Chlorfenapyr is a liposoluble insecticide belonging to the pyrrole family. Chlorfenapyr is activated when the N-ethoxymethyl side chain breaks, forming a toxic metabolite, which uncouples oxidative phosphorylation in the mitochondria, inhibits the production of adenosine triphosphate (ATP), and leads to the death of cells and target organisms.[1] Symptoms of chlorfenapyr poisoning in patients are mild and atypical in the early stage, especially in patients receiving low dose exposure; however, such cases are rare and may be ignored by physicians, often leading to delayed treatment.[2,3] Once the hypothalamus thermoregulatory center and medulla oblongata respiratory and heartbeat centers are involved, patients often develop hyperthermic coma, leading to death.[2⇓⇓-5] At present, there is no specific antidote or effective treatment for chlorfenapyr poisoning. The reported mortality rate is 38.0%-87.5%.[6⇓-8] As the toxicokinetics of chlorfenapyr are unclear, the clearance effect of blood purification technology on absorbed chlorfenapyr remains unknown. Here, we reported a chlorfenapyr poisoning patient treated with hemoperfusion (HP) and continuous veno-venous hemodiafiltration (CVVHDF). The blood toxicant concentrations were monitored during treatment. These data may help physicians decide whether to use HP and CVVHDF for patients with chlorfenapyr poisoning.
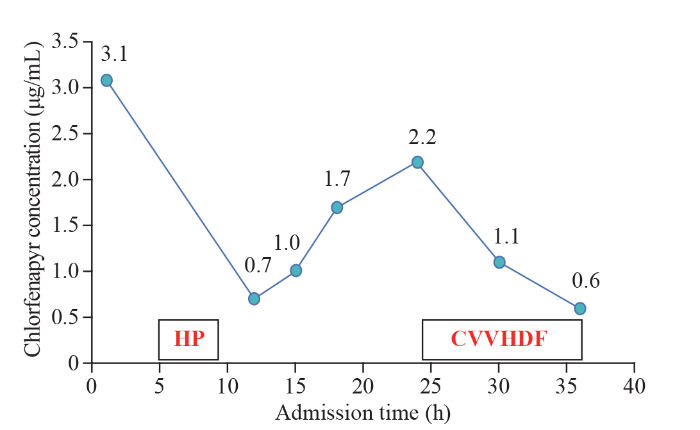
A 15-year-old female (62 kg body weight, body mass index [BMI] 23) with no prior medical history was admitted 12 d after a suicidal overdose of 100 mL of chlorfenapyr. One hour after ingesting the poison, the patient was sent to the local hospital for gastric lavage with 15,000 mL of clean water. After gastric lavage, the patient refused further treatment and was discharged against medical advice. She stayed at home for 3 d and then returned to school for 7 d. On the 11th day after ingesting the poison, the patient experienced fatigue and headache and sought medical attention in our emergency department. In the examination room, the patient’s consciousness was blurred, and the laboratory findings are shown in Table 1. On the 12th day after ingesting the poison, she was admitted to our department. Physical examination after admission revealed the temperature 38.5 °C, pulse 82 beats/min, blood pressure 109/64 mmHg (1 mmHg=0.133 kPa), and oxygen saturation (SpO2) 99%, with light coma, sweating, a positive meningeal irritation sign, muscle strength of both upper limbs at level III, lower limbs at level I, and pathological signs of both lower limbs being positive. A liquid chromatography-mass spectrometer (LC-MS) (API-3200; ABI, USA) was used for toxicity analysis. The concentration of chlorfenapyr in venous blood was 3.1 μg/mL but was undetectable in gastric juice and urine. Brain computed tomography (CT) showed cerebral cortex swelling, cerebral sulcus disappearance, and white matter density reduction. Electroencephalography (EEG) showed no α rhythm, and the background showed predominantly low- and medium-amplitude θ waves. Electromyography revealed that there was no skin sympathetic response (SSR) in the extremities, the sensory motor nerve conduction in the extremities was normal, and the peripheral sensory motor nerves were not damaged. Electrocardiogram (ECG) showed sinus arrhythmia. Her chest and abdominal CT findings were normal. The arterial blood gas analysis, routine blood examination, liver and kidney function, and myocardial enzyme data are shown in Table 1. The patient was given mannitol dehydration to reduce intracranial pressure, ATP, neurotrophic nerve cooling and other treatments, along with HP for 4 h (Jianfan HA resin hemoperfusion device, model HA330, two cans in total, each lasted for 2 h). The blood concentration of chlorfenapyr was 0.7 μg/mL 3 h after the procedure. Continuous blood purification treatment (mode CVVHDF, blood flow of 180 mL/h, replacement fluid velocity of 2,000 mL/h, heparin anticoagulation) was performed 24 h after admission for 12 h. During this period, the blood chlorfenapyr concentration was monitored, and chlorfenapyr was not detected in the replacement waste liquid. The concentration of chlorfenapyr in the blood after admission are shown in Figure 1. On the second day after admission, the patient was in a deep coma and had a stiff neck and unequal bilateral pupils; the maximum temperature increased to 42 °C, the heart rate gradually increased to 160-170 beats/min, and the non-invasive finger oxygen saturation gradually decreased. The patient was treated with oral endotracheal intubation connected with ventilator-assisted ventilation, an ice cap and ice blanket cooling, and ventricular rate control. However, the patient developed ventricular fibrillation 39 h after admission and died after cardiopulmonary resuscitation failure.
