INTRODUCTION
Chlorfenapyr is a white-tan crystalline solid developed by the American Cyanamid Company.[3] Its International Union of Pure and Applied Chemistry name is 4-bromo-2-(4-chlorophenyl)-1-ethoxymethyl-5-(trifluoromethyl)pyrrole-3-carbonitrile. Its molecular formula is C15H11BrClF3N2O, and its relative molecular weight is 407.61 g/mol. It is a pyrrole class broad-spectrum insecticide (supplementary Figure 1A) currently registered in 19 countries for the control of various insects and mites.[4] Chlorfenapyr has a more significant insecticidal effect than the traditional insecticide phoxim;[5] however, it also poses a threat to human health and is classified as a medium risk by the World Health Organization (WHO). Chlorfenapyr has poor water solubility, dissolves in organic solvents, degrades slowly in soil, and is not volatile; this exacerbates the problems caused by insecticide residues in the environment.[6] Chlorfenapyr is passively degraded into CL303268, CL325195, CL152832, CL152835, CL325157, and other metabolites. Among these compounds, CL303268 (tralopyril) is the most toxic (supplementary Figure 1B),[7] with a relative molecular weight of 349.53 g/mol and the same solubility as chlorfenapyr.
Chlorfenapyr does not cause any cross-resistance to neurotoxic insecticides.[5,8] Chlorfenapyr has been reported to have greater toxicity with increasing temperature, which predicts the seasonality of pesticide spraying. Chlorfenapyr has been used to control mites on Lepidoptera, Diptera, Coleoptera, Hemiptera, and other crops and trees.[3] Its metabolite, tralopyril, was accepted in 2014 as a new-generation alternative antifouling biocide for effectively controlling biological fouling.[9⇓⇓-12] Tralopyril is often released into the water environment through splashing or soil drainage.[13⇓⇓-16]
Given the high mortality rate associated with chlorfenapyr poisoning, this study aimed to review the mechanisms, clinical presentations, and treatment strategies for chlorfenapyr poisoning.
METHODS
