors.[17] Snake venom serine proteases mainly affect coagulation factors.[9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
6
2017
... Snake venom is a complex high-efficiency toxin mixture characterized by its pale yellow or milky white, translucent, and viscous liquid appearance. It is mainly composed of proteins (such as enzymes, polypeptides, and glycoproteins) and non-protein ingredients (such as lipids, metal ions, and biogenic amines). Proteins account for 90%-95% of the dry weight of snake venom.[7-8] Each venomous snake contains a diverse array of toxins, ranging from 20 to over 100 different components, and the types of toxins vary within and between species,[9-10] depending on the region, season, and age of the snake.[11-12] The toxins of the Cobra family are dominated by three-finger toxins and phospholipase A2 (PLA2), while those of the Viper family are dominated by snake venom metalloproteinases, PLA2, and snake venom serine proteases.[13] Snake venom proteins can produce various toxic effects depending on their targets.[9,14-15] For example, PLA2 mainly acts on various receptors in the plasma membrane and axonal membrane of muscle cells.[16] Snake venom metalloproteinases have multiple targets, the most notable being type IV collagen and coagulation factors.[17] Snake venom serine proteases mainly affect coagulation factors.[9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
... Hemotoxins are commonly found in toxins of Viperidae, cobras, and Salviidae. These most commonly affect the clotting cascade and platelets.[15] Snake venom factor V activator, factor X activator, prothrombin activator, and procoagulant enzymes result in coagulable blood. Factor IX/X binding protein, protein C activator, thrombin inhibitor, and PLA2 exhibit anticoagulant properties; fibrinolytic enzymes and plasminogen activator have fibrinolytic activity. Snake venom metalloproteinases, disintegrins, and C-type lectins directly damage the vascular wall.[20] Snake venom may promote or inhibit platelet aggregation. For example, serine proteases promote platelet aggregation; disintegrin and 5-nucleotidases inhibit platelet aggregation; and PLA2s, metalloproteinases, C-type lectin-like proteins, and different subtypes of L-amino acid oxidases inhibit or promote platelet aggregation.[21] Snake venom is absorbed through lymphatic vessels and capillaries, and macromolecules are absorbed through the lymphatic system. Enzyme toxins such as metalloproteinases impair the integrity of lymphatic vessels, increasing their permeability and promoting or aggravating local edema.[15] ...
... [15] ...
... Toxins act on the neuromuscular junction of skeletal muscle. The main targets of toxins are acetylcholine receptors on motor nerve endings (presynaptic membrane) and motor endplates (postsynaptic membrane), resulting in flaccid paralysis, which is observed in most Elapidae and a few viper venom bites. β-bungarotoxin, PLA2, and dendrimer toxins act on the presynaptic membrane; α-bungarotoxin, weak toxin, black green ironhead toxin, and PLA2 act on the postsynaptic membrane; acetylcholinesterase acts on the synaptic cleft.[15] Most venomous snakes contain only a single neurotoxin that binds to the presynaptic membrane (such as the Sri Lankan viper, many-banded krait, and Coastal Taipan) or the postsynaptic membrane (such as the king cobra and cobra). Many-banded krait venom contains α-bungarotoxin, β-bungarotoxin, κ-bungarotoxin, and γ-bungarotoxin, which affect both presynaptic and postsynaptic membranes.[1,22] Most toxins have a high affinity for binding to neuromuscular receptors and are not easily dissociated, especially after binding to presynaptic receptors, which impedes clinical recovery. However, the effects of postsynaptic neurotoxins can be rapidly reversed by antivenom serum. [23-24] The three-finger toxin PLA2 and weak toxin of the Bengal cobra have certain effects on the autonomic nervous system.[15] ...
... [15] ...
... No clinical study can clearly determine the ideal dosage of antivenom, and there is no unified standard around the world. The dosage is mainly determined based on the patient’s condition, guidelines, or clinical experience. The dosage should be determined by clinicians on the basis of differences in snake species, region, severity, and timing of treatment. According to the North American protocol, the initial dose is 4-6 vials, and for cases with potentially fatal injuries, such as shock or severe active bleeding, the initial dose is increased to 8-12 vials. The median initial control dose is nine vials (interquartile range 6-15 vials).[67-68] In China, where monovalent antivenom is available, an initial dose of 2-4 vials seems reasonable on the basis of domestic and overseas experience; the dose can be increased on the basis of the severity of poisoning.[15,47] Appropriately increasing the initial dose may help combat potential snake venom in the blood and is as safe as low initial doses.[69] However, the administration of >5 vials in a single dose can increase the risk of adverse reactions.[62] ...
Updating phospholipase A2 biology
1
2020
... Snake venom is a complex high-efficiency toxin mixture characterized by its pale yellow or milky white, translucent, and viscous liquid appearance. It is mainly composed of proteins (such as enzymes, polypeptides, and glycoproteins) and non-protein ingredients (such as lipids, metal ions, and biogenic amines). Proteins account for 90%-95% of the dry weight of snake venom.[7-8] Each venomous snake contains a diverse array of toxins, ranging from 20 to over 100 different components, and the types of toxins vary within and between species,[9-10] depending on the region, season, and age of the snake.[11-12] The toxins of the Cobra family are dominated by three-finger toxins and phospholipase A2 (PLA2), while those of the Viper family are dominated by snake venom metalloproteinases, PLA2, and snake venom serine proteases.[13] Snake venom proteins can produce various toxic effects depending on their targets.[9,14-15] For example, PLA2 mainly acts on various receptors in the plasma membrane and axonal membrane of muscle cells.[16] Snake venom metalloproteinases have multiple targets, the most notable being type IV collagen and coagulation factors.[17] Snake venom serine proteases mainly affect coagulation factors.[9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
Snake venom metalloproteinases (SVMPs): a structure-function update
1
2020
... Snake venom is a complex high-efficiency toxin mixture characterized by its pale yellow or milky white, translucent, and viscous liquid appearance. It is mainly composed of proteins (such as enzymes, polypeptides, and glycoproteins) and non-protein ingredients (such as lipids, metal ions, and biogenic amines). Proteins account for 90%-95% of the dry weight of snake venom.[7-8] Each venomous snake contains a diverse array of toxins, ranging from 20 to over 100 different components, and the types of toxins vary within and between species,[9-10] depending on the region, season, and age of the snake.[11-12] The toxins of the Cobra family are dominated by three-finger toxins and phospholipase A2 (PLA2), while those of the Viper family are dominated by snake venom metalloproteinases, PLA2, and snake venom serine proteases.[13] Snake venom proteins can produce various toxic effects depending on their targets.[9,14-15] For example, PLA2 mainly acts on various receptors in the plasma membrane and axonal membrane of muscle cells.[16] Snake venom metalloproteinases have multiple targets, the most notable being type IV collagen and coagulation factors.[17] Snake venom serine proteases mainly affect coagulation factors.[9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
Structure, function and evolution of three-finger toxins: mini proteins with multiple targets
1
2010
... Snake venom is a complex high-efficiency toxin mixture characterized by its pale yellow or milky white, translucent, and viscous liquid appearance. It is mainly composed of proteins (such as enzymes, polypeptides, and glycoproteins) and non-protein ingredients (such as lipids, metal ions, and biogenic amines). Proteins account for 90%-95% of the dry weight of snake venom.[7-8] Each venomous snake contains a diverse array of toxins, ranging from 20 to over 100 different components, and the types of toxins vary within and between species,[9-10] depending on the region, season, and age of the snake.[11-12] The toxins of the Cobra family are dominated by three-finger toxins and phospholipase A2 (PLA2), while those of the Viper family are dominated by snake venom metalloproteinases, PLA2, and snake venom serine proteases.[13] Snake venom proteins can produce various toxic effects depending on their targets.[9,14-15] For example, PLA2 mainly acts on various receptors in the plasma membrane and axonal membrane of muscle cells.[16] Snake venom metalloproteinases have multiple targets, the most notable being type IV collagen and coagulation factors.[17] Snake venom serine proteases mainly affect coagulation factors.[9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
Amplification of snake venom toxicity by endogenous signaling pathways
1
2020
... Snake venom is a complex high-efficiency toxin mixture characterized by its pale yellow or milky white, translucent, and viscous liquid appearance. It is mainly composed of proteins (such as enzymes, polypeptides, and glycoproteins) and non-protein ingredients (such as lipids, metal ions, and biogenic amines). Proteins account for 90%-95% of the dry weight of snake venom.[7-8] Each venomous snake contains a diverse array of toxins, ranging from 20 to over 100 different components, and the types of toxins vary within and between species,[9-10] depending on the region, season, and age of the snake.[11-12] The toxins of the Cobra family are dominated by three-finger toxins and phospholipase A2 (PLA2), while those of the Viper family are dominated by snake venom metalloproteinases, PLA2, and snake venom serine proteases.[13] Snake venom proteins can produce various toxic effects depending on their targets.[9,14-15] For example, PLA2 mainly acts on various receptors in the plasma membrane and axonal membrane of muscle cells.[16] Snake venom metalloproteinases have multiple targets, the most notable being type IV collagen and coagulation factors.[17] Snake venom serine proteases mainly affect coagulation factors.[9] Three-finger toxins act on nicotinic and muscarinic acetylcholine receptors, and acetylcholinesterase, and block neuromuscular impulse transmission.[18] Some toxins may have relatively low toxicity when isolated, but when mixed with other toxins in the venom, they enhance each other’s toxicity. This synergistic effect amplifies overall venom toxicity.[19] ...
Snake venoms and hemostasis
1
2005
... Hemotoxins are commonly found in toxins of Viperidae, cobras, and Salviidae. These most commonly affect the clotting cascade and platelets.[15] Snake venom factor V activator, factor X activator, prothrombin activator, and procoagulant enzymes result in coagulable blood. Factor IX/X binding protein, protein C activator, thrombin inhibitor, and PLA2 exhibit anticoagulant properties; fibrinolytic enzymes and plasminogen activator have fibrinolytic activity. Snake venom metalloproteinases, disintegrins, and C-type lectins directly damage the vascular wall.[20] Snake venom may promote or inhibit platelet aggregation. For example, serine proteases promote platelet aggregation; disintegrin and 5-nucleotidases inhibit platelet aggregation; and PLA2s, metalloproteinases, C-type lectin-like proteins, and different subtypes of L-amino acid oxidases inhibit or promote platelet aggregation.[21] Snake venom is absorbed through lymphatic vessels and capillaries, and macromolecules are absorbed through the lymphatic system. Enzyme toxins such as metalloproteinases impair the integrity of lymphatic vessels, increasing their permeability and promoting or aggravating local edema.[15] ...
Snake venoms and coagulopathy
1
2005
... Hemotoxins are commonly found in toxins of Viperidae, cobras, and Salviidae. These most commonly affect the clotting cascade and platelets.[15] Snake venom factor V activator, factor X activator, prothrombin activator, and procoagulant enzymes result in coagulable blood. Factor IX/X binding protein, protein C activator, thrombin inhibitor, and PLA2 exhibit anticoagulant properties; fibrinolytic enzymes and plasminogen activator have fibrinolytic activity. Snake venom metalloproteinases, disintegrins, and C-type lectins directly damage the vascular wall.[20] Snake venom may promote or inhibit platelet aggregation. For example, serine proteases promote platelet aggregation; disintegrin and 5-nucleotidases inhibit platelet aggregation; and PLA2s, metalloproteinases, C-type lectin-like proteins, and different subtypes of L-amino acid oxidases inhibit or promote platelet aggregation.[21] Snake venom is absorbed through lymphatic vessels and capillaries, and macromolecules are absorbed through the lymphatic system. Enzyme toxins such as metalloproteinases impair the integrity of lymphatic vessels, increasing their permeability and promoting or aggravating local edema.[15] ...
Neuromuscular weakness and paralysis produced by snakebite envenoming: mechanisms and proposed standards for clinical assessment
1
2023
... Toxins act on the neuromuscular junction of skeletal muscle. The main targets of toxins are acetylcholine receptors on motor nerve endings (presynaptic membrane) and motor endplates (postsynaptic membrane), resulting in flaccid paralysis, which is observed in most Elapidae and a few viper venom bites. β-bungarotoxin, PLA2, and dendrimer toxins act on the presynaptic membrane; α-bungarotoxin, weak toxin, black green ironhead toxin, and PLA2 act on the postsynaptic membrane; acetylcholinesterase acts on the synaptic cleft.[15] Most venomous snakes contain only a single neurotoxin that binds to the presynaptic membrane (such as the Sri Lankan viper, many-banded krait, and Coastal Taipan) or the postsynaptic membrane (such as the king cobra and cobra). Many-banded krait venom contains α-bungarotoxin, β-bungarotoxin, κ-bungarotoxin, and γ-bungarotoxin, which affect both presynaptic and postsynaptic membranes.[1,22] Most toxins have a high affinity for binding to neuromuscular receptors and are not easily dissociated, especially after binding to presynaptic receptors, which impedes clinical recovery. However, the effects of postsynaptic neurotoxins can be rapidly reversed by antivenom serum. [23-24] The three-finger toxin PLA2 and weak toxin of the Bengal cobra have certain effects on the autonomic nervous system.[15] ...
Snake bite
2
2010
... Toxins act on the neuromuscular junction of skeletal muscle. The main targets of toxins are acetylcholine receptors on motor nerve endings (presynaptic membrane) and motor endplates (postsynaptic membrane), resulting in flaccid paralysis, which is observed in most Elapidae and a few viper venom bites. β-bungarotoxin, PLA2, and dendrimer toxins act on the presynaptic membrane; α-bungarotoxin, weak toxin, black green ironhead toxin, and PLA2 act on the postsynaptic membrane; acetylcholinesterase acts on the synaptic cleft.[15] Most venomous snakes contain only a single neurotoxin that binds to the presynaptic membrane (such as the Sri Lankan viper, many-banded krait, and Coastal Taipan) or the postsynaptic membrane (such as the king cobra and cobra). Many-banded krait venom contains α-bungarotoxin, β-bungarotoxin, κ-bungarotoxin, and γ-bungarotoxin, which affect both presynaptic and postsynaptic membranes.[1,22] Most toxins have a high affinity for binding to neuromuscular receptors and are not easily dissociated, especially after binding to presynaptic receptors, which impedes clinical recovery. However, the effects of postsynaptic neurotoxins can be rapidly reversed by antivenom serum. [23-24] The three-finger toxin PLA2 and weak toxin of the Bengal cobra have certain effects on the autonomic nervous system.[15] ...
... Most acute adverse reactions occur within 1-2 h after the initiation of antivenom treatment. Close monitoring for adverse reactions, focusing on observing the aggravation and amelioration of toxic symptoms, signs, and laboratory indices, is necessary to facilitate the timely administration of additional treatment or calibration of medication. Depending on the severity of envenomation, patients should be monitored at least 2, 6, 12, and 24 h after the first use of antivenom. Blood routine, coagulation function, (cardiac) muscle enzymes, and other laboratory tests should be repeated at 6, 12, and 24 h.[74] After a sufficient neutralizing dose of antivenom is used, the median recovery time for coagulation dysfunction is 6 h.[7,23,77] Therefore, indications for additional medication include persistence of toxic symptoms 6 h after the first dose of antivenom, re-aggravation of coagulation dysfunction after transient recovery, recurrence of bleeding 1-2 h after cessation of bleeding, and continued deterioration of neurological or cardiovascular function for 1 h after the first dose of antivenom. ...
Neurotoxicity in snakebite—the limits of our knowledge
1
2013
... Toxins act on the neuromuscular junction of skeletal muscle. The main targets of toxins are acetylcholine receptors on motor nerve endings (presynaptic membrane) and motor endplates (postsynaptic membrane), resulting in flaccid paralysis, which is observed in most Elapidae and a few viper venom bites. β-bungarotoxin, PLA2, and dendrimer toxins act on the presynaptic membrane; α-bungarotoxin, weak toxin, black green ironhead toxin, and PLA2 act on the postsynaptic membrane; acetylcholinesterase acts on the synaptic cleft.[15] Most venomous snakes contain only a single neurotoxin that binds to the presynaptic membrane (such as the Sri Lankan viper, many-banded krait, and Coastal Taipan) or the postsynaptic membrane (such as the king cobra and cobra). Many-banded krait venom contains α-bungarotoxin, β-bungarotoxin, κ-bungarotoxin, and γ-bungarotoxin, which affect both presynaptic and postsynaptic membranes.[1,22] Most toxins have a high affinity for binding to neuromuscular receptors and are not easily dissociated, especially after binding to presynaptic receptors, which impedes clinical recovery. However, the effects of postsynaptic neurotoxins can be rapidly reversed by antivenom serum. [23-24] The three-finger toxin PLA2 and weak toxin of the Bengal cobra have certain effects on the autonomic nervous system.[15] ...
Snake envenomation-induced acute kidney injury: prognosis and long-term renal outcomes
1
2022
... Bites of some venomous snakes, notably viperaceous snakes, can cause acute kidney injury. PLA2, metalloproteinase, hyaluronidase, and serine proteinase present in the venom of Vipera russelli, sea snake, and rattlesnake can cause injury to glomeruli, renal tubules, and renal vessels. Some non-enzymatic components can also directly cause nephrotoxicity.[25] ...
Snakebite envenomation and heart: systematic review
2
2022
... PLA2 is present in the venom of almost all venomous snakes. It can synergically induce hypotension with snake venom natriuretic peptide, bradykinin-enhancing peptide, vascular endothelial growth factor, snake venom metalloproteinase, and serine proteinase. The three-finger toxin induces hypotension by blocking L-type calcium ion channels and interacting with adrenergic and muscarinic receptors. Venom adenosine triphosphatase (ATPase), adenosine-5-diphosphate (ADP enzyme), and nucleotidase catalyze the decomposition of ATP into ADP, adenosine monophosphate (AMP), and adenosine, further promote a proinflammatory response (vascular dilation and increased permeability) and reduce blood pressure.[26] Serine proteinases and metalloproteinases can induce bleeding and coagulation disorders, produce snake venom-induced consumption coagulopathy (VICC), and reduce effective blood volume. Some snake venoms cause vasoconstriction, microthrombi formation, microangiopathic hemolytic anemia, and thrombocytopenia, thereby reducing effective circulation.[27] Other mechanisms of cardiovascular damage include direct injury to the myocardial cell membrane, the induction of arrhythmia, secondary hypercoagulability-induced coronary syndrome and coronary spasm, secondary hyperkalemia resulting from acute renal failure, and the proinflammatory effect of hypersensitivity reactions to snake venom.[26,28] ...
... [26,28] ...
Hypotensive snake venom components-a mini-review
2
2019
... PLA2 is present in the venom of almost all venomous snakes. It can synergically induce hypotension with snake venom natriuretic peptide, bradykinin-enhancing peptide, vascular endothelial growth factor, snake venom metalloproteinase, and serine proteinase. The three-finger toxin induces hypotension by blocking L-type calcium ion channels and interacting with adrenergic and muscarinic receptors. Venom adenosine triphosphatase (ATPase), adenosine-5-diphosphate (ADP enzyme), and nucleotidase catalyze the decomposition of ATP into ADP, adenosine monophosphate (AMP), and adenosine, further promote a proinflammatory response (vascular dilation and increased permeability) and reduce blood pressure.[26] Serine proteinases and metalloproteinases can induce bleeding and coagulation disorders, produce snake venom-induced consumption coagulopathy (VICC), and reduce effective blood volume. Some snake venoms cause vasoconstriction, microthrombi formation, microangiopathic hemolytic anemia, and thrombocytopenia, thereby reducing effective circulation.[27] Other mechanisms of cardiovascular damage include direct injury to the myocardial cell membrane, the induction of arrhythmia, secondary hypercoagulability-induced coronary syndrome and coronary spasm, secondary hyperkalemia resulting from acute renal failure, and the proinflammatory effect of hypersensitivity reactions to snake venom.[26,28] ...