Table 1. Laboratory findings
| Variables (normal range) | 1 h after admission | 25 h after admission |
|---|---|---|
| White blood cell count ([3.5-9.5]×109/L) | 7.0×109/L | 12.4×109/L |
| Neutrophil ([1.8-6.3]×109/L) | 6.2×109/L | 10.3×109/L |
| Hemoglobin (115-150 g/L) | 104 g/L | 104 g/L |
| Platelet ([125-350]×109/L) | 276×109/L | 209×109/L |
| Blood urea nitrogen (2.6-7.5 mmol/L) | 3.9 mmol/L | 2.5 mmol/L |
| Creatinine (50-110 μmol/L) | 46 μmol/L | 32 μmol/L |
| Alanine transaminase (7-40 U/L) | 33 U/L | 81 U/L |
| Total bilirubin (≤21.0 μmol/L) | 14.7 μmol/L | 7.2 μmol/L |
| Amylase (0-220 U/L) | 45 U/L | 180 U/L |
| C-reactive protein (≤3.0 mg/L) | 0.3 mg/L | 2.7 mg/L |
| Creatine kinase (40-200 U/L) | 7,541 U/L | 7,299 U/L |
| Troponin-I (0-0.16 ng/mL) | <0.01 ng/mL | <0.01 ng/mL |
| Myosin (0-61.5 ng/mL) | >2,000 ng/mL | 1,519 ng/mL |
| Creatine kinase isoenzyme (≤25 U/L) | 75 U/L | 83 U/L |
| Blood gas analysis (artery) | ||
| pH (7.35-7.45) | 7.46 | 7.35 |
| PO2 (80-100 mmHg) | 95 mmHg | 174 mmHg |
| PCO2 (35-45 mmHg) | 31 mmHg | 44.0 mmHg |
| Bicarbonate (22-27 mmol/L) | 23.4 mmol/L | 25.6 mmol/L |
| Lactate (0.5-1.6 mmol/L) | 1.7 mmol/L | 2.4 mmol/L |
Figure 1.
Figure 1.
Chlorfenapyr concentration after admission to our hospital. HP: hemoperfusion; CVVHDF: continuous veno-venous hemodiafiltration.
Our case showed that (1) HP and CVVHDF can effectively remove the absorbed chlorfenapyr in the body. After HP treatment, the concentration of chlorfenapyr in the blood decreased by 77.4%, from 3.1 μg/mL to 0.7 μg/mL. After CVVHDF treatment, the blood chlorfenapyr concentration decreased by 54.5%, from 2.2 μg/mL to 0.6 μg/mL. (2) The effects of chlorfenapyr can persist in the peripheral environment for as long as 34 weeks.[1] High concentrations of chlorfenapyr can be detected in the blood 12 d after ingestion, indicating that it is slowly metabolized in the human body. (3) After HP treatment, the blood toxicity concentration decreased significantly but gradually increased over the next 12 h, indicating that chlorfenapyr is extensively stored in tissues and organs and is released into the blood once the blood toxicity concentration decreases. This may be related to the fat solubility of chlorfenapyr. (4) On the 12th day after ingesting the poison, the concentration of chlorfenapyr in the blood was 3.1 μg/mL, but it was not detected in the gastric juice or urine, indicating that chlorfenapyr was absent in the intestinal liver circulation and was not excreted in the urine in its original form, which may be one of the reasons for its slow metabolism. CVVHDF can reduce the concentration of blood poison, but no poison was detected in the replacement waste liquid, indicating that dialysis cannot remove chlorfenapyr and continuous veno-venous hemofiltration (CVVH) should be used for a better effect.
Chlorfenapyr is highly toxic, with a long half-life and a minimum lethal dose of 5 mL.[4] The main cause of death is damage to the central nervous system, especially the cardiovascular, respiratory and hypothalamic thermoregulatory centers in the medulla oblongata.[3,8] Therefore, early elimination of the toxin is crucial, including elimination of the toxin in the gastrointestinal tract and the absorbed toxin in the body. However, whether direct supplementation with large doses of ATP is effective remains unknown.
The patient did not receive treatment immediately after ingesting the poison and visited the doctor after developing consciousness disorders. Due to the relative rarity and high mortality rate of chlorfenapyr poisoning, it is necessary to conduct experimental studies on animals to determine its toxicology, toxicokinetics, and efficacy of blood purification to eliminate the toxin.
Funding: This study was supported by a grant from the National Key R&D Program of China (2019YFC16063000).
Ethical approval: The patient provided written informed consent for the publication of this case report and for the use of her medical data.
Conflicts of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Contributors: YQL and XXL contributed equally to this work. YQL and XXL drafted the manuscript. HCW, RZZ, ZYL, and LMH performed research; MN tested poisons; XGZ designed research study; XGZ and XBP had full access to the data reported in this study and had final responsibility for the decision to submit this report for publication.
Reference
Chlorfenapyr: a new insecticide with novel mode of action can control pyrethroid resistant malaria vectors
Magnetic resonance imaging and clinical features of chlorfenapyr-induced toxic leukoencephalopathy: a case report
A patient fatality following the ingestion of a small amount of chlorfenapyr
DOI:10.4103/0974-2700.136874
PMID:25114438
[Cited within: 3]

Chlorfenapyr has been used worldwide for agricultural pest control since 1995. Despite its widespread use, acute human poisoning data are insufficient; only a small number of fatalities from chlorfenapyr poisoning have been reported. The signs and symptoms of chlorfenapyr toxicity include nausea, vomiting, fever, rhabdomyolysis, among others. In addition, central nervous system effects in association with delayed toxicity have also been observed. Here, we detail a fatality resulting from delayed chlorfenapyr toxicity following the ingestion of a small amount of pesticide.
Malignant hyperthermia-like syndrome in acute chlorfenapyr poisoning - A case report
Clinical and radiological findings in chlorfenapyr poisoning
Vigilance against a highly lethal insecticide chlorfenapyr poisoning (report of 4 cases and literature review)
Acute chlorfenapyr poisoning
A fatal case of chlorfenapyr poisoning and a review of the literature