We conducted a review of the literature using PubMed, Web of Science, and SpringerLink from their beginnings to the end of October 2023. The inclusion criteria were systematic reviews, clinical guidelines, retrospective studies, and case reports on chlorfenapyr poisoning that focused on its mechanisms, clinical presentations, and treatment strategies. The references of the included studies were also examined to identify additional sources.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
RESULTS
Toxicological data
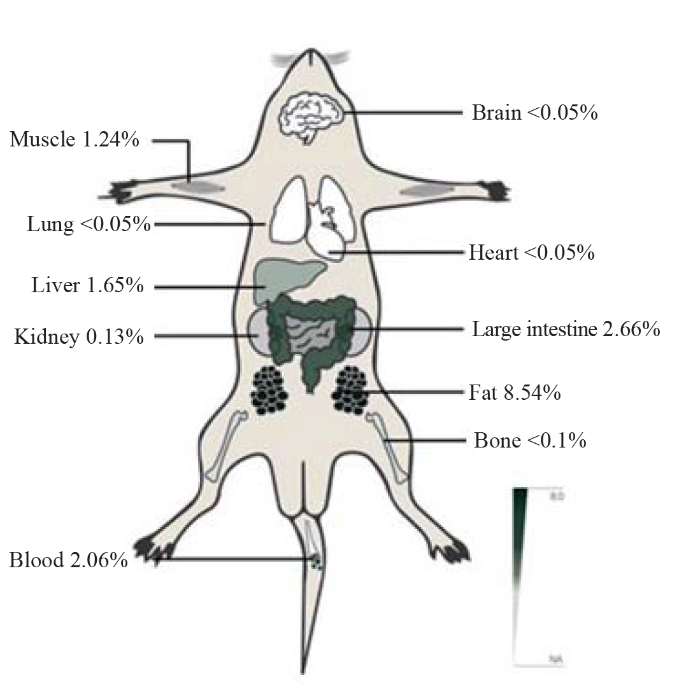
In total, 57 articles were identified in an initial search. Chlorfenapyr poisoning can occur through oral ingestion, with a median lethal dose (LD50) of 441 mg/kg for rats[17] and 45 mg/kg for mice.[18] Moreover, the LD50 of chlorfenapyr on rabbit skin is >2,000 mg/kg body weight (BW).[19] The absorption of chlorfenapyr in rats is relatively slow, plasma concentrations peak at 8-12 h after administration, and more than 90% of a dose is gradually transferred from the circulation organs to other organs within 168 h, with the final 75%-85% of the dose excreted in the stool and a small amount via urine. The half-life of plasma clearance is approximately 56 h.[20] Currently, there are no data on the protein binding rate or apparent volume of distribution (VD). After the rats were fed with carbon-14 labeled chlorfenapyr for 1, 8, 24, or 168 h, the concentrations of the radioisotope carbon 14 in the blood and organs of the rats were measured, and the researchers found that the apparent VD of chlorfenapyr was large.[21] To date, various pathways of chlorfenapyr poisoning, including oral administration, contact, inhalation, and intraperitoneal injections, have been reported.[22,23] Following oral administration, chlorfenapyr is absorbed into the blood via the digestive tract. Most poisons are removed from the body through the intestine, while some enter the hepatoenteric circulation and are metabolized by the liver. Figure 1 shows the residues distributed in tissues and organs throughout the body, and Figure 2 shows clinical manifestations of chlorfenapyr poisoning.[11]
Figure 1.
Figure 1.
The tissue radioactivity (% of dose ) in rats receiving 20 mg/kg (body weight) carbon-14 labeled chlorfenapyr.
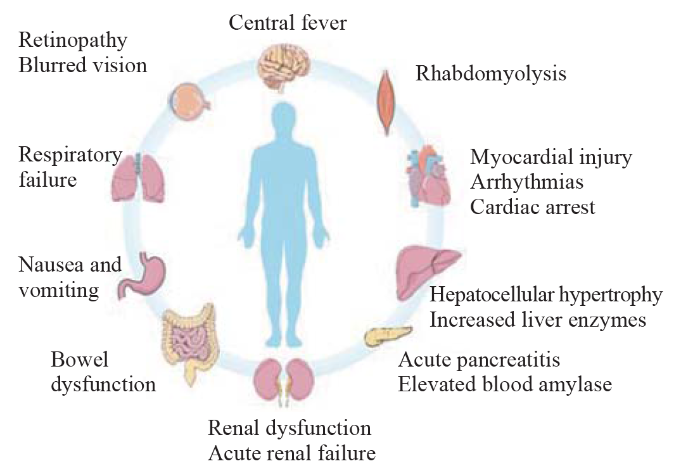
Figure 2.
Figure 2.
Clinical manifestations of chlorfenapyr poisoning.