... Neurotoxic snakes such as Bungarus multicinctus mainly cause flaccid and descending paralysis, gradually affecting muscles innervated by cranial nerves as well as cervical flexor muscles, medulla oblongata, respiratory muscles, trunk and limb muscles. Typical descending paralysis first involves the eyelid muscles, manifesting as bilateral ptosis, typically occurring within hours of the bite. Next, the external eye muscles are involved, causing diplopia, fixed pupil dilation, facial paralysis with slurred speech and difficulty opening the mouth. The palate, mandible, tongue, and throat are subsequently involved, resulting in the accumulation of pharyngeal secretions and the loss of the pharyngeal reflex. Then, the paralysis symptoms develop and continue to descend to the neck muscles and medulla oblongata muscles. The involvement of medulla oblongata causes difficulty in swallowing and the loss of airway protection function, leading to a high risk of aspiration or suffocation. Cervical muscle paralysis may manifest as a soft and weak neck. The involvement of respiratory muscles leads to shallow and rapid breathing, decreased ventilation capacity, abnormal abdominal breathing, use of auxiliary muscles, and cyanosis. Once severe dyspnea occurs, it will quickly lead to respiratory arrest. The time elapsed between snakebite and the occurrence of respiratory failure ranges from 30 min to more than 24 h, with an average of 6-12 h. Finally, the limb muscles are involved, manifesting as weakness. The proximal muscles are involved first, followed by the distal muscles. In severe cases, complete paralysis of the limbs may occur, with attenuation or disappearance of deep tendon reflexes. The recovery of neurological function usually occurs in the reverse sequence. Distal muscle strength first recovers, followed by a gradual recovery of proximal muscle strength, and finally upper eyelid ptosis and ophthalmoplegia.[1,27,31] ...
Snake venom three-finger toxins and their potential in drug development targeting cardiovascular diseases
1
2020
... PLA2 is present in the venom of almost all venomous snakes. It can synergically induce hypotension with snake venom natriuretic peptide, bradykinin-enhancing peptide, vascular endothelial growth factor, snake venom metalloproteinase, and serine proteinase. The three-finger toxin induces hypotension by blocking L-type calcium ion channels and interacting with adrenergic and muscarinic receptors. Venom adenosine triphosphatase (ATPase), adenosine-5-diphosphate (ADP enzyme), and nucleotidase catalyze the decomposition of ATP into ADP, adenosine monophosphate (AMP), and adenosine, further promote a proinflammatory response (vascular dilation and increased permeability) and reduce blood pressure.[26] Serine proteinases and metalloproteinases can induce bleeding and coagulation disorders, produce snake venom-induced consumption coagulopathy (VICC), and reduce effective blood volume. Some snake venoms cause vasoconstriction, microthrombi formation, microangiopathic hemolytic anemia, and thrombocytopenia, thereby reducing effective circulation.[27] Other mechanisms of cardiovascular damage include direct injury to the myocardial cell membrane, the induction of arrhythmia, secondary hypercoagulability-induced coronary syndrome and coronary spasm, secondary hyperkalemia resulting from acute renal failure, and the proinflammatory effect of hypersensitivity reactions to snake venom.[26,28] ...
Joint trauma system clinical practice guideline: global snake envenomation management
1
2020
... The clinical manifestations of venomous snakebites vary depending on the snake species or toxin content. Approximately 20% (1.75%-50%) of venomous snakebites are “dry bites”.[3] The severity of toxic manifestations depends on the amount of venom released by the snake and the time elapsed between the bite and medical treatment.[29] The main clinical manifestations are the neurotoxic triad (bilateral ptosis, descending paralysis, dyspnea/acute respiratory failure), the hematotoxic triad (consumptive coagulopathy, local bleeding, and systemic bleeding), and the cytotoxic triad (severe pain, progressive swelling, and tissue damage). Some Colubridae are known to produce varying degrees of toxic effects.[30] Most victims present with mild pain, tooth marks or lacerations, local mild reactive edema, or small amounts of bleeding, which usually resolve within 24-36 h. A minority of cases exhibit severe symptoms. ...
1
2022
... The clinical manifestations of venomous snakebites vary depending on the snake species or toxin content. Approximately 20% (1.75%-50%) of venomous snakebites are “dry bites”.[3] The severity of toxic manifestations depends on the amount of venom released by the snake and the time elapsed between the bite and medical treatment.[29] The main clinical manifestations are the neurotoxic triad (bilateral ptosis, descending paralysis, dyspnea/acute respiratory failure), the hematotoxic triad (consumptive coagulopathy, local bleeding, and systemic bleeding), and the cytotoxic triad (severe pain, progressive swelling, and tissue damage). Some Colubridae are known to produce varying degrees of toxic effects.[30] Most victims present with mild pain, tooth marks or lacerations, local mild reactive edema, or small amounts of bleeding, which usually resolve within 24-36 h. A minority of cases exhibit severe symptoms. ...
Antivenom for neuromuscular paralysis resulting from snake envenoming
1
2017
... Neurotoxic snakes such as Bungarus multicinctus mainly cause flaccid and descending paralysis, gradually affecting muscles innervated by cranial nerves as well as cervical flexor muscles, medulla oblongata, respiratory muscles, trunk and limb muscles. Typical descending paralysis first involves the eyelid muscles, manifesting as bilateral ptosis, typically occurring within hours of the bite. Next, the external eye muscles are involved, causing diplopia, fixed pupil dilation, facial paralysis with slurred speech and difficulty opening the mouth. The palate, mandible, tongue, and throat are subsequently involved, resulting in the accumulation of pharyngeal secretions and the loss of the pharyngeal reflex. Then, the paralysis symptoms develop and continue to descend to the neck muscles and medulla oblongata muscles. The involvement of medulla oblongata causes difficulty in swallowing and the loss of airway protection function, leading to a high risk of aspiration or suffocation. Cervical muscle paralysis may manifest as a soft and weak neck. The involvement of respiratory muscles leads to shallow and rapid breathing, decreased ventilation capacity, abnormal abdominal breathing, use of auxiliary muscles, and cyanosis. Once severe dyspnea occurs, it will quickly lead to respiratory arrest. The time elapsed between snakebite and the occurrence of respiratory failure ranges from 30 min to more than 24 h, with an average of 6-12 h. Finally, the limb muscles are involved, manifesting as weakness. The proximal muscles are involved first, followed by the distal muscles. In severe cases, complete paralysis of the limbs may occur, with attenuation or disappearance of deep tendon reflexes. The recovery of neurological function usually occurs in the reverse sequence. Distal muscle strength first recovers, followed by a gradual recovery of proximal muscle strength, and finally upper eyelid ptosis and ophthalmoplegia.[1,27,31] ...
Systematic review and meta-analysis of global prevalence of neurotoxic and hemotoxic snakebite envenomation
1
2022
... Hemotoxic snakes such as Trimeresurus stejnegeri mainly cause coagulopathy, referred to as VICC. VICC manifests as wound bleeding or even uncontrollable bleeding, petechiae, ecchymosis, hematemesis, black stool, hemoptysis, and hematuria. Severe cases may develop bleeding in important organs (such as the brain) and hypovolemic shock.[32] ...
Snake envenomation
2
2022
... The main symptoms are localized pain and swelling that spread from the site of the bite to the surrounding areas, with the appearance of blisters, skin necrosis or infection, and local bruising. If the swelling exceeds the elastic limit of the skin, it can induce fascia compartment syndrome-like symptoms such as pain, passive stretching pain, sensory abnormalities, and limb paralysis. Rarely, there may be skin pallor and a lack of pulses.[33-34] ...
... In children, the toxin load after a snakebite is comparable to that of adults. Owing to their different physical constitution and smaller blood volume, the blood concentration of toxins in children is higher than that in adults. Therefore, a relatively larger first dose of antivenom may be needed to neutralize the potentially higher toxin load.[33] ...
Diagnosis and treatment of acute extremity compartment syndrome
1
2015
... The main symptoms are localized pain and swelling that spread from the site of the bite to the surrounding areas, with the appearance of blisters, skin necrosis or infection, and local bruising. If the swelling exceeds the elastic limit of the skin, it can induce fascia compartment syndrome-like symptoms such as pain, passive stretching pain, sensory abnormalities, and limb paralysis. Rarely, there may be skin pallor and a lack of pulses.[33-34] ...
Snake bite associated with acute kidney injury
1
2021
... Cardiovascular manifestations include palpitations, hypotension, shock, and arrhythmia. Urinary manifestations include acute kidney injury or uremia symptoms such as low back pain, hematuria, hemoglobinuria, myoglobinuria, and oliguria/anuria. Skeletal musclar manifestations include generalized pain, muscle stiffness, tenderness, and difficulty opening the mouth. Endocrine system involvement may present with hypopituitarism, shock, hypoglycemia, secondary hair loss, sexual dysfunction, amenorrhea, testicular atrophy, or hypothyroidism.[35] The venom of venomous cobras, such as the spitting cobra and the Chinese cobra, can cause severe eye pain, inability to open the eyes, excessive lacrimation, conjunctival congestion, blepharitis, eyelid spasm, corneal erosion and other manifestations of snake venom-related ophthalmia. Delayed treatment or a lack of treatment may lead to corneal opacity, anterior chamber effusion, and blindness.[36] ...
Venom ophthalmia caused by venoms of spitting elapid and other snakes: Report of ten cases with review of epidemiology, clinical features, pathophysiology and management
2
2010
... Cardiovascular manifestations include palpitations, hypotension, shock, and arrhythmia. Urinary manifestations include acute kidney injury or uremia symptoms such as low back pain, hematuria, hemoglobinuria, myoglobinuria, and oliguria/anuria. Skeletal musclar manifestations include generalized pain, muscle stiffness, tenderness, and difficulty opening the mouth. Endocrine system involvement may present with hypopituitarism, shock, hypoglycemia, secondary hair loss, sexual dysfunction, amenorrhea, testicular atrophy, or hypothyroidism.[35] The venom of venomous cobras, such as the spitting cobra and the Chinese cobra, can cause severe eye pain, inability to open the eyes, excessive lacrimation, conjunctival congestion, blepharitis, eyelid spasm, corneal erosion and other manifestations of snake venom-related ophthalmia. Delayed treatment or a lack of treatment may lead to corneal opacity, anterior chamber effusion, and blindness.[36] ...
... There are two methods for additional administration: one is on-demand administration, which means that an additional dose is administered based on the progress of envenomation monitored every 6-8 h. If there is no progression of symptoms, there is no need to administer additional doses. The other method is scheduled administration, which means that, regardless of the progress, additional doses (2 doses) are administered every 6-8 h. After three consecutive administrations, additional doses are administered according to the status of patients. For cases of fatal poisoning, shortening the time interval between the first administration and additional administration can be considered, e.g., according to the monitoring results at the 2nd, 4th, 6th, 12th, and 24th hour. In the case of progression of poisoning, an additional dose can be administered again. After 24 h, an additional dose is administered according to the need.[36] ...
Expert consensus on diagnosis and treatment of acute hemorrhagic coagulation disorder
1
2020
... Routine blood examination reveals an increase in white blood cell and neutrophil counts, with a leftward shift in the nucleus (indicating immature neutrophils), which is often caused by stress in the early stages. In patients with significant bleeding or hemolysis, there may be a decrease in red blood cell count and hemoglobin. Patients bitten by some snake species are prone to thrombocytopenia. Patients with VICC may also present thrombocytopenia. Hematuria, black urine, oliguria, anuria, proteinuria, or casts suggest acute kidney injury. Intravascular hemolysis may present as hemoglobinuria. Muscle injury or dissolution may present as myoglobinuria and hyperkalemia. Abnormal results of coagulation-fibrinolytic system tests, such as international normalized ratio (INR), prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen, D-dimer, bleeding time (BT), thrombin time, antithrombin III (AT-III), and protamine paracoagulant (3P) tests, suggest blood poisoning from snakebites. Thromboelastography (TEG) can reflect dynamic changes in blood coagulation or fibrinolytic processes, including the formation and development of blood clots and the retraction and dissolution of blood clots, and provide information on the speed, intensity, and stability of thrombogenesis. It is suitable for evaluating coagulopathy after hematotoxic snakebites and can be used as an auxiliary test.[37⇓-39] ...
Use of thromboelastography in clinical practice
1
2020
... Routine blood examination reveals an increase in white blood cell and neutrophil counts, with a leftward shift in the nucleus (indicating immature neutrophils), which is often caused by stress in the early stages. In patients with significant bleeding or hemolysis, there may be a decrease in red blood cell count and hemoglobin. Patients bitten by some snake species are prone to thrombocytopenia. Patients with VICC may also present thrombocytopenia. Hematuria, black urine, oliguria, anuria, proteinuria, or casts suggest acute kidney injury. Intravascular hemolysis may present as hemoglobinuria. Muscle injury or dissolution may present as myoglobinuria and hyperkalemia. Abnormal results of coagulation-fibrinolytic system tests, such as international normalized ratio (INR), prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen, D-dimer, bleeding time (BT), thrombin time, antithrombin III (AT-III), and protamine paracoagulant (3P) tests, suggest blood poisoning from snakebites. Thromboelastography (TEG) can reflect dynamic changes in blood coagulation or fibrinolytic processes, including the formation and development of blood clots and the retraction and dissolution of blood clots, and provide information on the speed, intensity, and stability of thrombogenesis. It is suitable for evaluating coagulopathy after hematotoxic snakebites and can be used as an auxiliary test.[37⇓-39] ...
Thromboelastography in the management of snakebite-induced coagulopathy: a case series and literature review
1
2018
... Routine blood examination reveals an increase in white blood cell and neutrophil counts, with a leftward shift in the nucleus (indicating immature neutrophils), which is often caused by stress in the early stages. In patients with significant bleeding or hemolysis, there may be a decrease in red blood cell count and hemoglobin. Patients bitten by some snake species are prone to thrombocytopenia. Patients with VICC may also present thrombocytopenia. Hematuria, black urine, oliguria, anuria, proteinuria, or casts suggest acute kidney injury. Intravascular hemolysis may present as hemoglobinuria. Muscle injury or dissolution may present as myoglobinuria and hyperkalemia. Abnormal results of coagulation-fibrinolytic system tests, such as international normalized ratio (INR), prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen, D-dimer, bleeding time (BT), thrombin time, antithrombin III (AT-III), and protamine paracoagulant (3P) tests, suggest blood poisoning from snakebites. Thromboelastography (TEG) can reflect dynamic changes in blood coagulation or fibrinolytic processes, including the formation and development of blood clots and the retraction and dissolution of blood clots, and provide information on the speed, intensity, and stability of thrombogenesis. It is suitable for evaluating coagulopathy after hematotoxic snakebites and can be used as an auxiliary test.[37⇓-39] ...
The 20-minute whole blood clotting test (20WBCT) for snakebite coagulopathy-a systematic review and meta-analysis of diagnostic test accuracy
1
2021
... Routine blood biochemical tests, liver and kidney function tests, and (cardiac) muscle enzyme profiles are performed to assess the internal environment and vital organ function. Blood gas analysis can help evaluate respiratory function and electrolyte abnormalities; blood lactate levels can reflect changes in circulatory perfusion and tissue metabolism. The 20-minute whole blood agglutination test is a highly specific (specificity 98%) and sensitive (sensitivity 82%) bedside test for detecting coagulopathy after snakebite.[40] It is simple and easy to perform and is particularly suitable for resource-limited settings and for those who have not had laboratory tests for coagulation function for a long time, but the positive rate is low, within 3 h. [41] ...
Diagnostic characteristics of the 20-minute whole blood clotting test in detecting venom-induced consumptive coagulopathy following carpet viper envenoming
1
2023
... Routine blood biochemical tests, liver and kidney function tests, and (cardiac) muscle enzyme profiles are performed to assess the internal environment and vital organ function. Blood gas analysis can help evaluate respiratory function and electrolyte abnormalities; blood lactate levels can reflect changes in circulatory perfusion and tissue metabolism. The 20-minute whole blood agglutination test is a highly specific (specificity 98%) and sensitive (sensitivity 82%) bedside test for detecting coagulopathy after snakebite.[40] It is simple and easy to perform and is particularly suitable for resource-limited settings and for those who have not had laboratory tests for coagulation function for a long time, but the positive rate is low, within 3 h. [41] ...
Infrared thermography to diagnose and manage venomous animal bites and stings
1
2017
... An electrocardiogram (ECG) examination can detect cardiac manifestations, such as changes in cardiac rhythm, myocardial ischemia or infarction, and hyperkalemia. A chest X-ray or chest computed tomography (CT) scan can assess lung damage, especially pulmonary edema, pulmonary hemorrhage, and pleural effusion. CT or magnetic resonance imaging (MRI) is useful for detecting internal bleeding or other structural changes, such as intracranial hemorrhage, cerebral infarction, and chest and abdominal hematoma. Ultrasound is suitable for detecting serous cavity effusion or hematoma and assessing cardiac function. Electromyography can help evaluate neuromuscular lesions, and electroencephalography can be used to evaluate “brain death-like changes” after a neurotoxic snakebite. If possible, infrared thermography can be used to visually assess the local inflammatory response at the bite site.[42-43] For those with confirmed or suspected snake venom injection into the eyes, an ophthalmologist should be consulted to assess the eye damage caused by snake venom-related ophthalmia. ...
Point-of-care infrared thermal imaging for differentiating venomous snakebites from non-venomous and dry bites
1
2021
... An electrocardiogram (ECG) examination can detect cardiac manifestations, such as changes in cardiac rhythm, myocardial ischemia or infarction, and hyperkalemia. A chest X-ray or chest computed tomography (CT) scan can assess lung damage, especially pulmonary edema, pulmonary hemorrhage, and pleural effusion. CT or magnetic resonance imaging (MRI) is useful for detecting internal bleeding or other structural changes, such as intracranial hemorrhage, cerebral infarction, and chest and abdominal hematoma. Ultrasound is suitable for detecting serous cavity effusion or hematoma and assessing cardiac function. Electromyography can help evaluate neuromuscular lesions, and electroencephalography can be used to evaluate “brain death-like changes” after a neurotoxic snakebite. If possible, infrared thermography can be used to visually assess the local inflammatory response at the bite site.[42-43] For those with confirmed or suspected snake venom injection into the eyes, an ophthalmologist should be consulted to assess the eye damage caused by snake venom-related ophthalmia. ...
The BRAVO clinical study protocol: oral varespladib for inhibition of secretory phospholipase A 2 in the treatment of snakebite envenoming
1
2022
... The Snakebite Severity Scale (SSS) is a method used to quantify the severity of snakebites based on clinical and laboratory results.[44,45] A score of 0 to 3 indicates mild snakebite, 4 to 7 indicates moderate snakebite, and ≥8 indicates severe snakebite. The SSS is an objective tool with detailed classification. It has been adopted by most countries, mainly for academic research. However, owing to its slight complexity, its original design intention is as a research tool rather than a clinical evaluation tool. Therefore, it is more suitable for population studies of snakebite incidents. If used for clinical diagnosis of patients, the SSS is effective in distinguishing severe poisoning but has poor discrimination for mild and moderate poisoning.[46] It is not specific for certain symptoms and signs, so its reliability is poor. The modified SSS incorporates indicators of kidney damage, improving its discrimination ability (Table 2). However, the score still has poor discrimination for neurotoxic snakebites. For evaluating and classifying neurotoxic snakebites, it is recommended to directly refer to the neurological assessment score in this modified SSS. ...
Validation of a severity score for the assessment of crotalid snakebite
1
1996
... The Snakebite Severity Scale (SSS) is a method used to quantify the severity of snakebites based on clinical and laboratory results.[44,45] A score of 0 to 3 indicates mild snakebite, 4 to 7 indicates moderate snakebite, and ≥8 indicates severe snakebite. The SSS is an objective tool with detailed classification. It has been adopted by most countries, mainly for academic research. However, owing to its slight complexity, its original design intention is as a research tool rather than a clinical evaluation tool. Therefore, it is more suitable for population studies of snakebite incidents. If used for clinical diagnosis of patients, the SSS is effective in distinguishing severe poisoning but has poor discrimination for mild and moderate poisoning.[46] It is not specific for certain symptoms and signs, so its reliability is poor. The modified SSS incorporates indicators of kidney damage, improving its discrimination ability (Table 2). However, the score still has poor discrimination for neurotoxic snakebites. For evaluating and classifying neurotoxic snakebites, it is recommended to directly refer to the neurological assessment score in this modified SSS. ...
Limitations of the snakebite severity score
1
1996
... The Snakebite Severity Scale (SSS) is a method used to quantify the severity of snakebites based on clinical and laboratory results.[44,45] A score of 0 to 3 indicates mild snakebite, 4 to 7 indicates moderate snakebite, and ≥8 indicates severe snakebite. The SSS is an objective tool with detailed classification. It has been adopted by most countries, mainly for academic research. However, owing to its slight complexity, its original design intention is as a research tool rather than a clinical evaluation tool. Therefore, it is more suitable for population studies of snakebite incidents. If used for clinical diagnosis of patients, the SSS is effective in distinguishing severe poisoning but has poor discrimination for mild and moderate poisoning.[46] It is not specific for certain symptoms and signs, so its reliability is poor. The modified SSS incorporates indicators of kidney damage, improving its discrimination ability (Table 2). However, the score still has poor discrimination for neurotoxic snakebites. For evaluating and classifying neurotoxic snakebites, it is recommended to directly refer to the neurological assessment score in this modified SSS. ...
7
... The assessment of venomous snakebites has obvious time limits. If the time from bite to medical treatment is short, the degree of poisoning is relatively mild. With progression of time, the poisoning may become more serious. The following conditions suggest severe poisoning:[47] (1) snakebite caused by a very dangerous snake species or a snake with a large body; (2) wide distance between teeth, or multiple bites or multiple venomous snake bites; (3) rapid progression and spread of local swelling in the early stage; (4) early occurrence of lymph node swelling and pain, suggesting the spread of snake venom through the lymphatic system; (5) early occurrence of symptoms of systemic toxicity, such as low blood pressure or shock, nausea, vomiting, diarrhea, severe headache, heavy eyelid sensation, drowsiness, early ptosis or ophthalmoplegia; (6) early occurrence of spontaneous systemic bleeding; (7) no urine after the snakebite; (8) early appearance of hematuria, brown urine, or black urine. ...
... The following measures should be avoided for snakebite first aid: picking up or touching a seemingly dead venomous snake with bare hands; waiting for symptoms to occur to determine whether it is a venomous snakebite; using a tourniquet to tie up injured limbs;[51] using a knife to cut the wound (tooth marks); attempting to suck out the toxin; using ice or immersing the wound in ice water; drinking alcohol or coffee to alleviate pain; and treating the wound with cautery.[47,52] ...
... There is no absolute contraindication to the use of antivenom, especially for severely toxic patients. Skin tests are effective tools for screening patients with severe immediate reactions and should be performed before the first use of antivenom. Antivenom can be used only if the test is negative. Although antivenom skin tests cannot accurately predict allergic reactions,[47,62] they should be routinely performed before medication. For patients with a positive skin test, antivenom should be administered only when its benefits outweigh the risks. Adrenaline preconditioning might be considered for patients with a positive skin test alone. ...
... No clinical study can clearly determine the ideal dosage of antivenom, and there is no unified standard around the world. The dosage is mainly determined based on the patient’s condition, guidelines, or clinical experience. The dosage should be determined by clinicians on the basis of differences in snake species, region, severity, and timing of treatment. According to the North American protocol, the initial dose is 4-6 vials, and for cases with potentially fatal injuries, such as shock or severe active bleeding, the initial dose is increased to 8-12 vials. The median initial control dose is nine vials (interquartile range 6-15 vials).[67-68] In China, where monovalent antivenom is available, an initial dose of 2-4 vials seems reasonable on the basis of domestic and overseas experience; the dose can be increased on the basis of the severity of poisoning.[15,47] Appropriately increasing the initial dose may help combat potential snake venom in the blood and is as safe as low initial doses.[69] However, the administration of >5 vials in a single dose can increase the risk of adverse reactions.[62] ...
... For management of adverse reactions,[47,83 -84] it is recommended to immediately stop using antivenom and other medications and rapidly inject 500 mL of normal saline (10 mL/kg for children) to maintain an adequate blood volume. Place the patient at a supine position, keep the airway unobstructed, provide high-flow oxygen therapy, and if necessary, provide endotracheal intubation and ventilation support. Adrenaline is the first choice for treating allergic reactions to antivenom. For cardiac and respiratory arrest, rescue procedures comply with the cardiopulmonary resuscitation protocol. For mild cases, administer antihistamines and glucocorticoids; and for those with asthma, give inhalational β-agonists. Treatment of serum sickness mainly involves antihistamines and glucocorticoids; non-steroidal anti-inflammatory drugs can be used for pain relief. ...
... Neurotoxins produce myocardial paralytic effects by inhibiting the release of acetylcholine from the presynaptic membrane or by binding to acetylcholine receptors on the postsynaptic membrane. Anticholinesterase drugs such as neostigmine or pyridostigmine can inhibit acetylcholinesterase activity, reduce the hydrolysis of acetylcholine in the synaptic gap, and exert a complete cholinesteroid effect, which produces an excitatory effect on skeletal muscle and has a certain degree of efficacy in the reversal of some types of neurotoxin-induced myocardial paralysis.[128⇓-130] However, these methods are ineffective against presynaptic membrane toxins such as those produced by Bungarus caeruleus.[131] Neostigmine or pyridostigmine 0.02 mg/kg (0.04 mg/kg in children) is administered intramuscularly, with 0.5-2.5 mg repeated for 1-3 h if necessary, with the total daily dose not exceeding 10 mg. Because of the risk of increased airway secretions with neostigmine, 0.6 mg of atropine sulfate (50 μg/kg in children) may be administered intravenously prior to the medication.[47] Such medications should not delay the administration of antivenom and necessary tracheal intubation. ...
... The neck glands of venom-spraying cobras, Chinese cobras, and Rhabdophis tigrinus can spray toxins. If toxins are sprayed into the eyes, they can cause severe pain, photophobia, lacrimation, blurred vision, other irritating symptoms, and even corneal ulcers and secondary endophthalmitis.[144] Immediate low-pressure irrigation with large amounts of water is required at the scene, followed by thorough irrigation with normal saline or lactated Ringer’s solution after arrival at the hospital. Topical application of 0.5% adrenaline drops or 4% lidocaine eye drops can be used for pain relief. Ophthalmologic examination is required to assess corneal damage, and topical antibiotic drops such as chloramphenicol, tetracycline, or ciprofloxacin can be administered to prevent intraocular or corneal opacities. In principle, antivenom is not necessary, but for those with severe eye damage or signs of toxin absorption in the early stages, antivenom should be administered. Glucocorticoids are contraindicated because of the risk of herpes simplex keratitis.[47,145] ...
First aid and pre-hospital management of venomous snakebites
1
2018
... On-site first aid aims to ensure the safety and physical integrity of snakebite victims, delay toxin absorption, prevent complications, and ensure immediate transport of the victim to a medical center capable of treating snakebites to minimize additional harm. In order not to delay first aid, refer to the protocol for snakebites (Figure 1). The following first aid methods can be used:[1,48⇓-50] ...
The treatment of snake bites in a first aid setting: a systematic review
1
2016
... On-site first aid aims to ensure the safety and physical integrity of snakebite victims, delay toxin absorption, prevent complications, and ensure immediate transport of the victim to a medical center capable of treating snakebites to minimize additional harm. In order not to delay first aid, refer to the protocol for snakebites (Figure 1). The following first aid methods can be used:[1,48⇓-50] ...
1
... On-site first aid aims to ensure the safety and physical integrity of snakebite victims, delay toxin absorption, prevent complications, and ensure immediate transport of the victim to a medical center capable of treating snakebites to minimize additional harm. In order not to delay first aid, refer to the protocol for snakebites (Figure 1). The following first aid methods can be used:[1,48⇓-50] ...
Impact of tourniquet use on severity of snakebite envenoming in Chongqing, China: a single-center retrospective study
1
2024
... The following measures should be avoided for snakebite first aid: picking up or touching a seemingly dead venomous snake with bare hands; waiting for symptoms to occur to determine whether it is a venomous snakebite; using a tourniquet to tie up injured limbs;[51] using a knife to cut the wound (tooth marks); attempting to suck out the toxin; using ice or immersing the wound in ice water; drinking alcohol or coffee to alleviate pain; and treating the wound with cautery.[47,52] ...
Traditional first aid in a case of snake bite: more harm than good
1
2014
... The following measures should be avoided for snakebite first aid: picking up or touching a seemingly dead venomous snake with bare hands; waiting for symptoms to occur to determine whether it is a venomous snakebite; using a tourniquet to tie up injured limbs;[51] using a knife to cut the wound (tooth marks); attempting to suck out the toxin; using ice or immersing the wound in ice water; drinking alcohol or coffee to alleviate pain; and treating the wound with cautery.[47,52] ...
1
... The classical indications for the use of antivenom include patients with confirmed or suspected snakebites who have at least one of the following systemic or local toxic manifestations.[53] (1) Systemic toxic manifestations: coagulopathy (such as spontaneous bleeding in other parts of the body in addition to bite wounds, decreased platelet count, prolonged BT, decreased fibrinogen, INR >1.2, PT 4-5 s higher than the normal upper limit, and platelet count <10×109/L), neurological toxic manifestations (such as ptosis, external eye muscle paralysis, dilated pupils, muscle weakness or paralysis, and fasciculations), cardiovascular manifestations (such as hypotension, shock, arrhythmia, and abnormal ECG), manifestations of acute kidney injury or renal failure (such as oliguria or anuria, elevated blood urea nitrogen [BUN]/creatinine, black urine or brown urine), and other evidence of intravascular hemolysis (rhabdomyolysis [myalgia or hyperkalemia], hemoglobinuria, or myoglobinuria). (2) Local toxic manifestations: local swelling exceeding half of the bitten limb within 48 h of snakebite; swelling of fingers and toes after bite, with extensive blisters; rapid progression of swelling (such as swelling exceeding the wrist or ankle joint within a few hours of the hand or foot being bitten); swelling of draining lymph nodes after bite; and snakebites caused by local species known to cause necrosis, such as Chinese cobra, Agkistrodon acutus, and Asian cobra. ...
Older age and time to medical assistance are associated with severity and mortality of snakebites in the Brazilian Amazon: a case-control study
1
2015
... Snakebite is a time-critical emergency. The antivenom binds to snake venom to exert its anti-toxic effect, and the time of initiation of antivenom treatment is directly related to the prognosis. It is critical to shorten the time from the bite of a venomous snake to the use of antivenom.[54⇓-56] This can reverse VICC, hypotension, and postsynaptic neurotoxicity. Early medication can prevent or limit presynaptic neurotoxicity, rhabdomyolysis, and local tissue necrosis.[13] Delayed use of antivenoms increases the risk of death.[57] Antivenoms are antidotes. Early and sufficient antivenom can effectively prevent subsequent damage from snake venom. The sooner it is used, the less damage the venom causes to the tissue and the better the prognosis.[58] As long as it is confirmed or highly suspected that there is a venomous snakebite with progressive intoxication or abnormal laboratory results, antivenom should be administered immediately without waiting for the onset of typical toxic manifestations. If the amount of antivenom is insufficient, the unneutralized toxins in the tissue can still produce toxic manifestations nearly 200 h or longer after the bite.[59-60] Therefore, if antivenom is not used in sufficient amounts in the early stage, as long as the toxic damage continues, antivenom should still be considered several days or even longer after the bite. Antivenom has been shown to be effective even 17 d after the bite of a venomous snake.[61] ...
Antivenom availability, delays and use in Australia
1
2022
... Snakebite is a time-critical emergency. The antivenom binds to snake venom to exert its anti-toxic effect, and the time of initiation of antivenom treatment is directly related to the prognosis. It is critical to shorten the time from the bite of a venomous snake to the use of antivenom.[54⇓-56] This can reverse VICC, hypotension, and postsynaptic neurotoxicity. Early medication can prevent or limit presynaptic neurotoxicity, rhabdomyolysis, and local tissue necrosis.[13] Delayed use of antivenoms increases the risk of death.[57] Antivenoms are antidotes. Early and sufficient antivenom can effectively prevent subsequent damage from snake venom. The sooner it is used, the less damage the venom causes to the tissue and the better the prognosis.[58] As long as it is confirmed or highly suspected that there is a venomous snakebite with progressive intoxication or abnormal laboratory results, antivenom should be administered immediately without waiting for the onset of typical toxic manifestations. If the amount of antivenom is insufficient, the unneutralized toxins in the tissue can still produce toxic manifestations nearly 200 h or longer after the bite.[59-60] Therefore, if antivenom is not used in sufficient amounts in the early stage, as long as the toxic damage continues, antivenom should still be considered several days or even longer after the bite. Antivenom has been shown to be effective even 17 d after the bite of a venomous snake.[61] ...
Australian taipan (Oxyuranus spp.) envenoming: clinical effects and potential benefits of early antivenom therapy - Australian Snakebite Project (ASP-25)
1
2017
... Snakebite is a time-critical emergency. The antivenom binds to snake venom to exert its anti-toxic effect, and the time of initiation of antivenom treatment is directly related to the prognosis. It is critical to shorten the time from the bite of a venomous snake to the use of antivenom.[54⇓-56] This can reverse VICC, hypotension, and postsynaptic neurotoxicity. Early medication can prevent or limit presynaptic neurotoxicity, rhabdomyolysis, and local tissue necrosis.[13] Delayed use of antivenoms increases the risk of death.[57] Antivenoms are antidotes. Early and sufficient antivenom can effectively prevent subsequent damage from snake venom. The sooner it is used, the less damage the venom causes to the tissue and the better the prognosis.[58] As long as it is confirmed or highly suspected that there is a venomous snakebite with progressive intoxication or abnormal laboratory results, antivenom should be administered immediately without waiting for the onset of typical toxic manifestations. If the amount of antivenom is insufficient, the unneutralized toxins in the tissue can still produce toxic manifestations nearly 200 h or longer after the bite.[59-60] Therefore, if antivenom is not used in sufficient amounts in the early stage, as long as the toxic damage continues, antivenom should still be considered several days or even longer after the bite. Antivenom has been shown to be effective even 17 d after the bite of a venomous snake.[61] ...
Factors affecting snakebite mortality in north-eastern Nigeria
1
2011
... Snakebite is a time-critical emergency. The antivenom binds to snake venom to exert its anti-toxic effect, and the time of initiation of antivenom treatment is directly related to the prognosis. It is critical to shorten the time from the bite of a venomous snake to the use of antivenom.[54⇓-56] This can reverse VICC, hypotension, and postsynaptic neurotoxicity. Early medication can prevent or limit presynaptic neurotoxicity, rhabdomyolysis, and local tissue necrosis.[13] Delayed use of antivenoms increases the risk of death.[57] Antivenoms are antidotes. Early and sufficient antivenom can effectively prevent subsequent damage from snake venom. The sooner it is used, the less damage the venom causes to the tissue and the better the prognosis.[58] As long as it is confirmed or highly suspected that there is a venomous snakebite with progressive intoxication or abnormal laboratory results, antivenom should be administered immediately without waiting for the onset of typical toxic manifestations. If the amount of antivenom is insufficient, the unneutralized toxins in the tissue can still produce toxic manifestations nearly 200 h or longer after the bite.[59-60] Therefore, if antivenom is not used in sufficient amounts in the early stage, as long as the toxic damage continues, antivenom should still be considered several days or even longer after the bite. Antivenom has been shown to be effective even 17 d after the bite of a venomous snake.[61] ...
Bite-to-needle time - an extrapolative indicator of repercussion in patients with snakebite
1
2022
... Snakebite is a time-critical emergency. The antivenom binds to snake venom to exert its anti-toxic effect, and the time of initiation of antivenom treatment is directly related to the prognosis. It is critical to shorten the time from the bite of a venomous snake to the use of antivenom.[54⇓-56] This can reverse VICC, hypotension, and postsynaptic neurotoxicity. Early medication can prevent or limit presynaptic neurotoxicity, rhabdomyolysis, and local tissue necrosis.[13] Delayed use of antivenoms increases the risk of death.[57] Antivenoms are antidotes. Early and sufficient antivenom can effectively prevent subsequent damage from snake venom. The sooner it is used, the less damage the venom causes to the tissue and the better the prognosis.[58] As long as it is confirmed or highly suspected that there is a venomous snakebite with progressive intoxication or abnormal laboratory results, antivenom should be administered immediately without waiting for the onset of typical toxic manifestations. If the amount of antivenom is insufficient, the unneutralized toxins in the tissue can still produce toxic manifestations nearly 200 h or longer after the bite.[59-60] Therefore, if antivenom is not used in sufficient amounts in the early stage, as long as the toxic damage continues, antivenom should still be considered several days or even longer after the bite. Antivenom has been shown to be effective even 17 d after the bite of a venomous snake.[61] ...
Recurrence phenomena after immunoglobulin therapy for snake envenomations: Part 1. Pharmacokinetics and pharmacodynamics of immunoglobulin antivenoms and related antibodies
1
2001
... Snakebite is a time-critical emergency. The antivenom binds to snake venom to exert its anti-toxic effect, and the time of initiation of antivenom treatment is directly related to the prognosis. It is critical to shorten the time from the bite of a venomous snake to the use of antivenom.[54⇓-56] This can reverse VICC, hypotension, and postsynaptic neurotoxicity. Early medication can prevent or limit presynaptic neurotoxicity, rhabdomyolysis, and local tissue necrosis.[13] Delayed use of antivenoms increases the risk of death.[57] Antivenoms are antidotes. Early and sufficient antivenom can effectively prevent subsequent damage from snake venom. The sooner it is used, the less damage the venom causes to the tissue and the better the prognosis.[58] As long as it is confirmed or highly suspected that there is a venomous snakebite with progressive intoxication or abnormal laboratory results, antivenom should be administered immediately without waiting for the onset of typical toxic manifestations. If the amount of antivenom is insufficient, the unneutralized toxins in the tissue can still produce toxic manifestations nearly 200 h or longer after the bite.[59-60] Therefore, if antivenom is not used in sufficient amounts in the early stage, as long as the toxic damage continues, antivenom should still be considered several days or even longer after the bite. Antivenom has been shown to be effective even 17 d after the bite of a venomous snake.[61] ...
Unpredicted late-, new-onset thrombocytopenia and hypofibrinogenemia in Fab antivenom-treated rattlesnake envenomation
1
2020
... Snakebite is a time-critical emergency. The antivenom binds to snake venom to exert its anti-toxic effect, and the time of initiation of antivenom treatment is directly related to the prognosis. It is critical to shorten the time from the bite of a venomous snake to the use of antivenom.[54⇓-56] This can reverse VICC, hypotension, and postsynaptic neurotoxicity. Early medication can prevent or limit presynaptic neurotoxicity, rhabdomyolysis, and local tissue necrosis.[13] Delayed use of antivenoms increases the risk of death.[57] Antivenoms are antidotes. Early and sufficient antivenom can effectively prevent subsequent damage from snake venom. The sooner it is used, the less damage the venom causes to the tissue and the better the prognosis.[58] As long as it is confirmed or highly suspected that there is a venomous snakebite with progressive intoxication or abnormal laboratory results, antivenom should be administered immediately without waiting for the onset of typical toxic manifestations. If the amount of antivenom is insufficient, the unneutralized toxins in the tissue can still produce toxic manifestations nearly 200 h or longer after the bite.[59-60] Therefore, if antivenom is not used in sufficient amounts in the early stage, as long as the toxic damage continues, antivenom should still be considered several days or even longer after the bite. Antivenom has been shown to be effective even 17 d after the bite of a venomous snake.[61] ...
One case of re-recurrence of poisoning symptoms in Chinese cobra bite with literature review
1
2022
... Snakebite is a time-critical emergency. The antivenom binds to snake venom to exert its anti-toxic effect, and the time of initiation of antivenom treatment is directly related to the prognosis. It is critical to shorten the time from the bite of a venomous snake to the use of antivenom.[54⇓-56] This can reverse VICC, hypotension, and postsynaptic neurotoxicity. Early medication can prevent or limit presynaptic neurotoxicity, rhabdomyolysis, and local tissue necrosis.[13] Delayed use of antivenoms increases the risk of death.[57] Antivenoms are antidotes. Early and sufficient antivenom can effectively prevent subsequent damage from snake venom. The sooner it is used, the less damage the venom causes to the tissue and the better the prognosis.[58] As long as it is confirmed or highly suspected that there is a venomous snakebite with progressive intoxication or abnormal laboratory results, antivenom should be administered immediately without waiting for the onset of typical toxic manifestations. If the amount of antivenom is insufficient, the unneutralized toxins in the tissue can still produce toxic manifestations nearly 200 h or longer after the bite.[59-60] Therefore, if antivenom is not used in sufficient amounts in the early stage, as long as the toxic damage continues, antivenom should still be considered several days or even longer after the bite. Antivenom has been shown to be effective even 17 d after the bite of a venomous snake.[61] ...
Risk factors associated with snake antivenom reaction and the role of skin test
2
2020
... There is no absolute contraindication to the use of antivenom, especially for severely toxic patients. Skin tests are effective tools for screening patients with severe immediate reactions and should be performed before the first use of antivenom. Antivenom can be used only if the test is negative. Although antivenom skin tests cannot accurately predict allergic reactions,[47,62] they should be routinely performed before medication. For patients with a positive skin test, antivenom should be administered only when its benefits outweigh the risks. Adrenaline preconditioning might be considered for patients with a positive skin test alone. ...