Toxicological mechanisms
Chlorfenapyr and its metabolites can act on the mitochondria of cells, resulting in decreased mitochondrial membrane potential due to calcium overload. This interferes with the internal and external proton balance of the mitochondrial membrane, causing mitochondrial injury.[24] Chlorfenapyr reduces energy production by inhibiting the conversion of adenosine diphosphate to adenosine triphosphate (ATP), promoting mitochondria-mediated programmed cell death (PCD)[25] and contributing to DNA injury.[26] In human hepatocytes, chlorfenapyr promotes the expression of the cellular oxidative stress factor Bax/Bcl-2, leading to the release of cytochrome c (Cyt c) into the cytoplasm, the activation of Cas9/3, and the cleavage of poly(ADP-ribose) polymerase. The DNA injury and cell cycle arrest were detected in human hepatocytes treated with chlorfenapyr.[27] Moreover, the serum glutamate aminotransferase, gamma-glutamyl transpeptidase, urea nitrogen, and total protein levels increased in rats poisoned with chlorfenapyr. Hypertrophy and hepatocyte necrosis have also been observed.[28] These results suggest that chlorfenapyr induces PCD in hepatocytes. Uncoupling protein 1 (UCP1) is specifically and highly expressed in brown fat and is located in the inner mitochondrial membrane. After activation of UCP1, a large amount of heat generated through decoupling increases body temperature.[29] Sweating and hyperthermia caused by chlorfenapyr are related to the decoupling of mitochondrial oxidative phosphorylation. At 52 weeks following ingestion, the mature central nervous system in rats with chlorfenapyr poisoning exhibited pathological processes of vacuolar myelination and mild myelin swelling, demonstrating white matter degeneration and a high signal intensity on T2-weighted imaging andmagnetic resonance imaging (MRI).[28] Studies have shown that chlorfenapyr and its metabolites can also affect the muscle system and reproductive system.[9,30]
Clinical presentations
Owing to the atypical symptoms of acute chlorfenapyr poisoning and the lack of clinical research on this pesticide, there is no specific antidote available. The diagnosis and treatment of this disease are often delayed. Chlorfenapyr poisoning is characterized by delayed toxicity, and its metabolites can reduce ATP production by inhibiting oxidative phosphorylation in mitochondria, thereby causing severe injury to energy-intensive organs, including the nervous system, muscular system, and cardiovascular system (Figure 2).[30] Chlorfenapyr poisoning has an incubation period of 1-2 weeks. After absorption, chlorfenapyr is slowly released into the blood, causing delayed toxic reactions.[31] Chlorfenapyr poisoning is highly lethal; most patients develop high fever and experience mental disturbance within 4-21 d.[32] Therefore, patients with chlorfenapyr poisoning should be closely monitored, and early symptoms should not be ignored. Almost all patients with severe chlorfenapyr poisoning show progressive and aggravated symptoms of nervous system injury, including fatigue, high fever, and an altered state of consciousness,[33,35] indicating that the brain is one of the primary target organs.[36]
Focal neurologic deficits have also been reported in patients with chlorfenapyr poisoning. Kwon et al[33] reported a case of lower limb weakness with hypoesthesia 2 weeks after oral administration of 10 mL of 10% chlorfenapyr, which progressed to lower limb paralysis 10 d later. Another patient exhibited blurred vision, difficult urinating, and incoherence 2 d after skin exposure to chlorfenapyr.[37]
High fever and disturbances in consciousness are characteristic clinical manifestations of chlorfenapyr poisoning. The physical and pharmacological cooling effects are usually unsuccessful, which may indicate ATP depletion.[38] Respiratory and circulatory failures occur quickly in these patients, requiring ventilator-assisted ventilation and high doses of vasoactive drugs.
Chlorfenapyr causes mitochondrial dysfunction, which leads to myocardial injury. The heart is an energy-intensive organ with distinct clinical manifestations. Patients may have an increased heart rate and arrhythmias as the disease progresses.[37,24] Li et al[39] reported acute myocardial injury caused by chlorfenapyr. Twelve-lead electrocardiography after oral adminstration of chlorfenapyr (unknown dosage) revealed extensive ST-T changes and ventricular arrythmia with troponin elevation, suggesting myocardial injury and involvement of the cardiac conduction system. Notably, cardiac arrest is the direct cause of death in patients with chlorfenapyr poisoning;[30,32] however, the specific mechanism of cardiac injury has not yet been explored.
It is common for digestive system to be injured following the absorption of chlorfenapyr through the digestive tract. Nausea, vomiting, abdominal pain, abdominal distension, and other manifestations occur 1-2 d after chlorfenapyr ingestion.[35,40] The severity of injury is dose-related. Patients with acute poisoning often develop liver injury. The main pathological changes associated with liver injury are hepatocyte hypertrophy and necrosis.[28] Ku et al[41] reported acute pancreatitis after chlorfenapyr poisoning. There was no biliary obstruction in this patient, and amylase and lipase levels suddenly and progressively increased, suggesting that acute pancreatitis may have been caused by the direct toxicity of chlorfenapyr and the cumulative toxicity of its metabolites.