... No clinical study can clearly determine the ideal dosage of antivenom, and there is no unified standard around the world. The dosage is mainly determined based on the patient’s condition, guidelines, or clinical experience. The dosage should be determined by clinicians on the basis of differences in snake species, region, severity, and timing of treatment. According to the North American protocol, the initial dose is 4-6 vials, and for cases with potentially fatal injuries, such as shock or severe active bleeding, the initial dose is increased to 8-12 vials. The median initial control dose is nine vials (interquartile range 6-15 vials).[67-68] In China, where monovalent antivenom is available, an initial dose of 2-4 vials seems reasonable on the basis of domestic and overseas experience; the dose can be increased on the basis of the severity of poisoning.[15,47] Appropriately increasing the initial dose may help combat potential snake venom in the blood and is as safe as low initial doses.[69] However, the administration of >5 vials in a single dose can increase the risk of adverse reactions.[62] ...
Low dose subcutaneous adrenaline to prevent acute adverse reactions to antivenom serum in people bitten by snakes: randomised, placebo-controlled trial
1
1999
... Pre-treatment with low-dose adrenaline (0.25 mg via intramuscular injection) before antivenom can safely reduce the risk of adverse reactions in the early stage (within 1 h) and is still effective for up to 48 h.[63⇓-65] However, routine adrenaline pre-treatment is not necessary. Adrenaline pre-treatment should be used cautiously in infants, pregnant women, individuals with altered consciousness, and those with VICC accompanied by meningeal irritation or neurologic localization signs due to the high risk of adverse reactions.[66] ...
Antivenom use, premedication and early adverse reactions in the management of snake bites in rural Papua New Guinea
1
2007
... Pre-treatment with low-dose adrenaline (0.25 mg via intramuscular injection) before antivenom can safely reduce the risk of adverse reactions in the early stage (within 1 h) and is still effective for up to 48 h.[63⇓-65] However, routine adrenaline pre-treatment is not necessary. Adrenaline pre-treatment should be used cautiously in infants, pregnant women, individuals with altered consciousness, and those with VICC accompanied by meningeal irritation or neurologic localization signs due to the high risk of adverse reactions.[66] ...
Low-dose adrenaline, promethazine, and hydrocortisone in the prevention of acute adverse reactions to antivenom following snakebite: a randomised, double-blind, placebo-controlled trial
2
2011
... Pre-treatment with low-dose adrenaline (0.25 mg via intramuscular injection) before antivenom can safely reduce the risk of adverse reactions in the early stage (within 1 h) and is still effective for up to 48 h.[63⇓-65] However, routine adrenaline pre-treatment is not necessary. Adrenaline pre-treatment should be used cautiously in infants, pregnant women, individuals with altered consciousness, and those with VICC accompanied by meningeal irritation or neurologic localization signs due to the high risk of adverse reactions.[66] ...
... Adverse reactions are inherent reactions to xenogeneic proteins, with wide variability in incidence rates (2.9%-75.0%).[65,78 -79] The incidence rate of adverse reactions to antivenom in China is approximately 4.9%.[80] Negative skin tests do not rule out potential allergic reactions. Therefore, it is necessary to prepare emergency drugs and equipment, such as adrenaline, oxygen, and endotracheal intubation. It is recommended that the first dose of antivenom should be administered in a setting with rescue facilities. ...
Effect of pre-medication on early adverse reactions following antivenom use in snakebite: a systematic review and meta-analysis
1
2011
... Pre-treatment with low-dose adrenaline (0.25 mg via intramuscular injection) before antivenom can safely reduce the risk of adverse reactions in the early stage (within 1 h) and is still effective for up to 48 h.[63⇓-65] However, routine adrenaline pre-treatment is not necessary. Adrenaline pre-treatment should be used cautiously in infants, pregnant women, individuals with altered consciousness, and those with VICC accompanied by meningeal irritation or neurologic localization signs due to the high risk of adverse reactions.[66] ...
Unified treatment algorithm for the management of crotaline snakebite in the United States: results of an evidence-informed consensus workshop
1
2011
... No clinical study can clearly determine the ideal dosage of antivenom, and there is no unified standard around the world. The dosage is mainly determined based on the patient’s condition, guidelines, or clinical experience. The dosage should be determined by clinicians on the basis of differences in snake species, region, severity, and timing of treatment. According to the North American protocol, the initial dose is 4-6 vials, and for cases with potentially fatal injuries, such as shock or severe active bleeding, the initial dose is increased to 8-12 vials. The median initial control dose is nine vials (interquartile range 6-15 vials).[67-68] In China, where monovalent antivenom is available, an initial dose of 2-4 vials seems reasonable on the basis of domestic and overseas experience; the dose can be increased on the basis of the severity of poisoning.[15,47] Appropriately increasing the initial dose may help combat potential snake venom in the blood and is as safe as low initial doses.[69] However, the administration of >5 vials in a single dose can increase the risk of adverse reactions.[62] ...
North American snake envenomation
1
2017
... No clinical study can clearly determine the ideal dosage of antivenom, and there is no unified standard around the world. The dosage is mainly determined based on the patient’s condition, guidelines, or clinical experience. The dosage should be determined by clinicians on the basis of differences in snake species, region, severity, and timing of treatment. According to the North American protocol, the initial dose is 4-6 vials, and for cases with potentially fatal injuries, such as shock or severe active bleeding, the initial dose is increased to 8-12 vials. The median initial control dose is nine vials (interquartile range 6-15 vials).[67-68] In China, where monovalent antivenom is available, an initial dose of 2-4 vials seems reasonable on the basis of domestic and overseas experience; the dose can be increased on the basis of the severity of poisoning.[15,47] Appropriately increasing the initial dose may help combat potential snake venom in the blood and is as safe as low initial doses.[69] However, the administration of >5 vials in a single dose can increase the risk of adverse reactions.[62] ...
Dose of antivenom for the treatment of snakebite with neurotoxic envenoming: evidence from a randomised controlled trial in Nepal
1
2017
... No clinical study can clearly determine the ideal dosage of antivenom, and there is no unified standard around the world. The dosage is mainly determined based on the patient’s condition, guidelines, or clinical experience. The dosage should be determined by clinicians on the basis of differences in snake species, region, severity, and timing of treatment. According to the North American protocol, the initial dose is 4-6 vials, and for cases with potentially fatal injuries, such as shock or severe active bleeding, the initial dose is increased to 8-12 vials. The median initial control dose is nine vials (interquartile range 6-15 vials).[67-68] In China, where monovalent antivenom is available, an initial dose of 2-4 vials seems reasonable on the basis of domestic and overseas experience; the dose can be increased on the basis of the severity of poisoning.[15,47] Appropriately increasing the initial dose may help combat potential snake venom in the blood and is as safe as low initial doses.[69] However, the administration of >5 vials in a single dose can increase the risk of adverse reactions.[62] ...
Snakebite during pregnancy: a literature review
1
2010
... Venomous snakebites in pregnant women, while relatively rare, can increase the risk of adverse pregnancy outcomes, with a perinatal mortality rate of 5.6%-20.0% (both fetal and neonatal mortality) and a maternal mortality rate of 0-5%.[70-71] Antivenom can counteract or reduce the damage caused by toxins to the mother, thereby reducing the impact on the fetus.[72] Therefore, antivenom is not contraindicated for pregnant women with venomous snakebites, but close maternal and fetal monitoring is required. Fetal heart monitoring should be conducted for at least 8 h and might continue for one week, if feasible.[73] ...
Pregnancy outcomes after snakebite envenomations: a retrospective cohort in the Brazilian Amazonia
1
2022
... Venomous snakebites in pregnant women, while relatively rare, can increase the risk of adverse pregnancy outcomes, with a perinatal mortality rate of 5.6%-20.0% (both fetal and neonatal mortality) and a maternal mortality rate of 0-5%.[70-71] Antivenom can counteract or reduce the damage caused by toxins to the mother, thereby reducing the impact on the fetus.[72] Therefore, antivenom is not contraindicated for pregnant women with venomous snakebites, but close maternal and fetal monitoring is required. Fetal heart monitoring should be conducted for at least 8 h and might continue for one week, if feasible.[73] ...
IgG placental transfer in healthy and pathological pregnancies
1
2012
... Venomous snakebites in pregnant women, while relatively rare, can increase the risk of adverse pregnancy outcomes, with a perinatal mortality rate of 5.6%-20.0% (both fetal and neonatal mortality) and a maternal mortality rate of 0-5%.[70-71] Antivenom can counteract or reduce the damage caused by toxins to the mother, thereby reducing the impact on the fetus.[72] Therefore, antivenom is not contraindicated for pregnant women with venomous snakebites, but close maternal and fetal monitoring is required. Fetal heart monitoring should be conducted for at least 8 h and might continue for one week, if feasible.[73] ...
1
2022
... Venomous snakebites in pregnant women, while relatively rare, can increase the risk of adverse pregnancy outcomes, with a perinatal mortality rate of 5.6%-20.0% (both fetal and neonatal mortality) and a maternal mortality rate of 0-5%.[70-71] Antivenom can counteract or reduce the damage caused by toxins to the mother, thereby reducing the impact on the fetus.[72] Therefore, antivenom is not contraindicated for pregnant women with venomous snakebites, but close maternal and fetal monitoring is required. Fetal heart monitoring should be conducted for at least 8 h and might continue for one week, if feasible.[73] ...
Managing snakebite
2
2022
... The intravenous injection of antivenom can quickly achieve peak blood concentrations, making it the most rapid and effective route for the administration of antivenom. Opening venous access to healthy limbs is more conducive to the rapid entry of antivenom into the blood circulation. For patients with severe coagulopathy, deep venous or arterial puncture should be avoided (which might be considered after improvement of coagulopathy)[74] to prevent severe bleeding. Intravenous injection should be administered slowly (≤2 mL/min); for intravenous infusion, antivenom can be added to 100-250 mL of normal saline and administered within one hour, starting slowly and then quickly (25-50 mL/h for the first 10 min, and the rest quickly dripped). For patients who have undergone local compression fixation or ligation during the visit, the bandage should be removed several minutes after the administration of antivenom. However, for patients with irreversible damage, such as suspected necrosis in the local limb/finger, the bandage should be removed immediately. Antivenom is mostly a large molecular immunoglobulin that is slowly absorbed via intramuscular injection and has low bioavailability.[75] Intramuscular administration may induce local bleeding or hematoma in patients with VICC.[76] ...
... Most acute adverse reactions occur within 1-2 h after the initiation of antivenom treatment. Close monitoring for adverse reactions, focusing on observing the aggravation and amelioration of toxic symptoms, signs, and laboratory indices, is necessary to facilitate the timely administration of additional treatment or calibration of medication. Depending on the severity of envenomation, patients should be monitored at least 2, 6, 12, and 24 h after the first use of antivenom. Blood routine, coagulation function, (cardiac) muscle enzymes, and other laboratory tests should be repeated at 6, 12, and 24 h.[74] After a sufficient neutralizing dose of antivenom is used, the median recovery time for coagulation dysfunction is 6 h.[7,23,77] Therefore, indications for additional medication include persistence of toxic symptoms 6 h after the first dose of antivenom, re-aggravation of coagulation dysfunction after transient recovery, recurrence of bleeding 1-2 h after cessation of bleeding, and continued deterioration of neurological or cardiovascular function for 1 h after the first dose of antivenom. ...
Snake F(ab’)2 antivenom from hyperimmunized horse: pharmacokinetics following intravenous and intramuscular administrations in rabbits
1
1995
... The intravenous injection of antivenom can quickly achieve peak blood concentrations, making it the most rapid and effective route for the administration of antivenom. Opening venous access to healthy limbs is more conducive to the rapid entry of antivenom into the blood circulation. For patients with severe coagulopathy, deep venous or arterial puncture should be avoided (which might be considered after improvement of coagulopathy)[74] to prevent severe bleeding. Intravenous injection should be administered slowly (≤2 mL/min); for intravenous infusion, antivenom can be added to 100-250 mL of normal saline and administered within one hour, starting slowly and then quickly (25-50 mL/h for the first 10 min, and the rest quickly dripped). For patients who have undergone local compression fixation or ligation during the visit, the bandage should be removed several minutes after the administration of antivenom. However, for patients with irreversible damage, such as suspected necrosis in the local limb/finger, the bandage should be removed immediately. Antivenom is mostly a large molecular immunoglobulin that is slowly absorbed via intramuscular injection and has low bioavailability.[75] Intramuscular administration may induce local bleeding or hematoma in patients with VICC.[76] ...
Snake antivenoms-toward better understanding of the administration route
1
2023
... The intravenous injection of antivenom can quickly achieve peak blood concentrations, making it the most rapid and effective route for the administration of antivenom. Opening venous access to healthy limbs is more conducive to the rapid entry of antivenom into the blood circulation. For patients with severe coagulopathy, deep venous or arterial puncture should be avoided (which might be considered after improvement of coagulopathy)[74] to prevent severe bleeding. Intravenous injection should be administered slowly (≤2 mL/min); for intravenous infusion, antivenom can be added to 100-250 mL of normal saline and administered within one hour, starting slowly and then quickly (25-50 mL/h for the first 10 min, and the rest quickly dripped). For patients who have undergone local compression fixation or ligation during the visit, the bandage should be removed several minutes after the administration of antivenom. However, for patients with irreversible damage, such as suspected necrosis in the local limb/finger, the bandage should be removed immediately. Antivenom is mostly a large molecular immunoglobulin that is slowly absorbed via intramuscular injection and has low bioavailability.[75] Intramuscular administration may induce local bleeding or hematoma in patients with VICC.[76] ...
Blood venom antigen levels after Malayan pit viper bite
1
1987
... Most acute adverse reactions occur within 1-2 h after the initiation of antivenom treatment. Close monitoring for adverse reactions, focusing on observing the aggravation and amelioration of toxic symptoms, signs, and laboratory indices, is necessary to facilitate the timely administration of additional treatment or calibration of medication. Depending on the severity of envenomation, patients should be monitored at least 2, 6, 12, and 24 h after the first use of antivenom. Blood routine, coagulation function, (cardiac) muscle enzymes, and other laboratory tests should be repeated at 6, 12, and 24 h.[74] After a sufficient neutralizing dose of antivenom is used, the median recovery time for coagulation dysfunction is 6 h.[7,23,77] Therefore, indications for additional medication include persistence of toxic symptoms 6 h after the first dose of antivenom, re-aggravation of coagulation dysfunction after transient recovery, recurrence of bleeding 1-2 h after cessation of bleeding, and continued deterioration of neurological or cardiovascular function for 1 h after the first dose of antivenom. ...
Early adverse reactions to snake antivenom: poison center data analysis
1
2022
... Adverse reactions are inherent reactions to xenogeneic proteins, with wide variability in incidence rates (2.9%-75.0%).[65,78 -79] The incidence rate of adverse reactions to antivenom in China is approximately 4.9%.[80] Negative skin tests do not rule out potential allergic reactions. Therefore, it is necessary to prepare emergency drugs and equipment, such as adrenaline, oxygen, and endotracheal intubation. It is recommended that the first dose of antivenom should be administered in a setting with rescue facilities. ...
A retrospective study of antivenom-associated adverse reaction and anaphylaxis at Ngwelezana Hospital, South Africa
1
2022
... Adverse reactions are inherent reactions to xenogeneic proteins, with wide variability in incidence rates (2.9%-75.0%).[65,78 -79] The incidence rate of adverse reactions to antivenom in China is approximately 4.9%.[80] Negative skin tests do not rule out potential allergic reactions. Therefore, it is necessary to prepare emergency drugs and equipment, such as adrenaline, oxygen, and endotracheal intubation. It is recommended that the first dose of antivenom should be administered in a setting with rescue facilities. ...
Adverse reactions to four types of monovalent antivenom used in the treatment of snakebite envenoming in South China
1
2022
... Adverse reactions are inherent reactions to xenogeneic proteins, with wide variability in incidence rates (2.9%-75.0%).[65,78 -79] The incidence rate of adverse reactions to antivenom in China is approximately 4.9%.[80] Negative skin tests do not rule out potential allergic reactions. Therefore, it is necessary to prepare emergency drugs and equipment, such as adrenaline, oxygen, and endotracheal intubation. It is recommended that the first dose of antivenom should be administered in a setting with rescue facilities. ...
Antivenom therapy: efficacy of premedication for the prevention of adverse reactions
1
2018
... Adverse reactions to antivenom can be classified into three types.[81-82] (1) Anaphylactic reactions, such as rash, urticaria, nausea and vomiting, pain, and even anaphylactic shock, often occur within minutes to several hours after administration, with most occurring within 1-2 h. In severe cases, symptoms such as hypotension or asthma may occur. (2) Allergen reactions, which occur within 1-2 h after administration, manifest as chills, rigors, fever, vascular dilation, and blood pressure drop. Children may develop febrile convulsions. (3) Serum sickness, which occurs 5-20 d (average 7 d) after administration of antivenom, manifests as fever, rash, nausea, vomiting, itching, fatigue, myalgia, joint swelling and pain, and lymphadenopathy. ...
Antivenom: an immunotherapy for the treatment of snakebite envenoming in sub-Saharan Africa
1
2022
... Adverse reactions to antivenom can be classified into three types.[81-82] (1) Anaphylactic reactions, such as rash, urticaria, nausea and vomiting, pain, and even anaphylactic shock, often occur within minutes to several hours after administration, with most occurring within 1-2 h. In severe cases, symptoms such as hypotension or asthma may occur. (2) Allergen reactions, which occur within 1-2 h after administration, manifest as chills, rigors, fever, vascular dilation, and blood pressure drop. Children may develop febrile convulsions. (3) Serum sickness, which occurs 5-20 d (average 7 d) after administration of antivenom, manifests as fever, rash, nausea, vomiting, itching, fatigue, myalgia, joint swelling and pain, and lymphadenopathy. ...
EAACI guidelines: Anaphylaxis (2021 update)
1
2022
... For management of adverse reactions,[47,83 -84] it is recommended to immediately stop using antivenom and other medications and rapidly inject 500 mL of normal saline (10 mL/kg for children) to maintain an adequate blood volume. Place the patient at a supine position, keep the airway unobstructed, provide high-flow oxygen therapy, and if necessary, provide endotracheal intubation and ventilation support. Adrenaline is the first choice for treating allergic reactions to antivenom. For cardiac and respiratory arrest, rescue procedures comply with the cardiopulmonary resuscitation protocol. For mild cases, administer antihistamines and glucocorticoids; and for those with asthma, give inhalational β-agonists. Treatment of serum sickness mainly involves antihistamines and glucocorticoids; non-steroidal anti-inflammatory drugs can be used for pain relief. ...
A clinical practice guideline for the emergency management of anaphylaxis (2020)
1
2022
... For management of adverse reactions,[47,83 -84] it is recommended to immediately stop using antivenom and other medications and rapidly inject 500 mL of normal saline (10 mL/kg for children) to maintain an adequate blood volume. Place the patient at a supine position, keep the airway unobstructed, provide high-flow oxygen therapy, and if necessary, provide endotracheal intubation and ventilation support. Adrenaline is the first choice for treating allergic reactions to antivenom. For cardiac and respiratory arrest, rescue procedures comply with the cardiopulmonary resuscitation protocol. For mild cases, administer antihistamines and glucocorticoids; and for those with asthma, give inhalational β-agonists. Treatment of serum sickness mainly involves antihistamines and glucocorticoids; non-steroidal anti-inflammatory drugs can be used for pain relief. ...
Role of surgical intervention in the management of crotaline snake envenomation
1
2001
... There is no need for wound treatment, or only water local rinse is needed for pre-hospital treatment of venomous snakebites. The purpose of wound debridement in venomous snakebites is to find and remove possible residual broken teeth, clean the wound contamination or infection focus, and remove local necrotic tissue. Most venomous snakebite teeth marks do not require treatment. For example, bites of many-banded kraits, banded kraits, sea snakes, and bamboo snakes do not require wound treatment or only require saline washing at the site of the tooth mark. Only a very small number of snakebites may need debridement. For example, the cytotoxicity of the Chinese cobra can easily cause wound tissue necrosis and infection.[85⇓-87] Other venomous snakebites may require debridement depending on the wound infection or necrosis. For those bitten by simple neurotoxic snakes, a local incision is not necessary.[88] Incision debridement must be considered after using sufficient antivenom. Early sufficient antivenom can prevent tissue damage and reduce intraventricular pressure.[89] ...
Surgery in management of snake envenomation in children
1
2011
... There is no need for wound treatment, or only water local rinse is needed for pre-hospital treatment of venomous snakebites. The purpose of wound debridement in venomous snakebites is to find and remove possible residual broken teeth, clean the wound contamination or infection focus, and remove local necrotic tissue. Most venomous snakebite teeth marks do not require treatment. For example, bites of many-banded kraits, banded kraits, sea snakes, and bamboo snakes do not require wound treatment or only require saline washing at the site of the tooth mark. Only a very small number of snakebites may need debridement. For example, the cytotoxicity of the Chinese cobra can easily cause wound tissue necrosis and infection.[85⇓-87] Other venomous snakebites may require debridement depending on the wound infection or necrosis. For those bitten by simple neurotoxic snakes, a local incision is not necessary.[88] Incision debridement must be considered after using sufficient antivenom. Early sufficient antivenom can prevent tissue damage and reduce intraventricular pressure.[89] ...