Chlorfenapyr reduces energy production. Failure of the necessary energy supply leads to multiple organ failure and eventually death. Striated muscles are high-energy and oxygen-consuming organs. Most patients with chlorfenapyr poisoning have symptoms of rhabdomyolysis, including muscle soreness and fatigue.[40] Chlorfenapyr also causes kidney injury, and some patients may develop renal dysfunction and acute renal failure at later stages.[30]
Diagnosis
The diagnosis of chlorfenapyr poisoning requires knowing the exact amounts of toxicants and their metabolites. These can be achieved by testing vomit, excreta, serum, and residual poisons using gas chromatography-tandem mass spectrometry.[43] Chung et al[38] showed that low levels of serum chlorfenapyr were detected 4 h after poisoning but were not detected 4-7 d after poisoning. They also observed a delayed increase in serum CL303268 (tralopyril) levels, suggesting a greater correlation between metabolites and symptoms compared with serum chlorfenapyr.
Timely and effective laboratory and imaging examinations are helpful for determining disease severity and providing guidance for treatment measures. After analyzing the laboratory results of reported poisoning cases, we found that at the early stage of the disease (less than 6 d), the abnormalities were liver and kidney injuries (increased levels of blood urea nitrogen, creatinine, aspartate aminotransferase, and alanine aminotransferase), as well as elevated levels of myoglobin and creatine kinase. Some patients also exhibited increased levels of inflammatory markers, as well as electrolytes and acid-base balance disorders (e.g., hyperkalemia and metabolic acidosis). However, at the later stage (7-14 d), more signs of neurotoxicity and cardiotoxicity were shown, and they were more significant according to the imaging and electrocardiogram findings.[33,35,42]
Electroencephalography has been performed on patients with chlorfenapyr poisoning and has demonstrated widespread slow delta activity.[33] White matter lesions may also affect body temperature regulation.[44] Therefore, in the diagnosis and evaluation of chlorfenapyr poisoning, MRI is particularly important for determining the course of the disease and patient prognosis. Imaging studies have shown that there are extensive and symmetrical signal intensity abnormalities in the white matter of the brain and cerebellum, including the internal capsule, optic nerve, corpus callosum, corticospinal tract, and brainstem; in addition, spinal MRI shows diffuse swelling of the spinal cord.[34]
Hyperpyrexia-neurological syndrome, including Parkinson’s hyperpyrexia syndrome, neuro-blocker malignant syndrome, and 5-hydroxytryptamine syndrome, often manifests as high fever, fatigue, sweating, and altered mental status, among other symptoms.[45⇓⇓-48] This type of disorder can be identified based on specific medical and medication histories.
Toxic leukoencephalopathy is a disease characterized by structural changes in the white matter caused by various pathogenic factors, mainly myelin sheath injury. Clinical manifestations include inattention, forgetfulness, and personality changes that can progress to dementia, coma, and even death.[34] Moreover, imaging studies of chlorfenapyr poisoning should focus on the white matter tracts, which are helpful for differential diagnosis.[49]
Other poisons, like cyanide, aluminum phosphide, and sodium pentachlorophenol, inhibit mitochondrial oxygen metabolism through different pathways.[50,51] After chlorfenapyr poisoning, high-energy oxygen-consuming organs are injured, and respiratory circulatory failure occurs in severe cases,[52] similar to the poisoning of sodium pentachlorophenol, a mitochondrial phosphoric acid decoupling poison.[53] Patients with pesticide poisoning can be diagnosed and the poison itself can be identified based on the characteristic clinical manifestations.
Treatment strategies
Currently, there is a lack of effective antidotes for chlorfenapyr poisoning, leading to high mortality rates.
Early emesis, gastric lavage, and catharsis after poisoning are essential first steps. The effects of chlorfenapyr if taken orally are delayed.[30] Based on experimental data from rats, it is speculated that the apparent VD of chlorfenapyr is large and that sufficient hemoperfusion of tralopyril can effectively reduce the distribution of toxins. Multiple early hemoperfusions are beneficial for the long-term survival of patients with chlorfenapyr poisoning.[54] Sequential hemoperfusion through hemodialysis can also be used.[55] Relatively expensive blood transfusions have also been reported to clear chlorfenapyr; however, their efficacy has not yet been determined. In severe cases, hemoperfusion should be considered. For patients with delayed intoxication or hemodynamic instability, the purification method of continuous renal replacement therapy, which can have a better curative effect by extending the filtration time, should be considered.[36] Owing to the lack of data on the apparent VD, protein binding rate, and clearance rate of chlorfenapyr, clinicians should consider a more efficient blood purification method.
Creatine kinase and myoglobin levels can be used as monitoring indicators after poisoning for >3 weeks.[31] Early extracorporeal membrane oxygenation is important for reducing the risk of death from cardiac arrest in patients with high-dose intake or severe illness.[56] The application of excitatory substances to the central nervous system and exogenous ATP supplementation may be helpful in patients with chlorfenapyr poisoning.[57] Acetylcysteine antioxidants, the protection of mitochondria using coenzyme Q, the promotion of oxidative phosphorylation, vitamin supplementation, and glucocorticoids have all been studied for the treatment of chlorfenapyr poisoning.[24,30,35]
CONCLUSION
Chlorfenapyr is a pyrrole insecticide that is often mixed with other pesticides. Although it has been identified as a moderately toxic pesticide by the WHO, the mortality rate of poisoned patients is extremely high. Further research is needed to identify fast and efficient detoxification methods. High fever and consciousness disorders are featured clinical manifestations of chlorfenapyr poisoning. Respiratory failure occurs immediately, ultimately leading to death from cardiac arrest, which is difficult to prevent. To explore treatment options, we should focus on reconstructing intracellular oxidative phosphorylation coupling. Patients with chlorfenapyr poisoning should be closely monitored, and early symptoms should not be ignored. It is important to identify early biomarkers of chlorfenapyr poisoning to determine its prognosis. As there is no specific antidote for chlorfenapyr poisoning, we can consider developing new drugs that block the conversion of chlorfenapyr to tralopyril, reducing its toxic effects. We believe that further research will help clinicians make early diagnoses and treatments and eventually improve patient outcomes.
Funding: This study was supported by the Research Foundation of Ningbo No. 2 Hospital (2023HMKY49) and Ningbo Key Support Medical Discipline (2022-F16).
Ethical approval: Not needed.
Conflicts of interest: The authors do not have a financial interest or relationship to disclose regarding this research project.
Contributors: JC (Ji Cheng) and YLC (Yulu Chen) contributed equally to this work. JC, YLC, and LWD conceived the study concept and design. JC and YLC was involved in the drafting and critical revision of the manuscript. All authors contributed to the design and interpretation of the study and to further drafts.
All the supplementary files in this paper are available at http://wjem.com.cn.