Snake bite management: a scoping review of the literature
1
2021
... There is no need for wound treatment, or only water local rinse is needed for pre-hospital treatment of venomous snakebites. The purpose of wound debridement in venomous snakebites is to find and remove possible residual broken teeth, clean the wound contamination or infection focus, and remove local necrotic tissue. Most venomous snakebite teeth marks do not require treatment. For example, bites of many-banded kraits, banded kraits, sea snakes, and bamboo snakes do not require wound treatment or only require saline washing at the site of the tooth mark. Only a very small number of snakebites may need debridement. For example, the cytotoxicity of the Chinese cobra can easily cause wound tissue necrosis and infection.[85⇓-87] Other venomous snakebites may require debridement depending on the wound infection or necrosis. For those bitten by simple neurotoxic snakes, a local incision is not necessary.[88] Incision debridement must be considered after using sufficient antivenom. Early sufficient antivenom can prevent tissue damage and reduce intraventricular pressure.[89] ...
Clinical manifestations and treatments of Protobothrops mucrosquamatus bite and associated factors for wound necrosis and subsequent debridement and finger or toe amputation surgery
1
2021
... There is no need for wound treatment, or only water local rinse is needed for pre-hospital treatment of venomous snakebites. The purpose of wound debridement in venomous snakebites is to find and remove possible residual broken teeth, clean the wound contamination or infection focus, and remove local necrotic tissue. Most venomous snakebite teeth marks do not require treatment. For example, bites of many-banded kraits, banded kraits, sea snakes, and bamboo snakes do not require wound treatment or only require saline washing at the site of the tooth mark. Only a very small number of snakebites may need debridement. For example, the cytotoxicity of the Chinese cobra can easily cause wound tissue necrosis and infection.[85⇓-87] Other venomous snakebites may require debridement depending on the wound infection or necrosis. For those bitten by simple neurotoxic snakes, a local incision is not necessary.[88] Incision debridement must be considered after using sufficient antivenom. Early sufficient antivenom can prevent tissue damage and reduce intraventricular pressure.[89] ...
Surgical considerations in the management of pit viper snake envenomation
2
2013
... There is no need for wound treatment, or only water local rinse is needed for pre-hospital treatment of venomous snakebites. The purpose of wound debridement in venomous snakebites is to find and remove possible residual broken teeth, clean the wound contamination or infection focus, and remove local necrotic tissue. Most venomous snakebite teeth marks do not require treatment. For example, bites of many-banded kraits, banded kraits, sea snakes, and bamboo snakes do not require wound treatment or only require saline washing at the site of the tooth mark. Only a very small number of snakebites may need debridement. For example, the cytotoxicity of the Chinese cobra can easily cause wound tissue necrosis and infection.[85⇓-87] Other venomous snakebites may require debridement depending on the wound infection or necrosis. For those bitten by simple neurotoxic snakes, a local incision is not necessary.[88] Incision debridement must be considered after using sufficient antivenom. Early sufficient antivenom can prevent tissue damage and reduce intraventricular pressure.[89] ...
... NPWT, also known as vacuum sealing drainage (VSD) or vacuum-assisted wound closure (VAC), entails the use of a closed negative pressure treatment system that provides subatmospheric pressure to acute and chronic open wounds. The system consists of a polyurethane foam sponge, a semiocclusive barrier, a liquid collection system, and a negative pressure pump. NPWT promotes wound healing by promoting tissue deformation, drainage of extracellular inflammatory fluid, stabilization of the wound environment, and micro-deformation.[95-96] It is an effective treatment for acute and chronic wounds such as diabetic foot ulcers, pressure ulcers, chronic wounds, and skin grafts.[97⇓⇓⇓-101] However, several high-quality studies have shown that NPWT is not superior to standard therapy and may even cause more complications,[102⇓⇓-105] such as toxic shock syndrome, infection, pain, bleeding, adjacent tissue ischemia, and hemodynamic instability.[89] ...
Is there a role for fasciotomy in Crotalinae envenomations in North America?
1
2011
... Most cases of snake venom-induced compartment syndrome (SVCS) are caused by direct injection of toxins into muscle tissue. Risk factors for SVCS include venomous snakebites, bites on fingers or toes, the use of ice packs or ice compresses on wounds, insufficient or delayed use of antivenom, and younger children.[90] Only a small number of patients develop acute compartment syndrome (ACS). ...
An online survey of non-compressible torso hemorrhage: training is needed
1
2022
... Most of the conscious patients with ACS only present with pain and sensory abnormalities; manifestations of arterial ischemia, such as pallor, numbness, and pulselessness, are rare or occur only in the later stages of severe ACS. Therefore, it is not possible to determine compartment syndrome on the basis of “soft signs”, such as local swelling and hardening, disproportionate pain, and stretch pain. The indications for decompression of the SVCS include at least the following four criteria:[1] (1) coagulopathy is corrected or significantly improved; (2) clinical indications consistent with ACS; (3) pressure difference (△P = diastolic pressure - intra-fascial pressure) ≤30 mmHg or absolute pressure in the fascia compartment >40 mmHg; and (4) presence of signs of neurological and/or vascular damage and blood flow impairment. △P>30 mmHg can be used as an exclusion criterion for SVCS.[91] ...
Compartment syndrome after crotalid envenomation in the United States: a review of the North American snakebite registry from 2013 to 2021 on behalf of the Toxic snakebite study group
1
2023
... For patients with SVCS, in whom SVCS is not relieved after sufficient antivenom, incision and decompression are reasonable.[92] However, it should not be performed as a preventive measure,[93] as an incision increases the risk of bleeding, neurovascular or tendon injury, and infection and may even aggravate local muscle necrosis, prolong hospitalization time, and increase hospitalization costs. In addition, it may also cause wound scar formation, fibrosis, and surgical accident-induced nerve or vascular injury or lead to long-term adverse effects such as impaired physical function, unaesthetic appearance, pain, or sensory disturbance.[94-95] ...
Fasciotomy worsens the amount of myonecrosis in a porcine model of crotaline envenomation
1
2004
... For patients with SVCS, in whom SVCS is not relieved after sufficient antivenom, incision and decompression are reasonable.[92] However, it should not be performed as a preventive measure,[93] as an incision increases the risk of bleeding, neurovascular or tendon injury, and infection and may even aggravate local muscle necrosis, prolong hospitalization time, and increase hospitalization costs. In addition, it may also cause wound scar formation, fibrosis, and surgical accident-induced nerve or vascular injury or lead to long-term adverse effects such as impaired physical function, unaesthetic appearance, pain, or sensory disturbance.[94-95] ...
A chance to cut is not always a chance to cure- fasciotomy in the treatment of rattlesnake envenomation: a retrospective poison center study
1
2015
... For patients with SVCS, in whom SVCS is not relieved after sufficient antivenom, incision and decompression are reasonable.[92] However, it should not be performed as a preventive measure,[93] as an incision increases the risk of bleeding, neurovascular or tendon injury, and infection and may even aggravate local muscle necrosis, prolong hospitalization time, and increase hospitalization costs. In addition, it may also cause wound scar formation, fibrosis, and surgical accident-induced nerve or vascular injury or lead to long-term adverse effects such as impaired physical function, unaesthetic appearance, pain, or sensory disturbance.[94-95] ...
Negative pressure wound therapy: mechanism of action and clinical applications
2
2021
... For patients with SVCS, in whom SVCS is not relieved after sufficient antivenom, incision and decompression are reasonable.[92] However, it should not be performed as a preventive measure,[93] as an incision increases the risk of bleeding, neurovascular or tendon injury, and infection and may even aggravate local muscle necrosis, prolong hospitalization time, and increase hospitalization costs. In addition, it may also cause wound scar formation, fibrosis, and surgical accident-induced nerve or vascular injury or lead to long-term adverse effects such as impaired physical function, unaesthetic appearance, pain, or sensory disturbance.[94-95] ...
... NPWT, also known as vacuum sealing drainage (VSD) or vacuum-assisted wound closure (VAC), entails the use of a closed negative pressure treatment system that provides subatmospheric pressure to acute and chronic open wounds. The system consists of a polyurethane foam sponge, a semiocclusive barrier, a liquid collection system, and a negative pressure pump. NPWT promotes wound healing by promoting tissue deformation, drainage of extracellular inflammatory fluid, stabilization of the wound environment, and micro-deformation.[95-96] It is an effective treatment for acute and chronic wounds such as diabetic foot ulcers, pressure ulcers, chronic wounds, and skin grafts.[97⇓⇓⇓-101] However, several high-quality studies have shown that NPWT is not superior to standard therapy and may even cause more complications,[102⇓⇓-105] such as toxic shock syndrome, infection, pain, bleeding, adjacent tissue ischemia, and hemodynamic instability.[89] ...
Negative pressure wound therapy with instillation: international consensus guidelines update
1
2020
... NPWT, also known as vacuum sealing drainage (VSD) or vacuum-assisted wound closure (VAC), entails the use of a closed negative pressure treatment system that provides subatmospheric pressure to acute and chronic open wounds. The system consists of a polyurethane foam sponge, a semiocclusive barrier, a liquid collection system, and a negative pressure pump. NPWT promotes wound healing by promoting tissue deformation, drainage of extracellular inflammatory fluid, stabilization of the wound environment, and micro-deformation.[95-96] It is an effective treatment for acute and chronic wounds such as diabetic foot ulcers, pressure ulcers, chronic wounds, and skin grafts.[97⇓⇓⇓-101] However, several high-quality studies have shown that NPWT is not superior to standard therapy and may even cause more complications,[102⇓⇓-105] such as toxic shock syndrome, infection, pain, bleeding, adjacent tissue ischemia, and hemodynamic instability.[89] ...
Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial
1
2005
... NPWT, also known as vacuum sealing drainage (VSD) or vacuum-assisted wound closure (VAC), entails the use of a closed negative pressure treatment system that provides subatmospheric pressure to acute and chronic open wounds. The system consists of a polyurethane foam sponge, a semiocclusive barrier, a liquid collection system, and a negative pressure pump. NPWT promotes wound healing by promoting tissue deformation, drainage of extracellular inflammatory fluid, stabilization of the wound environment, and micro-deformation.[95-96] It is an effective treatment for acute and chronic wounds such as diabetic foot ulcers, pressure ulcers, chronic wounds, and skin grafts.[97⇓⇓⇓-101] However, several high-quality studies have shown that NPWT is not superior to standard therapy and may even cause more complications,[102⇓⇓-105] such as toxic shock syndrome, infection, pain, bleeding, adjacent tissue ischemia, and hemodynamic instability.[89] ...
Comparison of vacuum sealing drainage and traditional therapy for treatment of diabetic foot ulcers: a meta-analysis
1
2019
... NPWT, also known as vacuum sealing drainage (VSD) or vacuum-assisted wound closure (VAC), entails the use of a closed negative pressure treatment system that provides subatmospheric pressure to acute and chronic open wounds. The system consists of a polyurethane foam sponge, a semiocclusive barrier, a liquid collection system, and a negative pressure pump. NPWT promotes wound healing by promoting tissue deformation, drainage of extracellular inflammatory fluid, stabilization of the wound environment, and micro-deformation.[95-96] It is an effective treatment for acute and chronic wounds such as diabetic foot ulcers, pressure ulcers, chronic wounds, and skin grafts.[97⇓⇓⇓-101] However, several high-quality studies have shown that NPWT is not superior to standard therapy and may even cause more complications,[102⇓⇓-105] such as toxic shock syndrome, infection, pain, bleeding, adjacent tissue ischemia, and hemodynamic instability.[89] ...
Negative pressure wound therapy vs conventional wound treatment in subcutaneous abdominal wound healing impairment: the SAWHI randomized clinical trial
1
2020
... NPWT, also known as vacuum sealing drainage (VSD) or vacuum-assisted wound closure (VAC), entails the use of a closed negative pressure treatment system that provides subatmospheric pressure to acute and chronic open wounds. The system consists of a polyurethane foam sponge, a semiocclusive barrier, a liquid collection system, and a negative pressure pump. NPWT promotes wound healing by promoting tissue deformation, drainage of extracellular inflammatory fluid, stabilization of the wound environment, and micro-deformation.[95-96] It is an effective treatment for acute and chronic wounds such as diabetic foot ulcers, pressure ulcers, chronic wounds, and skin grafts.[97⇓⇓⇓-101] However, several high-quality studies have shown that NPWT is not superior to standard therapy and may even cause more complications,[102⇓⇓-105] such as toxic shock syndrome, infection, pain, bleeding, adjacent tissue ischemia, and hemodynamic instability.[89] ...
Comparison of vacuum sealing drainage and conventional drainage for postoperative drainage in closed calcaneal fracture: a randomized controlled trial
1
2022
... NPWT, also known as vacuum sealing drainage (VSD) or vacuum-assisted wound closure (VAC), entails the use of a closed negative pressure treatment system that provides subatmospheric pressure to acute and chronic open wounds. The system consists of a polyurethane foam sponge, a semiocclusive barrier, a liquid collection system, and a negative pressure pump. NPWT promotes wound healing by promoting tissue deformation, drainage of extracellular inflammatory fluid, stabilization of the wound environment, and micro-deformation.[95-96] It is an effective treatment for acute and chronic wounds such as diabetic foot ulcers, pressure ulcers, chronic wounds, and skin grafts.[97⇓⇓⇓-101] However, several high-quality studies have shown that NPWT is not superior to standard therapy and may even cause more complications,[102⇓⇓-105] such as toxic shock syndrome, infection, pain, bleeding, adjacent tissue ischemia, and hemodynamic instability.[89] ...
Vacuum sealing drainage with instillation in the treatment of necrotising soft-tissue infection: a retrospective analysis
1
2020
... NPWT, also known as vacuum sealing drainage (VSD) or vacuum-assisted wound closure (VAC), entails the use of a closed negative pressure treatment system that provides subatmospheric pressure to acute and chronic open wounds. The system consists of a polyurethane foam sponge, a semiocclusive barrier, a liquid collection system, and a negative pressure pump. NPWT promotes wound healing by promoting tissue deformation, drainage of extracellular inflammatory fluid, stabilization of the wound environment, and micro-deformation.[95-96] It is an effective treatment for acute and chronic wounds such as diabetic foot ulcers, pressure ulcers, chronic wounds, and skin grafts.[97⇓⇓⇓-101] However, several high-quality studies have shown that NPWT is not superior to standard therapy and may even cause more complications,[102⇓⇓-105] such as toxic shock syndrome, infection, pain, bleeding, adjacent tissue ischemia, and hemodynamic instability.[89] ...
Negative pressure wound therapy versus standard treatment in patients with acute conflict-related extremity wounds: a pragmatic, multisite, randomised controlled trial
1
2020
... NPWT, also known as vacuum sealing drainage (VSD) or vacuum-assisted wound closure (VAC), entails the use of a closed negative pressure treatment system that provides subatmospheric pressure to acute and chronic open wounds. The system consists of a polyurethane foam sponge, a semiocclusive barrier, a liquid collection system, and a negative pressure pump. NPWT promotes wound healing by promoting tissue deformation, drainage of extracellular inflammatory fluid, stabilization of the wound environment, and micro-deformation.[95-96] It is an effective treatment for acute and chronic wounds such as diabetic foot ulcers, pressure ulcers, chronic wounds, and skin grafts.[97⇓⇓⇓-101] However, several high-quality studies have shown that NPWT is not superior to standard therapy and may even cause more complications,[102⇓⇓-105] such as toxic shock syndrome, infection, pain, bleeding, adjacent tissue ischemia, and hemodynamic instability.[89] ...
Effect of negative pressure wound therapy vs. standard wound management on 12-month disability among adults with severe open fracture of the lower limb: the WOLLF randomized clinical trial
1
2018
... NPWT, also known as vacuum sealing drainage (VSD) or vacuum-assisted wound closure (VAC), entails the use of a closed negative pressure treatment system that provides subatmospheric pressure to acute and chronic open wounds. The system consists of a polyurethane foam sponge, a semiocclusive barrier, a liquid collection system, and a negative pressure pump. NPWT promotes wound healing by promoting tissue deformation, drainage of extracellular inflammatory fluid, stabilization of the wound environment, and micro-deformation.[95-96] It is an effective treatment for acute and chronic wounds such as diabetic foot ulcers, pressure ulcers, chronic wounds, and skin grafts.[97⇓⇓⇓-101] However, several high-quality studies have shown that NPWT is not superior to standard therapy and may even cause more complications,[102⇓⇓-105] such as toxic shock syndrome, infection, pain, bleeding, adjacent tissue ischemia, and hemodynamic instability.[89] ...
Effect of incisional negative pressure wound therapy vs. standard wound dressing on deep surgical site infection after surgery for lower limb fractures associated with major trauma: the WHIST randomized clinical trial
1
2020
... NPWT, also known as vacuum sealing drainage (VSD) or vacuum-assisted wound closure (VAC), entails the use of a closed negative pressure treatment system that provides subatmospheric pressure to acute and chronic open wounds. The system consists of a polyurethane foam sponge, a semiocclusive barrier, a liquid collection system, and a negative pressure pump. NPWT promotes wound healing by promoting tissue deformation, drainage of extracellular inflammatory fluid, stabilization of the wound environment, and micro-deformation.[95-96] It is an effective treatment for acute and chronic wounds such as diabetic foot ulcers, pressure ulcers, chronic wounds, and skin grafts.[97⇓⇓⇓-101] However, several high-quality studies have shown that NPWT is not superior to standard therapy and may even cause more complications,[102⇓⇓-105] such as toxic shock syndrome, infection, pain, bleeding, adjacent tissue ischemia, and hemodynamic instability.[89] ...
The management of degloving injuries of the limb with full thickness skin grafting using vacuum sealing drainage or traditional compression dressing: a comparative cohort study
1
2019
... NPWT, also known as vacuum sealing drainage (VSD) or vacuum-assisted wound closure (VAC), entails the use of a closed negative pressure treatment system that provides subatmospheric pressure to acute and chronic open wounds. The system consists of a polyurethane foam sponge, a semiocclusive barrier, a liquid collection system, and a negative pressure pump. NPWT promotes wound healing by promoting tissue deformation, drainage of extracellular inflammatory fluid, stabilization of the wound environment, and micro-deformation.[95-96] It is an effective treatment for acute and chronic wounds such as diabetic foot ulcers, pressure ulcers, chronic wounds, and skin grafts.[97⇓⇓⇓-101] However, several high-quality studies have shown that NPWT is not superior to standard therapy and may even cause more complications,[102⇓⇓-105] such as toxic shock syndrome, infection, pain, bleeding, adjacent tissue ischemia, and hemodynamic instability.[89] ...
Negative pressure wound therapy for skin necrosis prevention after snakebite in the emergency department: a retrospective cohort study
1
2021
... Small-scale clinical studies of NWPT for venomous snakebite wounds suggest that it can reduce wound necrosis, infection rates, and edema,[106⇓⇓-109] but there is a potential risk of complications. Therefore, further evidence is required to establish the effectiveness and safety of NPWT for the treatment of venomous snakebite wounds. Based on the available evidence, for the wound treatment of cytolytic venomous snakes (such as the Chinese cobra), this treatment option might be considered after sufficient use of antivenoms for a short period (<7 d). ...
Small incisions combined with negative-pressure wound therapy for treatment of Protobothrops mucrosquamatus bite envenomation: a new treatment strategy
1
2019
... Small-scale clinical studies of NWPT for venomous snakebite wounds suggest that it can reduce wound necrosis, infection rates, and edema,[106⇓⇓-109] but there is a potential risk of complications. Therefore, further evidence is required to establish the effectiveness and safety of NPWT for the treatment of venomous snakebite wounds. Based on the available evidence, for the wound treatment of cytolytic venomous snakes (such as the Chinese cobra), this treatment option might be considered after sufficient use of antivenoms for a short period (<7 d). ...
Clinical predictors of early surgical intervention in patients with venomous snakebites
1
2023
... Small-scale clinical studies of NWPT for venomous snakebite wounds suggest that it can reduce wound necrosis, infection rates, and edema,[106⇓⇓-109] but there is a potential risk of complications. Therefore, further evidence is required to establish the effectiveness and safety of NPWT for the treatment of venomous snakebite wounds. Based on the available evidence, for the wound treatment of cytolytic venomous snakes (such as the Chinese cobra), this treatment option might be considered after sufficient use of antivenoms for a short period (<7 d). ...
Effect of fluctuating negative-pressure closed drainage technique on the therapeutic efficacy of Chinese cobra bite patients
1
2021
... Small-scale clinical studies of NWPT for venomous snakebite wounds suggest that it can reduce wound necrosis, infection rates, and edema,[106⇓⇓-109] but there is a potential risk of complications. Therefore, further evidence is required to establish the effectiveness and safety of NPWT for the treatment of venomous snakebite wounds. Based on the available evidence, for the wound treatment of cytolytic venomous snakes (such as the Chinese cobra), this treatment option might be considered after sufficient use of antivenoms for a short period (<7 d). ...
3
2017
... Timely relief of pain is an important symptomatic treatment for snakebites. Currently, there are two main types of analgesics: non-steroidal anti-inflammatory drugs (NSAIDs) and opioids. NSAIDs can inhibit platelet aggregation and affect blood coagulation, which increases the risk of bleeding in patients with hematotoxic snakebites. Acetaminophen is an NSAID that inhibits the synthesis of prostaglandins in the central nervous system and blocks the impulse of pain nerve endings, resulting in analgesic effects.[110] Opioid analgesics produce strong analgesic effects by acting on opioid receptors in the central nervous system, but excessive or prolonged use can lead to respiratory depression, gastrointestinal reactions, and drug dependence.[110] ...
... [110] ...
... Glucocorticoids have an inhibitory effect on the inflammatory response. They attenuate or prevent inflammatory exudation, edema, and inflammatory cell infiltration during the acute inflammatory phase by directly constricting small blood vessels, inhibiting vasodilatation and fluid exudation, and inhibiting the effects of inflammatory cell aggregation and the release of oxygen free radicals from neutrophils.[110] A small-scale study has shown that adequate amounts of antivenom supplemented with short-term, low-dose oral steroids can help improve swelling and pain.[126] However, a randomized controlled trial has shown that glucocorticoids do not reduce limb swelling.[127] Glucocorticoids should not be routinely used for swelling reduction in venomous snakebites unless they are used for serum sickness or snake venom-associated adrenal insufficiency. ...
1
2023
... After using sufficient amounts of antivenom, providing positional drainage can facilitate the reabsorption of fluid in the tissue space of the swollen area, thereby reducing local pressure, relieving swelling, and alleviating swelling-related pain. The swelling of the affected limb can be reduced by elevating it not lower than the sternal angle.[1,111] ...
A cross-sectional survey of snake oral bacterial flora from Hong Kong, SAR, China
1
2011
... Multiple aerobic and anaerobic Gram-positive or Gram-negative pathogenic microorganisms are found in viper oral flora cultures.[112-113] The reported incidence of secondary bacterial infection in venomous snakebite wounds (10.3%-47.5%)[114⇓-116] is much higher than that in non-venomous snakebite wounds.[114] The infection rate in Chinese cobra bite wounds can be as high as 80.9%.[117] However, there is no need for routine infection prevention after snakebites. Once infected, approximately 68% of wounds require surgical intervention.[117] Anti-infection treatment is only suitable for patients with localized or suspected infections (such as abscess formation, increased wound secretion or odor, cellulitis, or positive microbial culture of secretions) or patients with localized tissue necrosis/gangrene.[13] Patients with confirmed infections should be administered anti-infection treatment promptly to prevent or ameliorate local tissue necrosis, systemic infection, and sepsis.[118] Patients with high fibrinogen, elevated alanine aminotransferase or C-reactive protein levels, and severe poisoning are at an increased risk of wound infection.[119] Common pathogens include Morganella, Enterococcus, Bacteroides fragilis, Escherichia coli, and Staphylococcus aureus. Empirical anti-infection treatment options include amoxicillin/clavulanic acid, fluoroquinolones, cefazolin, third-generation antibiotics against spore-forming bacteria, and aminoglycosides.[120⇓-122] Antimicrobial agents should be used based on clinical findings and drug sensitivity results. ...
Assessment of cultivable oral bacterial flora from important venomous snakes of India and their antibiotic susceptibilities
1
2017
... Multiple aerobic and anaerobic Gram-positive or Gram-negative pathogenic microorganisms are found in viper oral flora cultures.[112-113] The reported incidence of secondary bacterial infection in venomous snakebite wounds (10.3%-47.5%)[114⇓-116] is much higher than that in non-venomous snakebite wounds.[114] The infection rate in Chinese cobra bite wounds can be as high as 80.9%.[117] However, there is no need for routine infection prevention after snakebites. Once infected, approximately 68% of wounds require surgical intervention.[117] Anti-infection treatment is only suitable for patients with localized or suspected infections (such as abscess formation, increased wound secretion or odor, cellulitis, or positive microbial culture of secretions) or patients with localized tissue necrosis/gangrene.[13] Patients with confirmed infections should be administered anti-infection treatment promptly to prevent or ameliorate local tissue necrosis, systemic infection, and sepsis.[118] Patients with high fibrinogen, elevated alanine aminotransferase or C-reactive protein levels, and severe poisoning are at an increased risk of wound infection.[119] Common pathogens include Morganella, Enterococcus, Bacteroides fragilis, Escherichia coli, and Staphylococcus aureus. Empirical anti-infection treatment options include amoxicillin/clavulanic acid, fluoroquinolones, cefazolin, third-generation antibiotics against spore-forming bacteria, and aminoglycosides.[120⇓-122] Antimicrobial agents should be used based on clinical findings and drug sensitivity results. ...
The incidence of infection complicating snakebites in tropical Australia: implications for clinical management and antimicrobial prophylaxis
2
2023
... Multiple aerobic and anaerobic Gram-positive or Gram-negative pathogenic microorganisms are found in viper oral flora cultures.[112-113] The reported incidence of secondary bacterial infection in venomous snakebite wounds (10.3%-47.5%)[114⇓-116] is much higher than that in non-venomous snakebite wounds.[114] The infection rate in Chinese cobra bite wounds can be as high as 80.9%.[117] However, there is no need for routine infection prevention after snakebites. Once infected, approximately 68% of wounds require surgical intervention.[117] Anti-infection treatment is only suitable for patients with localized or suspected infections (such as abscess formation, increased wound secretion or odor, cellulitis, or positive microbial culture of secretions) or patients with localized tissue necrosis/gangrene.[13] Patients with confirmed infections should be administered anti-infection treatment promptly to prevent or ameliorate local tissue necrosis, systemic infection, and sepsis.[118] Patients with high fibrinogen, elevated alanine aminotransferase or C-reactive protein levels, and severe poisoning are at an increased risk of wound infection.[119] Common pathogens include Morganella, Enterococcus, Bacteroides fragilis, Escherichia coli, and Staphylococcus aureus. Empirical anti-infection treatment options include amoxicillin/clavulanic acid, fluoroquinolones, cefazolin, third-generation antibiotics against spore-forming bacteria, and aminoglycosides.[120⇓-122] Antimicrobial agents should be used based on clinical findings and drug sensitivity results. ...
... [114] The infection rate in Chinese cobra bite wounds can be as high as 80.9%.[117] However, there is no need for routine infection prevention after snakebites. Once infected, approximately 68% of wounds require surgical intervention.[117] Anti-infection treatment is only suitable for patients with localized or suspected infections (such as abscess formation, increased wound secretion or odor, cellulitis, or positive microbial culture of secretions) or patients with localized tissue necrosis/gangrene.[13] Patients with confirmed infections should be administered anti-infection treatment promptly to prevent or ameliorate local tissue necrosis, systemic infection, and sepsis.[118] Patients with high fibrinogen, elevated alanine aminotransferase or C-reactive protein levels, and severe poisoning are at an increased risk of wound infection.[119] Common pathogens include Morganella, Enterococcus, Bacteroides fragilis, Escherichia coli, and Staphylococcus aureus. Empirical anti-infection treatment options include amoxicillin/clavulanic acid, fluoroquinolones, cefazolin, third-generation antibiotics against spore-forming bacteria, and aminoglycosides.[120⇓-122] Antimicrobial agents should be used based on clinical findings and drug sensitivity results. ...
Epidemiology of secondary infection after snakebites in center-west Brazil
1
2023
... Multiple aerobic and anaerobic Gram-positive or Gram-negative pathogenic microorganisms are found in viper oral flora cultures.[112-113] The reported incidence of secondary bacterial infection in venomous snakebite wounds (10.3%-47.5%)[114⇓-116] is much higher than that in non-venomous snakebite wounds.[114] The infection rate in Chinese cobra bite wounds can be as high as 80.9%.[117] However, there is no need for routine infection prevention after snakebites. Once infected, approximately 68% of wounds require surgical intervention.[117] Anti-infection treatment is only suitable for patients with localized or suspected infections (such as abscess formation, increased wound secretion or odor, cellulitis, or positive microbial culture of secretions) or patients with localized tissue necrosis/gangrene.[13] Patients with confirmed infections should be administered anti-infection treatment promptly to prevent or ameliorate local tissue necrosis, systemic infection, and sepsis.[118] Patients with high fibrinogen, elevated alanine aminotransferase or C-reactive protein levels, and severe poisoning are at an increased risk of wound infection.[119] Common pathogens include Morganella, Enterococcus, Bacteroides fragilis, Escherichia coli, and Staphylococcus aureus. Empirical anti-infection treatment options include amoxicillin/clavulanic acid, fluoroquinolones, cefazolin, third-generation antibiotics against spore-forming bacteria, and aminoglycosides.[120⇓-122] Antimicrobial agents should be used based on clinical findings and drug sensitivity results. ...
Snakebite-associated infections: a systematic review and meta-analysis
1
2024
... Multiple aerobic and anaerobic Gram-positive or Gram-negative pathogenic microorganisms are found in viper oral flora cultures.[112-113] The reported incidence of secondary bacterial infection in venomous snakebite wounds (10.3%-47.5%)[114⇓-116] is much higher than that in non-venomous snakebite wounds.[114] The infection rate in Chinese cobra bite wounds can be as high as 80.9%.[117] However, there is no need for routine infection prevention after snakebites. Once infected, approximately 68% of wounds require surgical intervention.[117] Anti-infection treatment is only suitable for patients with localized or suspected infections (such as abscess formation, increased wound secretion or odor, cellulitis, or positive microbial culture of secretions) or patients with localized tissue necrosis/gangrene.[13] Patients with confirmed infections should be administered anti-infection treatment promptly to prevent or ameliorate local tissue necrosis, systemic infection, and sepsis.[118] Patients with high fibrinogen, elevated alanine aminotransferase or C-reactive protein levels, and severe poisoning are at an increased risk of wound infection.[119] Common pathogens include Morganella, Enterococcus, Bacteroides fragilis, Escherichia coli, and Staphylococcus aureus. Empirical anti-infection treatment options include amoxicillin/clavulanic acid, fluoroquinolones, cefazolin, third-generation antibiotics against spore-forming bacteria, and aminoglycosides.[120⇓-122] Antimicrobial agents should be used based on clinical findings and drug sensitivity results. ...
Naja atra snakebite in Taiwan, China
2
2018
... Multiple aerobic and anaerobic Gram-positive or Gram-negative pathogenic microorganisms are found in viper oral flora cultures.[112-113] The reported incidence of secondary bacterial infection in venomous snakebite wounds (10.3%-47.5%)[114⇓-116] is much higher than that in non-venomous snakebite wounds.[114] The infection rate in Chinese cobra bite wounds can be as high as 80.9%.[117] However, there is no need for routine infection prevention after snakebites. Once infected, approximately 68% of wounds require surgical intervention.[117] Anti-infection treatment is only suitable for patients with localized or suspected infections (such as abscess formation, increased wound secretion or odor, cellulitis, or positive microbial culture of secretions) or patients with localized tissue necrosis/gangrene.[13] Patients with confirmed infections should be administered anti-infection treatment promptly to prevent or ameliorate local tissue necrosis, systemic infection, and sepsis.[118] Patients with high fibrinogen, elevated alanine aminotransferase or C-reactive protein levels, and severe poisoning are at an increased risk of wound infection.[119] Common pathogens include Morganella, Enterococcus, Bacteroides fragilis, Escherichia coli, and Staphylococcus aureus. Empirical anti-infection treatment options include amoxicillin/clavulanic acid, fluoroquinolones, cefazolin, third-generation antibiotics against spore-forming bacteria, and aminoglycosides.[120⇓-122] Antimicrobial agents should be used based on clinical findings and drug sensitivity results. ...
... [117] Anti-infection treatment is only suitable for patients with localized or suspected infections (such as abscess formation, increased wound secretion or odor, cellulitis, or positive microbial culture of secretions) or patients with localized tissue necrosis/gangrene.[13] Patients with confirmed infections should be administered anti-infection treatment promptly to prevent or ameliorate local tissue necrosis, systemic infection, and sepsis.[118] Patients with high fibrinogen, elevated alanine aminotransferase or C-reactive protein levels, and severe poisoning are at an increased risk of wound infection.[119] Common pathogens include Morganella, Enterococcus, Bacteroides fragilis, Escherichia coli, and Staphylococcus aureus. Empirical anti-infection treatment options include amoxicillin/clavulanic acid, fluoroquinolones, cefazolin, third-generation antibiotics against spore-forming bacteria, and aminoglycosides.[120⇓-122] Antimicrobial agents should be used based on clinical findings and drug sensitivity results. ...
Oral bacteria and their antibiotic susceptibilities in Taiwan, China venomous snakes
1
2022
... Multiple aerobic and anaerobic Gram-positive or Gram-negative pathogenic microorganisms are found in viper oral flora cultures.[112-113] The reported incidence of secondary bacterial infection in venomous snakebite wounds (10.3%-47.5%)[114⇓-116] is much higher than that in non-venomous snakebite wounds.[114] The infection rate in Chinese cobra bite wounds can be as high as 80.9%.[117] However, there is no need for routine infection prevention after snakebites. Once infected, approximately 68% of wounds require surgical intervention.[117] Anti-infection treatment is only suitable for patients with localized or suspected infections (such as abscess formation, increased wound secretion or odor, cellulitis, or positive microbial culture of secretions) or patients with localized tissue necrosis/gangrene.[13] Patients with confirmed infections should be administered anti-infection treatment promptly to prevent or ameliorate local tissue necrosis, systemic infection, and sepsis.[118] Patients with high fibrinogen, elevated alanine aminotransferase or C-reactive protein levels, and severe poisoning are at an increased risk of wound infection.[119] Common pathogens include Morganella, Enterococcus, Bacteroides fragilis, Escherichia coli, and Staphylococcus aureus. Empirical anti-infection treatment options include amoxicillin/clavulanic acid, fluoroquinolones, cefazolin, third-generation antibiotics against spore-forming bacteria, and aminoglycosides.[120⇓-122] Antimicrobial agents should be used based on clinical findings and drug sensitivity results. ...
Poor efficacy of preemptive amoxicillin clavulanate for preventing secondary infection from Bothrops snakebites in the Brazilian Amazon: a randomized controlled clinical trial
1
2017
... Multiple aerobic and anaerobic Gram-positive or Gram-negative pathogenic microorganisms are found in viper oral flora cultures.[112-113] The reported incidence of secondary bacterial infection in venomous snakebite wounds (10.3%-47.5%)[114⇓-116] is much higher than that in non-venomous snakebite wounds.[114] The infection rate in Chinese cobra bite wounds can be as high as 80.9%.[117] However, there is no need for routine infection prevention after snakebites. Once infected, approximately 68% of wounds require surgical intervention.[117] Anti-infection treatment is only suitable for patients with localized or suspected infections (such as abscess formation, increased wound secretion or odor, cellulitis, or positive microbial culture of secretions) or patients with localized tissue necrosis/gangrene.[13] Patients with confirmed infections should be administered anti-infection treatment promptly to prevent or ameliorate local tissue necrosis, systemic infection, and sepsis.[118] Patients with high fibrinogen, elevated alanine aminotransferase or C-reactive protein levels, and severe poisoning are at an increased risk of wound infection.[119] Common pathogens include Morganella, Enterococcus, Bacteroides fragilis, Escherichia coli, and Staphylococcus aureus. Empirical anti-infection treatment options include amoxicillin/clavulanic acid, fluoroquinolones, cefazolin, third-generation antibiotics against spore-forming bacteria, and aminoglycosides.[120⇓-122] Antimicrobial agents should be used based on clinical findings and drug sensitivity results. ...
Wound infections of snakebites from the venomous Protobothrops mucrosquamatus and Viridovipera stejnegeri in Taiwan, China: bacteriology, antibiotic susceptibility, and predicting the need for antibiotics-a bite study
1
2020
... Multiple aerobic and anaerobic Gram-positive or Gram-negative pathogenic microorganisms are found in viper oral flora cultures.[112-113] The reported incidence of secondary bacterial infection in venomous snakebite wounds (10.3%-47.5%)[114⇓-116] is much higher than that in non-venomous snakebite wounds.[114] The infection rate in Chinese cobra bite wounds can be as high as 80.9%.[117] However, there is no need for routine infection prevention after snakebites. Once infected, approximately 68% of wounds require surgical intervention.[117] Anti-infection treatment is only suitable for patients with localized or suspected infections (such as abscess formation, increased wound secretion or odor, cellulitis, or positive microbial culture of secretions) or patients with localized tissue necrosis/gangrene.[13] Patients with confirmed infections should be administered anti-infection treatment promptly to prevent or ameliorate local tissue necrosis, systemic infection, and sepsis.[118] Patients with high fibrinogen, elevated alanine aminotransferase or C-reactive protein levels, and severe poisoning are at an increased risk of wound infection.[119] Common pathogens include Morganella, Enterococcus, Bacteroides fragilis, Escherichia coli, and Staphylococcus aureus. Empirical anti-infection treatment options include amoxicillin/clavulanic acid, fluoroquinolones, cefazolin, third-generation antibiotics against spore-forming bacteria, and aminoglycosides.[120⇓-122] Antimicrobial agents should be used based on clinical findings and drug sensitivity results. ...
Wound infections from Taiwan, China cobra (Naja atra) bites: determining bacteriology, antibiotic susceptibility, and the use of antibiotics-a cobra BITE study
1
2021
... Multiple aerobic and anaerobic Gram-positive or Gram-negative pathogenic microorganisms are found in viper oral flora cultures.[112-113] The reported incidence of secondary bacterial infection in venomous snakebite wounds (10.3%-47.5%)[114⇓-116] is much higher than that in non-venomous snakebite wounds.[114] The infection rate in Chinese cobra bite wounds can be as high as 80.9%.[117] However, there is no need for routine infection prevention after snakebites. Once infected, approximately 68% of wounds require surgical intervention.[117] Anti-infection treatment is only suitable for patients with localized or suspected infections (such as abscess formation, increased wound secretion or odor, cellulitis, or positive microbial culture of secretions) or patients with localized tissue necrosis/gangrene.[13] Patients with confirmed infections should be administered anti-infection treatment promptly to prevent or ameliorate local tissue necrosis, systemic infection, and sepsis.[118] Patients with high fibrinogen, elevated alanine aminotransferase or C-reactive protein levels, and severe poisoning are at an increased risk of wound infection.[119] Common pathogens include Morganella, Enterococcus, Bacteroides fragilis, Escherichia coli, and Staphylococcus aureus. Empirical anti-infection treatment options include amoxicillin/clavulanic acid, fluoroquinolones, cefazolin, third-generation antibiotics against spore-forming bacteria, and aminoglycosides.[120⇓-122] Antimicrobial agents should be used based on clinical findings and drug sensitivity results. ...
Bacteriological studies of venomous snakebite wounds in Hangzhou, southeast China
1
2022
... Multiple aerobic and anaerobic Gram-positive or Gram-negative pathogenic microorganisms are found in viper oral flora cultures.[112-113] The reported incidence of secondary bacterial infection in venomous snakebite wounds (10.3%-47.5%)[114⇓-116] is much higher than that in non-venomous snakebite wounds.[114] The infection rate in Chinese cobra bite wounds can be as high as 80.9%.[117] However, there is no need for routine infection prevention after snakebites. Once infected, approximately 68% of wounds require surgical intervention.[117] Anti-infection treatment is only suitable for patients with localized or suspected infections (such as abscess formation, increased wound secretion or odor, cellulitis, or positive microbial culture of secretions) or patients with localized tissue necrosis/gangrene.[13] Patients with confirmed infections should be administered anti-infection treatment promptly to prevent or ameliorate local tissue necrosis, systemic infection, and sepsis.[118] Patients with high fibrinogen, elevated alanine aminotransferase or C-reactive protein levels, and severe poisoning are at an increased risk of wound infection.[119] Common pathogens include Morganella, Enterococcus, Bacteroides fragilis, Escherichia coli, and Staphylococcus aureus. Empirical anti-infection treatment options include amoxicillin/clavulanic acid, fluoroquinolones, cefazolin, third-generation antibiotics against spore-forming bacteria, and aminoglycosides.[120⇓-122] Antimicrobial agents should be used based on clinical findings and drug sensitivity results. ...
Tetanus complicating snakebite in northern Nigeria: clinical presentation and public health implications
1
2003
... Tetanus is a bacterial infection caused by Clostridium tetani, which typically invades the body through skin wounds or mucous membranes. While venomous (or non-venomous) snakebites are unlikely to cause tetanus, there is a potential risk due to the relatively thin teeth of venomous snakes and the potential presence of clostridia in the oral cavity of snakes, which can contaminate the wound.[123⇓-125] Given the serious risk of tetanus, it is essential to implement routine tetanus prophylaxis for both venomous and non-venomous snakebites. Note that tetanus antitoxin or immunoglobulin should be administered separately from antivenom (usually administered after antivenom) because of the risk of allergic reactions. The interval should be at least one hour to avoid adverse reactions. After administration, patients should be closely monitored for potential adverse reactions. ...
Childhood tetanus complicating snakebite: a case report from a semi-urban area, northwestern Nigeria
1
2023
... Tetanus is a bacterial infection caused by Clostridium tetani, which typically invades the body through skin wounds or mucous membranes. While venomous (or non-venomous) snakebites are unlikely to cause tetanus, there is a potential risk due to the relatively thin teeth of venomous snakes and the potential presence of clostridia in the oral cavity of snakes, which can contaminate the wound.[123⇓-125] Given the serious risk of tetanus, it is essential to implement routine tetanus prophylaxis for both venomous and non-venomous snakebites. Note that tetanus antitoxin or immunoglobulin should be administered separately from antivenom (usually administered after antivenom) because of the risk of allergic reactions. The interval should be at least one hour to avoid adverse reactions. After administration, patients should be closely monitored for potential adverse reactions. ...
Rare case of tetanus from snakebite wound
1
2022
... Tetanus is a bacterial infection caused by Clostridium tetani, which typically invades the body through skin wounds or mucous membranes. While venomous (or non-venomous) snakebites are unlikely to cause tetanus, there is a potential risk due to the relatively thin teeth of venomous snakes and the potential presence of clostridia in the oral cavity of snakes, which can contaminate the wound.[123⇓-125] Given the serious risk of tetanus, it is essential to implement routine tetanus prophylaxis for both venomous and non-venomous snakebites. Note that tetanus antitoxin or immunoglobulin should be administered separately from antivenom (usually administered after antivenom) because of the risk of allergic reactions. The interval should be at least one hour to avoid adverse reactions. After administration, patients should be closely monitored for potential adverse reactions. ...
Role of steroid on management of limb swelling and local pain in haematotoxic snake bite
1
2022
... Glucocorticoids have an inhibitory effect on the inflammatory response. They attenuate or prevent inflammatory exudation, edema, and inflammatory cell infiltration during the acute inflammatory phase by directly constricting small blood vessels, inhibiting vasodilatation and fluid exudation, and inhibiting the effects of inflammatory cell aggregation and the release of oxygen free radicals from neutrophils.[110] A small-scale study has shown that adequate amounts of antivenom supplemented with short-term, low-dose oral steroids can help improve swelling and pain.[126] However, a randomized controlled trial has shown that glucocorticoids do not reduce limb swelling.[127] Glucocorticoids should not be routinely used for swelling reduction in venomous snakebites unless they are used for serum sickness or snake venom-associated adrenal insufficiency. ...
The role of prednisolone in reducing limb oedema in children bitten by green pit vipers: a randomized, controlled trial
1
2008
... Glucocorticoids have an inhibitory effect on the inflammatory response. They attenuate or prevent inflammatory exudation, edema, and inflammatory cell infiltration during the acute inflammatory phase by directly constricting small blood vessels, inhibiting vasodilatation and fluid exudation, and inhibiting the effects of inflammatory cell aggregation and the release of oxygen free radicals from neutrophils.[110] A small-scale study has shown that adequate amounts of antivenom supplemented with short-term, low-dose oral steroids can help improve swelling and pain.[126] However, a randomized controlled trial has shown that glucocorticoids do not reduce limb swelling.[127] Glucocorticoids should not be routinely used for swelling reduction in venomous snakebites unless they are used for serum sickness or snake venom-associated adrenal insufficiency. ...
Role of neostigmine in neurotoxic snake bite
1
2021
... Neurotoxins produce myocardial paralytic effects by inhibiting the release of acetylcholine from the presynaptic membrane or by binding to acetylcholine receptors on the postsynaptic membrane. Anticholinesterase drugs such as neostigmine or pyridostigmine can inhibit acetylcholinesterase activity, reduce the hydrolysis of acetylcholine in the synaptic gap, and exert a complete cholinesteroid effect, which produces an excitatory effect on skeletal muscle and has a certain degree of efficacy in the reversal of some types of neurotoxin-induced myocardial paralysis.[128⇓-130] However, these methods are ineffective against presynaptic membrane toxins such as those produced by Bungarus caeruleus.[131] Neostigmine or pyridostigmine 0.02 mg/kg (0.04 mg/kg in children) is administered intramuscularly, with 0.5-2.5 mg repeated for 1-3 h if necessary, with the total daily dose not exceeding 10 mg. Because of the risk of increased airway secretions with neostigmine, 0.6 mg of atropine sulfate (50 μg/kg in children) may be administered intravenously prior to the medication.[47] Such medications should not delay the administration of antivenom and necessary tracheal intubation. ...
Reversal of experimental paralysis in a human by intranasal neostigmine aerosol suggests a novel approach to the early treatment of neurotoxic envenomation
1
2013
... Neurotoxins produce myocardial paralytic effects by inhibiting the release of acetylcholine from the presynaptic membrane or by binding to acetylcholine receptors on the postsynaptic membrane. Anticholinesterase drugs such as neostigmine or pyridostigmine can inhibit acetylcholinesterase activity, reduce the hydrolysis of acetylcholine in the synaptic gap, and exert a complete cholinesteroid effect, which produces an excitatory effect on skeletal muscle and has a certain degree of efficacy in the reversal of some types of neurotoxin-induced myocardial paralysis.[128⇓-130] However, these methods are ineffective against presynaptic membrane toxins such as those produced by Bungarus caeruleus.[131] Neostigmine or pyridostigmine 0.02 mg/kg (0.04 mg/kg in children) is administered intramuscularly, with 0.5-2.5 mg repeated for 1-3 h if necessary, with the total daily dose not exceeding 10 mg. Because of the risk of increased airway secretions with neostigmine, 0.6 mg of atropine sulfate (50 μg/kg in children) may be administered intravenously prior to the medication.[47] Such medications should not delay the administration of antivenom and necessary tracheal intubation. ...
Recurrent neurotoxity in Naja kaouthia envenomation: a case report from Assam, India
1
2023
... Neurotoxins produce myocardial paralytic effects by inhibiting the release of acetylcholine from the presynaptic membrane or by binding to acetylcholine receptors on the postsynaptic membrane. Anticholinesterase drugs such as neostigmine or pyridostigmine can inhibit acetylcholinesterase activity, reduce the hydrolysis of acetylcholine in the synaptic gap, and exert a complete cholinesteroid effect, which produces an excitatory effect on skeletal muscle and has a certain degree of efficacy in the reversal of some types of neurotoxin-induced myocardial paralysis.[128⇓-130] However, these methods are ineffective against presynaptic membrane toxins such as those produced by Bungarus caeruleus.[131] Neostigmine or pyridostigmine 0.02 mg/kg (0.04 mg/kg in children) is administered intramuscularly, with 0.5-2.5 mg repeated for 1-3 h if necessary, with the total daily dose not exceeding 10 mg. Because of the risk of increased airway secretions with neostigmine, 0.6 mg of atropine sulfate (50 μg/kg in children) may be administered intravenously prior to the medication.[47] Such medications should not delay the administration of antivenom and necessary tracheal intubation. ...
Role of neostigmine and polyvalent antivenom in Indian common krait (Bungarus caeruleus) bite
1
2010
... Neurotoxins produce myocardial paralytic effects by inhibiting the release of acetylcholine from the presynaptic membrane or by binding to acetylcholine receptors on the postsynaptic membrane. Anticholinesterase drugs such as neostigmine or pyridostigmine can inhibit acetylcholinesterase activity, reduce the hydrolysis of acetylcholine in the synaptic gap, and exert a complete cholinesteroid effect, which produces an excitatory effect on skeletal muscle and has a certain degree of efficacy in the reversal of some types of neurotoxin-induced myocardial paralysis.[128⇓-130] However, these methods are ineffective against presynaptic membrane toxins such as those produced by Bungarus caeruleus.[131] Neostigmine or pyridostigmine 0.02 mg/kg (0.04 mg/kg in children) is administered intramuscularly, with 0.5-2.5 mg repeated for 1-3 h if necessary, with the total daily dose not exceeding 10 mg. Because of the risk of increased airway secretions with neostigmine, 0.6 mg of atropine sulfate (50 μg/kg in children) may be administered intravenously prior to the medication.[47] Such medications should not delay the administration of antivenom and necessary tracheal intubation. ...
A randomized controlled trial of fresh frozen plasma for treating venom-induced consumption coagulopathy in cases of Australian snakebite (ASP-18)
1
2013
... Snake venom-induced consumptive coagulopathy or coagulopathy is a severe reaction after a venomous snakebite, and the efficacy of blood products is highly controversial. A multicenter randomized controlled trial has shown that fresh frozen plasma, which is used as an adjunct to antivenom, can accelerate the recovery of coagulation function but does not shorten the length of hospital stay and that early (<8 h) administration may be less effective.[132] A retrospective study has shown that fresh frozen plasma may accelerate the recovery of coagulation function and reduce bleeding and the amount of antivenom needed.[133] However, fresh frozen plasma was not found to accelerate the recovery of coagulation function in patients with viper bites.[134] Recent studies have shown that fresh frozen plasma and cryoprecipitate do not improve coagulation function,[135] and do not ameliorate coagulation disorders in those without bleeding.[136] Therefore, blood products such as fresh frozen plasma or cryoprecipitate should not be routinely used for venomous snakebites accompanied by VICC, but they can be considered adjuvant therapies for patients with severe coagulopathy who require emergency surgery, invasive procedures, those with insufficient antivenom usage, or those who have active bleeding. A variety of snake venom enzyme components can inhibit or activate platelets, producing antiplatelet or platelet aggregation effects.[137] Using sufficient antivenom can rapidly restore platelet levels, and platelet transfusion can be considered for those with severe thrombocytopenia with bleeding or those who require emergency surgery. ...
The role of fresh frozen plasma in reducing the volume of anti-snake venom in snakebite envenomation
1
2018
... Snake venom-induced consumptive coagulopathy or coagulopathy is a severe reaction after a venomous snakebite, and the efficacy of blood products is highly controversial. A multicenter randomized controlled trial has shown that fresh frozen plasma, which is used as an adjunct to antivenom, can accelerate the recovery of coagulation function but does not shorten the length of hospital stay and that early (<8 h) administration may be less effective.[132] A retrospective study has shown that fresh frozen plasma may accelerate the recovery of coagulation function and reduce bleeding and the amount of antivenom needed.[133] However, fresh frozen plasma was not found to accelerate the recovery of coagulation function in patients with viper bites.[134] Recent studies have shown that fresh frozen plasma and cryoprecipitate do not improve coagulation function,[135] and do not ameliorate coagulation disorders in those without bleeding.[136] Therefore, blood products such as fresh frozen plasma or cryoprecipitate should not be routinely used for venomous snakebites accompanied by VICC, but they can be considered adjuvant therapies for patients with severe coagulopathy who require emergency surgery, invasive procedures, those with insufficient antivenom usage, or those who have active bleeding. A variety of snake venom enzyme components can inhibit or activate platelets, producing antiplatelet or platelet aggregation effects.[137] Using sufficient antivenom can rapidly restore platelet levels, and platelet transfusion can be considered for those with severe thrombocytopenia with bleeding or those who require emergency surgery. ...
A randomized controlled trial of fresh frozen plasma for coagulopathy in Russell’s viper (Daboia russelii) envenoming
1
2017
... Snake venom-induced consumptive coagulopathy or coagulopathy is a severe reaction after a venomous snakebite, and the efficacy of blood products is highly controversial. A multicenter randomized controlled trial has shown that fresh frozen plasma, which is used as an adjunct to antivenom, can accelerate the recovery of coagulation function but does not shorten the length of hospital stay and that early (<8 h) administration may be less effective.[132] A retrospective study has shown that fresh frozen plasma may accelerate the recovery of coagulation function and reduce bleeding and the amount of antivenom needed.[133] However, fresh frozen plasma was not found to accelerate the recovery of coagulation function in patients with viper bites.[134] Recent studies have shown that fresh frozen plasma and cryoprecipitate do not improve coagulation function,[135] and do not ameliorate coagulation disorders in those without bleeding.[136] Therefore, blood products such as fresh frozen plasma or cryoprecipitate should not be routinely used for venomous snakebites accompanied by VICC, but they can be considered adjuvant therapies for patients with severe coagulopathy who require emergency surgery, invasive procedures, those with insufficient antivenom usage, or those who have active bleeding. A variety of snake venom enzyme components can inhibit or activate platelets, producing antiplatelet or platelet aggregation effects.[137] Using sufficient antivenom can rapidly restore platelet levels, and platelet transfusion can be considered for those with severe thrombocytopenia with bleeding or those who require emergency surgery. ...
Effectiveness of clotting factor replacement therapy after antivenom treatment on coagulopathic envenomation following green pit viper bites: a retrospective observational study
1
2022
... Snake venom-induced consumptive coagulopathy or coagulopathy is a severe reaction after a venomous snakebite, and the efficacy of blood products is highly controversial. A multicenter randomized controlled trial has shown that fresh frozen plasma, which is used as an adjunct to antivenom, can accelerate the recovery of coagulation function but does not shorten the length of hospital stay and that early (<8 h) administration may be less effective.[132] A retrospective study has shown that fresh frozen plasma may accelerate the recovery of coagulation function and reduce bleeding and the amount of antivenom needed.[133] However, fresh frozen plasma was not found to accelerate the recovery of coagulation function in patients with viper bites.[134] Recent studies have shown that fresh frozen plasma and cryoprecipitate do not improve coagulation function,[135] and do not ameliorate coagulation disorders in those without bleeding.[136] Therefore, blood products such as fresh frozen plasma or cryoprecipitate should not be routinely used for venomous snakebites accompanied by VICC, but they can be considered adjuvant therapies for patients with severe coagulopathy who require emergency surgery, invasive procedures, those with insufficient antivenom usage, or those who have active bleeding. A variety of snake venom enzyme components can inhibit or activate platelets, producing antiplatelet or platelet aggregation effects.[137] Using sufficient antivenom can rapidly restore platelet levels, and platelet transfusion can be considered for those with severe thrombocytopenia with bleeding or those who require emergency surgery. ...
Risk factor, monitoring, and treatment for snakebite induced coagulopathy: a multicenter retrospective study
1
2020
... Snake venom-induced consumptive coagulopathy or coagulopathy is a severe reaction after a venomous snakebite, and the efficacy of blood products is highly controversial. A multicenter randomized controlled trial has shown that fresh frozen plasma, which is used as an adjunct to antivenom, can accelerate the recovery of coagulation function but does not shorten the length of hospital stay and that early (<8 h) administration may be less effective.[132] A retrospective study has shown that fresh frozen plasma may accelerate the recovery of coagulation function and reduce bleeding and the amount of antivenom needed.[133] However, fresh frozen plasma was not found to accelerate the recovery of coagulation function in patients with viper bites.[134] Recent studies have shown that fresh frozen plasma and cryoprecipitate do not improve coagulation function,[135] and do not ameliorate coagulation disorders in those without bleeding.[136] Therefore, blood products such as fresh frozen plasma or cryoprecipitate should not be routinely used for venomous snakebites accompanied by VICC, but they can be considered adjuvant therapies for patients with severe coagulopathy who require emergency surgery, invasive procedures, those with insufficient antivenom usage, or those who have active bleeding. A variety of snake venom enzyme components can inhibit or activate platelets, producing antiplatelet or platelet aggregation effects.[137] Using sufficient antivenom can rapidly restore platelet levels, and platelet transfusion can be considered for those with severe thrombocytopenia with bleeding or those who require emergency surgery. ...
The role of platelets in hemostasis and the effects of snake venom toxins on platelet function
1
2017
... Snake venom-induced consumptive coagulopathy or coagulopathy is a severe reaction after a venomous snakebite, and the efficacy of blood products is highly controversial. A multicenter randomized controlled trial has shown that fresh frozen plasma, which is used as an adjunct to antivenom, can accelerate the recovery of coagulation function but does not shorten the length of hospital stay and that early (<8 h) administration may be less effective.[132] A retrospective study has shown that fresh frozen plasma may accelerate the recovery of coagulation function and reduce bleeding and the amount of antivenom needed.[133] However, fresh frozen plasma was not found to accelerate the recovery of coagulation function in patients with viper bites.[134] Recent studies have shown that fresh frozen plasma and cryoprecipitate do not improve coagulation function,[135] and do not ameliorate coagulation disorders in those without bleeding.[136] Therefore, blood products such as fresh frozen plasma or cryoprecipitate should not be routinely used for venomous snakebites accompanied by VICC, but they can be considered adjuvant therapies for patients with severe coagulopathy who require emergency surgery, invasive procedures, those with insufficient antivenom usage, or those who have active bleeding. A variety of snake venom enzyme components can inhibit or activate platelets, producing antiplatelet or platelet aggregation effects.[137] Using sufficient antivenom can rapidly restore platelet levels, and platelet transfusion can be considered for those with severe thrombocytopenia with bleeding or those who require emergency surgery. ...
1
... Plasma exchange, which entails separation and discarding of plasma to remove large molecules from the blood, may help remove large molecules of free snake venom from the blood.[138] A small-scale study has shown that plasma exchange can help remove snake venom from the body.[139] The efficacy of plasma exchange in treating snake venom-related thrombotic microvascular disease is uncertain.[140⇓⇓-143] ...
Snakebite-associated thrombotic microangiopathy: a spotlight on pharmaceutical interventions
1
2023
... Plasma exchange, which entails separation and discarding of plasma to remove large molecules from the blood, may help remove large molecules of free snake venom from the blood.[138] A small-scale study has shown that plasma exchange can help remove snake venom from the body.[139] The efficacy of plasma exchange in treating snake venom-related thrombotic microvascular disease is uncertain.[140⇓⇓-143] ...
Snakebite-associated thrombotic microangiopathy: a systematic review of clinical features, outcomes, and evidence for interventions including plasmapheresis
1
2020
... Plasma exchange, which entails separation and discarding of plasma to remove large molecules from the blood, may help remove large molecules of free snake venom from the blood.[138] A small-scale study has shown that plasma exchange can help remove snake venom from the body.[139] The efficacy of plasma exchange in treating snake venom-related thrombotic microvascular disease is uncertain.[140⇓⇓-143] ...
Role of therapeutic plasma exchange in snake bite associated thrombotic microangiopathy-a case report with review of literature
1
2019
... Plasma exchange, which entails separation and discarding of plasma to remove large molecules from the blood, may help remove large molecules of free snake venom from the blood.[138] A small-scale study has shown that plasma exchange can help remove snake venom from the body.[139] The efficacy of plasma exchange in treating snake venom-related thrombotic microvascular disease is uncertain.[140⇓⇓-143] ...
Snakebite-associated thrombotic microangiopathy: an Australian prospective cohort study
1
2022
... Plasma exchange, which entails separation and discarding of plasma to remove large molecules from the blood, may help remove large molecules of free snake venom from the blood.[138] A small-scale study has shown that plasma exchange can help remove snake venom from the body.[139] The efficacy of plasma exchange in treating snake venom-related thrombotic microvascular disease is uncertain.[140⇓⇓-143] ...
Snakebite-associated thrombotic microangiopathy: an Australian prospective cohort study [ASP30]
1
2022
... Plasma exchange, which entails separation and discarding of plasma to remove large molecules from the blood, may help remove large molecules of free snake venom from the blood.[138] A small-scale study has shown that plasma exchange can help remove snake venom from the body.[139] The efficacy of plasma exchange in treating snake venom-related thrombotic microvascular disease is uncertain.[140⇓⇓-143] ...
Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue
1
2013
... The neck glands of venom-spraying cobras, Chinese cobras, and Rhabdophis tigrinus can spray toxins. If toxins are sprayed into the eyes, they can cause severe pain, photophobia, lacrimation, blurred vision, other irritating symptoms, and even corneal ulcers and secondary endophthalmitis.[144] Immediate low-pressure irrigation with large amounts of water is required at the scene, followed by thorough irrigation with normal saline or lactated Ringer’s solution after arrival at the hospital. Topical application of 0.5% adrenaline drops or 4% lidocaine eye drops can be used for pain relief. Ophthalmologic examination is required to assess corneal damage, and topical antibiotic drops such as chloramphenicol, tetracycline, or ciprofloxacin can be administered to prevent intraocular or corneal opacities. In principle, antivenom is not necessary, but for those with severe eye damage or signs of toxin absorption in the early stages, antivenom should be administered. Glucocorticoids are contraindicated because of the risk of herpes simplex keratitis.[47,145] ...
Venom ophthalmia and ocular complications caused by snake venom
1
2020
... The neck glands of venom-spraying cobras, Chinese cobras, and Rhabdophis tigrinus can spray toxins. If toxins are sprayed into the eyes, they can cause severe pain, photophobia, lacrimation, blurred vision, other irritating symptoms, and even corneal ulcers and secondary endophthalmitis.[144] Immediate low-pressure irrigation with large amounts of water is required at the scene, followed by thorough irrigation with normal saline or lactated Ringer’s solution after arrival at the hospital. Topical application of 0.5% adrenaline drops or 4% lidocaine eye drops can be used for pain relief. Ophthalmologic examination is required to assess corneal damage, and topical antibiotic drops such as chloramphenicol, tetracycline, or ciprofloxacin can be administered to prevent intraocular or corneal opacities. In principle, antivenom is not necessary, but for those with severe eye damage or signs of toxin absorption in the early stages, antivenom should be administered. Glucocorticoids are contraindicated because of the risk of herpes simplex keratitis.[47,145] ...
Local antivenom treatment for ophthalmic injuries caused by a Naja atra
1
2010
... Traditional Chinese medicine has a long history of treating venomous snakebites. The oral and external administration of Chinese medicine, as well as acupuncture and cupping, could improve local and systemic symptoms and enhance therapeutic effects.[146] According to traditional Chinese medicine theory, the basic pathogenesis of snakebite toxicity is the lack of discharge of snake venom and the internalization of the toxin.[147] Snakebites are divided into three types: “wind toxins”, “fire toxins”, and “wind-fire toxins”. According to the syndrome type and clinical manifestations, the treatment of snakebites is based on the identification and application of methods for clearing away heat and detoxification, dispelling wind and opening up orifices, cooling the blood and stopping hemorrhage, and promoting diuresis and catharsis.[148] Alkaloids, flavonoids, phenols, and other active ingredients in some proprietary traditional Chinese medicine snakebite detoxification preparations have a certain inhibitory effect on enzyme snake venom components such as PLA2 and protein hydrolase.[149-150] Some traditional Chinese medicines have anti-inflammatory and antioxidant effects, which can help reduce swelling.[1] For ulcers caused by snakebites, in addition to sufficient antivenom and local debridement, traditional Chinese medicine that promote pus removal, tissue regeneration, and wound healing can be administered as adjunctive treatments.[151,152]A large-sample meta-analysis indicated that traditional Chinese medicines used for the treatment of pit viper bites can improve local symptoms and reduce swelling,[153] but more robust studies are required to confirm the reliability of the conclusions. ...
Clinical observation on 300 cases of pit viper bites treated with integrated therapy of Chinese medicine intervention
1
2011
... Traditional Chinese medicine has a long history of treating venomous snakebites. The oral and external administration of Chinese medicine, as well as acupuncture and cupping, could improve local and systemic symptoms and enhance therapeutic effects.[146] According to traditional Chinese medicine theory, the basic pathogenesis of snakebite toxicity is the lack of discharge of snake venom and the internalization of the toxin.[147] Snakebites are divided into three types: “wind toxins”, “fire toxins”, and “wind-fire toxins”. According to the syndrome type and clinical manifestations, the treatment of snakebites is based on the identification and application of methods for clearing away heat and detoxification, dispelling wind and opening up orifices, cooling the blood and stopping hemorrhage, and promoting diuresis and catharsis.[148] Alkaloids, flavonoids, phenols, and other active ingredients in some proprietary traditional Chinese medicine snakebite detoxification preparations have a certain inhibitory effect on enzyme snake venom components such as PLA2 and protein hydrolase.[149-150] Some traditional Chinese medicines have anti-inflammatory and antioxidant effects, which can help reduce swelling.[1] For ulcers caused by snakebites, in addition to sufficient antivenom and local debridement, traditional Chinese medicine that promote pus removal, tissue regeneration, and wound healing can be administered as adjunctive treatments.[151,152]A large-sample meta-analysis indicated that traditional Chinese medicines used for the treatment of pit viper bites can improve local symptoms and reduce swelling,[153] but more robust studies are required to confirm the reliability of the conclusions. ...
An overview of snakes in China
1
1999
... Traditional Chinese medicine has a long history of treating venomous snakebites. The oral and external administration of Chinese medicine, as well as acupuncture and cupping, could improve local and systemic symptoms and enhance therapeutic effects.[146] According to traditional Chinese medicine theory, the basic pathogenesis of snakebite toxicity is the lack of discharge of snake venom and the internalization of the toxin.[147] Snakebites are divided into three types: “wind toxins”, “fire toxins”, and “wind-fire toxins”. According to the syndrome type and clinical manifestations, the treatment of snakebites is based on the identification and application of methods for clearing away heat and detoxification, dispelling wind and opening up orifices, cooling the blood and stopping hemorrhage, and promoting diuresis and catharsis.[148] Alkaloids, flavonoids, phenols, and other active ingredients in some proprietary traditional Chinese medicine snakebite detoxification preparations have a certain inhibitory effect on enzyme snake venom components such as PLA2 and protein hydrolase.[149-150] Some traditional Chinese medicines have anti-inflammatory and antioxidant effects, which can help reduce swelling.[1] For ulcers caused by snakebites, in addition to sufficient antivenom and local debridement, traditional Chinese medicine that promote pus removal, tissue regeneration, and wound healing can be administered as adjunctive treatments.[151,152]A large-sample meta-analysis indicated that traditional Chinese medicines used for the treatment of pit viper bites can improve local symptoms and reduce swelling,[153] but more robust studies are required to confirm the reliability of the conclusions. ...
Expert consensus on Chinese medicine diagnostic and treatment protocol for venomous snake bites (2016 edition)
1
2017
... Traditional Chinese medicine has a long history of treating venomous snakebites. The oral and external administration of Chinese medicine, as well as acupuncture and cupping, could improve local and systemic symptoms and enhance therapeutic effects.[146] According to traditional Chinese medicine theory, the basic pathogenesis of snakebite toxicity is the lack of discharge of snake venom and the internalization of the toxin.[147] Snakebites are divided into three types: “wind toxins”, “fire toxins”, and “wind-fire toxins”. According to the syndrome type and clinical manifestations, the treatment of snakebites is based on the identification and application of methods for clearing away heat and detoxification, dispelling wind and opening up orifices, cooling the blood and stopping hemorrhage, and promoting diuresis and catharsis.[148] Alkaloids, flavonoids, phenols, and other active ingredients in some proprietary traditional Chinese medicine snakebite detoxification preparations have a certain inhibitory effect on enzyme snake venom components such as PLA2 and protein hydrolase.[149-150] Some traditional Chinese medicines have anti-inflammatory and antioxidant effects, which can help reduce swelling.[1] For ulcers caused by snakebites, in addition to sufficient antivenom and local debridement, traditional Chinese medicine that promote pus removal, tissue regeneration, and wound healing can be administered as adjunctive treatments.[151,152]A large-sample meta-analysis indicated that traditional Chinese medicines used for the treatment of pit viper bites can improve local symptoms and reduce swelling,[153] but more robust studies are required to confirm the reliability of the conclusions. ...
A review of Fujian snakebite prescriptions
1
2023
... Traditional Chinese medicine has a long history of treating venomous snakebites. The oral and external administration of Chinese medicine, as well as acupuncture and cupping, could improve local and systemic symptoms and enhance therapeutic effects.[146] According to traditional Chinese medicine theory, the basic pathogenesis of snakebite toxicity is the lack of discharge of snake venom and the internalization of the toxin.[147] Snakebites are divided into three types: “wind toxins”, “fire toxins”, and “wind-fire toxins”. According to the syndrome type and clinical manifestations, the treatment of snakebites is based on the identification and application of methods for clearing away heat and detoxification, dispelling wind and opening up orifices, cooling the blood and stopping hemorrhage, and promoting diuresis and catharsis.[148] Alkaloids, flavonoids, phenols, and other active ingredients in some proprietary traditional Chinese medicine snakebite detoxification preparations have a certain inhibitory effect on enzyme snake venom components such as PLA2 and protein hydrolase.[149-150] Some traditional Chinese medicines have anti-inflammatory and antioxidant effects, which can help reduce swelling.[1] For ulcers caused by snakebites, in addition to sufficient antivenom and local debridement, traditional Chinese medicine that promote pus removal, tissue regeneration, and wound healing can be administered as adjunctive treatments.[151,152]A large-sample meta-analysis indicated that traditional Chinese medicines used for the treatment of pit viper bites can improve local symptoms and reduce swelling,[153] but more robust studies are required to confirm the reliability of the conclusions. ...
Progress in the treatment of venomous snake bites by traditional Chinese medicine
1
2010
... Traditional Chinese medicine has a long history of treating venomous snakebites. The oral and external administration of Chinese medicine, as well as acupuncture and cupping, could improve local and systemic symptoms and enhance therapeutic effects.[146] According to traditional Chinese medicine theory, the basic pathogenesis of snakebite toxicity is the lack of discharge of snake venom and the internalization of the toxin.[147] Snakebites are divided into three types: “wind toxins”, “fire toxins”, and “wind-fire toxins”. According to the syndrome type and clinical manifestations, the treatment of snakebites is based on the identification and application of methods for clearing away heat and detoxification, dispelling wind and opening up orifices, cooling the blood and stopping hemorrhage, and promoting diuresis and catharsis.[148] Alkaloids, flavonoids, phenols, and other active ingredients in some proprietary traditional Chinese medicine snakebite detoxification preparations have a certain inhibitory effect on enzyme snake venom components such as PLA2 and protein hydrolase.[149-150] Some traditional Chinese medicines have anti-inflammatory and antioxidant effects, which can help reduce swelling.[1] For ulcers caused by snakebites, in addition to sufficient antivenom and local debridement, traditional Chinese medicine that promote pus removal, tissue regeneration, and wound healing can be administered as adjunctive treatments.[151,152]A large-sample meta-analysis indicated that traditional Chinese medicines used for the treatment of pit viper bites can improve local symptoms and reduce swelling,[153] but more robust studies are required to confirm the reliability of the conclusions. ...
Progress in the treatment of snakebite ulcers
1
2016
... Traditional Chinese medicine has a long history of treating venomous snakebites. The oral and external administration of Chinese medicine, as well as acupuncture and cupping, could improve local and systemic symptoms and enhance therapeutic effects.[146] According to traditional Chinese medicine theory, the basic pathogenesis of snakebite toxicity is the lack of discharge of snake venom and the internalization of the toxin.[147] Snakebites are divided into three types: “wind toxins”, “fire toxins”, and “wind-fire toxins”. According to the syndrome type and clinical manifestations, the treatment of snakebites is based on the identification and application of methods for clearing away heat and detoxification, dispelling wind and opening up orifices, cooling the blood and stopping hemorrhage, and promoting diuresis and catharsis.[148] Alkaloids, flavonoids, phenols, and other active ingredients in some proprietary traditional Chinese medicine snakebite detoxification preparations have a certain inhibitory effect on enzyme snake venom components such as PLA2 and protein hydrolase.[149-150] Some traditional Chinese medicines have anti-inflammatory and antioxidant effects, which can help reduce swelling.[1] For ulcers caused by snakebites, in addition to sufficient antivenom and local debridement, traditional Chinese medicine that promote pus removal, tissue regeneration, and wound healing can be administered as adjunctive treatments.[151,152]A large-sample meta-analysis indicated that traditional Chinese medicines used for the treatment of pit viper bites can improve local symptoms and reduce swelling,[153] but more robust studies are required to confirm the reliability of the conclusions. ...
Meta-analysis of the clinical efficacy of traditional Chinese medicine in the treatment of pit viper bites
2
2022
... Traditional Chinese medicine has a long history of treating venomous snakebites. The oral and external administration of Chinese medicine, as well as acupuncture and cupping, could improve local and systemic symptoms and enhance therapeutic effects.[146] According to traditional Chinese medicine theory, the basic pathogenesis of snakebite toxicity is the lack of discharge of snake venom and the internalization of the toxin.[147] Snakebites are divided into three types: “wind toxins”, “fire toxins”, and “wind-fire toxins”. According to the syndrome type and clinical manifestations, the treatment of snakebites is based on the identification and application of methods for clearing away heat and detoxification, dispelling wind and opening up orifices, cooling the blood and stopping hemorrhage, and promoting diuresis and catharsis.[148] Alkaloids, flavonoids, phenols, and other active ingredients in some proprietary traditional Chinese medicine snakebite detoxification preparations have a certain inhibitory effect on enzyme snake venom components such as PLA2 and protein hydrolase.[149-150] Some traditional Chinese medicines have anti-inflammatory and antioxidant effects, which can help reduce swelling.[1] For ulcers caused by snakebites, in addition to sufficient antivenom and local debridement, traditional Chinese medicine that promote pus removal, tissue regeneration, and wound healing can be administered as adjunctive treatments.[151,152]A large-sample meta-analysis indicated that traditional Chinese medicines used for the treatment of pit viper bites can improve local symptoms and reduce swelling,[153] but more robust studies are required to confirm the reliability of the conclusions. ...
... The incidence of psychological disorders in snakebite victims ranges from 8% to 43%, mainly post-traumatic stress disorder, depression, hysteria (or unspecified affective dissociative and conversion disorders), paranoia (or organic delusions or schizophrenia-like disorders), psychosocial disorders, hallucinations, acute psychosis, psychogenic convulsions, inattention, and aggressive behavior.[153] Most patients experience gradual remission as they recover from snakebites, and nearly 1/3 of patients may experience persistent symptoms for more than one year or even up to several years.[154-155] Psychological intervention is the main treatment, including psychological first aid (e.g., caring, listening, or soothing), psychoeducation, and cognitive-behavioral therapy.[156-157] Psychopharmacological medications, such as antidepressants, are administered when necessary.[158] ...
Mental health conditions after snakebite: a scoping review
1
2020
... The incidence of psychological disorders in snakebite victims ranges from 8% to 43%, mainly post-traumatic stress disorder, depression, hysteria (or unspecified affective dissociative and conversion disorders), paranoia (or organic delusions or schizophrenia-like disorders), psychosocial disorders, hallucinations, acute psychosis, psychogenic convulsions, inattention, and aggressive behavior.[153] Most patients experience gradual remission as they recover from snakebites, and nearly 1/3 of patients may experience persistent symptoms for more than one year or even up to several years.[154-155] Psychological intervention is the main treatment, including psychological first aid (e.g., caring, listening, or soothing), psychoeducation, and cognitive-behavioral therapy.[156-157] Psychopharmacological medications, such as antidepressants, are administered when necessary.[158] ...
Long-term health effects perceived by snakebite patients in rural Sri Lanka: a cohort study
1
2022
... The incidence of psychological disorders in snakebite victims ranges from 8% to 43%, mainly post-traumatic stress disorder, depression, hysteria (or unspecified affective dissociative and conversion disorders), paranoia (or organic delusions or schizophrenia-like disorders), psychosocial disorders, hallucinations, acute psychosis, psychogenic convulsions, inattention, and aggressive behavior.[153] Most patients experience gradual remission as they recover from snakebites, and nearly 1/3 of patients may experience persistent symptoms for more than one year or even up to several years.[154-155] Psychological intervention is the main treatment, including psychological first aid (e.g., caring, listening, or soothing), psychoeducation, and cognitive-behavioral therapy.[156-157] Psychopharmacological medications, such as antidepressants, are administered when necessary.[158] ...
Long-term effects of snake envenoming
1
2019
... The incidence of psychological disorders in snakebite victims ranges from 8% to 43%, mainly post-traumatic stress disorder, depression, hysteria (or unspecified affective dissociative and conversion disorders), paranoia (or organic delusions or schizophrenia-like disorders), psychosocial disorders, hallucinations, acute psychosis, psychogenic convulsions, inattention, and aggressive behavior.[153] Most patients experience gradual remission as they recover from snakebites, and nearly 1/3 of patients may experience persistent symptoms for more than one year or even up to several years.[154-155] Psychological intervention is the main treatment, including psychological first aid (e.g., caring, listening, or soothing), psychoeducation, and cognitive-behavioral therapy.[156-157] Psychopharmacological medications, such as antidepressants, are administered when necessary.[158] ...
Mental health response to community disasters: a systematic review
1
2013
... The incidence of psychological disorders in snakebite victims ranges from 8% to 43%, mainly post-traumatic stress disorder, depression, hysteria (or unspecified affective dissociative and conversion disorders), paranoia (or organic delusions or schizophrenia-like disorders), psychosocial disorders, hallucinations, acute psychosis, psychogenic convulsions, inattention, and aggressive behavior.[153] Most patients experience gradual remission as they recover from snakebites, and nearly 1/3 of patients may experience persistent symptoms for more than one year or even up to several years.[154-155] Psychological intervention is the main treatment, including psychological first aid (e.g., caring, listening, or soothing), psychoeducation, and cognitive-behavioral therapy.[156-157] Psychopharmacological medications, such as antidepressants, are administered when necessary.[158] ...
A randomized controlled trial of a brief intervention for delayed psychological effects in snakebite victims
1
2015
... The incidence of psychological disorders in snakebite victims ranges from 8% to 43%, mainly post-traumatic stress disorder, depression, hysteria (or unspecified affective dissociative and conversion disorders), paranoia (or organic delusions or schizophrenia-like disorders), psychosocial disorders, hallucinations, acute psychosis, psychogenic convulsions, inattention, and aggressive behavior.[153] Most patients experience gradual remission as they recover from snakebites, and nearly 1/3 of patients may experience persistent symptoms for more than one year or even up to several years.[154-155] Psychological intervention is the main treatment, including psychological first aid (e.g., caring, listening, or soothing), psychoeducation, and cognitive-behavioral therapy.[156-157] Psychopharmacological medications, such as antidepressants, are administered when necessary.[158] ...
Complex post-traumatic stress disorder
1
2022
... Nearly 5.8 billion people worldwide are at risk of being bitten by venomous snakes, predominantly in tropical and subtropical regions.[159] Snakebite prevention is the most important and cost-effective way to minimize the burden of snakebites. The main preventive measures are summarized below: strengthening the medical and health system, expanding the coverage of snakebite treatment in at-risk areas, and improving and standardizing snakebite treatment in medical institutions.[160] ...
WHO’s snakebite envenoming strategy for prevention and control
2
2019
... Nearly 5.8 billion people worldwide are at risk of being bitten by venomous snakes, predominantly in tropical and subtropical regions.[159] Snakebite prevention is the most important and cost-effective way to minimize the burden of snakebites. The main preventive measures are summarized below: strengthening the medical and health system, expanding the coverage of snakebite treatment in at-risk areas, and improving and standardizing snakebite treatment in medical institutions.[160] ...
... Enhancing community awareness regarding the prevention of snakebites, especially in high-risk communities. Students should be educated about snake habits and potential injuries, avoid close contact with snakes, and be trained in first aid related to snakebites.[160] Utilize mainstream or social media to raise awareness of the lifestyle of snake and potential harm caused by snakebites. As snakes are thermophiles, they are most active at temperatures of 26-34 °C. Snakes are more active from April to October, and the lower the temperature is, the less their activity. Snakes prepare for hibernation when the temperature is consistently below 15 °C. At temperatures exceeding 40%, snakes tend to find refuge in caves, rock crevices, bushes, or under dead trees and weeds to avoid heat. Snakes prefer warmer climates and are mostly diurnal (day-time hunters). For example, cobras and king cobra are more active during the daytime (09:00-15:00); gold-ringed snakes/silver-ringed snakes tend to be more active between 18:00-22:00; and the Medog green pit viper, pit viper, Agkistrodon acutus, Viperidae, pointed-scaled pit viper are often active in the morning and evening. Understanding their activity patterns can reduce the chance of encounters and consequently bites. ...
Effect of distance and delay in access to care on outcome of snakebite in rural north-eastern Nigeria
0
2015