Reference
Suicide, suicide attempts and pesticides: a major hidden public health problem
Malignant hyperthermia-like syndrome in acute chlorfenapyr poisoning - a case report
Mechanism and pathways of chlorfenapyr photocatalytic degradation in aqueous suspension of TiO2
Chlorfenapyr: a pyrrole insecticide for the control of pyrethroid or DDT resistant Anopheles gambiae (Diptera: Culicidae) mosquitoes
Chlorfenapyr, a potent alternative insecticide of phoxim to control Bradysia odoriphaga (Diptera: Sciaridae)
Green nano-phytoremediation and solubility improving agents for the remediation of chlorfenapyr contaminated soil and water
Dissipation behaviour, residue analysis, and dietary safety evaluation of chlorfenapyr on various vegetables in China
Effect of chlorfenapyr on cypermethrin-resistant culex pipiens pallens Coq mosquitoes
DOI:10.1016/j.actatropica.2014.12.002
PMID:25497774
[Cited within: 1]

Chlorfenapyr is a promising pyrrole insecticide with a unique mechanism of action that does not confer cross-resistance to neurotoxic insecticides. The effect of chlorfenapyr on pyrethorid-resistant Culex pipiens pallens Coq (Diptera: Culicidae) has not been fully investigated under laboratory conditions. In this study, cypermethrin-resistant C. p. pallens exhibited 376.79-fold and 395.40-fold increase in resistance to cypermethrin compared with susceptible strains after exposure for 24 and 48h, respectively. Larvae and adults were tested for susceptibility using dipping, topical, and impregnated paper methods as recommended by the WHO. No cross-resistance to chlorfenapyr was found. Increased mortality was apparent between 48 and 72h, indicating a slow rate of toxic activity. Synergism experiments with piperonyl butoxide (PBO) showed an antagonistic effect on chlorfenapyr toxicity. Mixtures of chlorfenapyr and cypermethrin could therefore provide additional benefits over either insecticide used alone. Mixtures of 5ng/ml chlorfenapyr and 500ng/ml cypermethrin exhibited a slight synergistic effect on cypermethrin-resistant mosquitoes (3.33, 6.84 and 2.34% after 24, 48 and 72h exposure, respectively. This activity was lost when the chlorfenapyr concentration was increased to 10 or 20ng/ml. Chlorfenapyr showed quite good results for pyrethroid-resistant C. p. pallens, and could improve public health by reducing the occurrence of mosquito bites and subsequently protecting against transmission of lymphatic filariasis and Japanese encephalitis. Copyright © 2014 Elsevier B.V. All rights reserved.
Tralopyril affects locomotor activity of zebrafish (Danio rerio) by impairing tail muscle tissue, the nervous system, and energy metabolism
LC-MS/MS determination of tralopyril in water samples
DOI:10.1016/j.chemosphere.2015.11.098
PMID:26694794
[Cited within: 1]

A targeted analytical method was established to determine tralopyril (4-bromo-2-(4-chlorophenyl)-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile) in water. This compound has been recently introduced as a biocide in ship antifouling paints, becoming a potential new environmental contaminant. The method presented here allows for the first time the direct determination of tralopyril in environmental samples without the need of a pre-concentration step. The injected sample is separated by a 30 min HPLC-gradient on a reversed phase column and the compound identified and quantified by negative ion LC-MS/MS. Tralopyril solutions in DMSO, seawater, river Glatt water and E3 medium (used for zebrafish experiments) were analysed to demonstrate the applicability of the method. The method provides good retention time reproducibility and a quantitation limit (LOQ) of 0.025 μg L(-1) for DMSO, seawater and E3 exposure medium and 0.05 μg L(-1) for river Glatt water. Calculated tralopyril half-lives were 6.1 h for seawater, 8.1 h for river Glatt water and 7.4 h for E3 medium at 18 °C.Copyright © 2015 Elsevier Ltd. All rights reserved.
Impact of tralopyril and triazolyl glycosylated chalcone in human retinal cells’ lipidome
Environmentally relevant concentrations of tralopyril affect carbohydrate metabolism and lipid metabolism of zebrafish (Danio rerio) by disrupting mitochondrial function
Long-term monitoring and characterization of resistance to chlorfenapyr in Plutella xylostella (Lepidoptera: Plutellidae) from China
Influence of pyranose and spacer arm structures on phloem mobility and insecticidal activity of new tralopyril derivatives
Acute toxicity of tralopyril, capsaicin and triphenylborane pyridine to marine invertebrates
DOI:10.1007/s10646-014-1276-9
PMID:24994544
[Cited within: 1]

A need for environmentally acceptable alternative antifouling (AF) biocides has arisen through restrictions in the use of many common biocides in the European Union through the Biocidal Product Regulation (Regulation EU No. 528/2012). Three such alternatives are triphenylborane pyridine (TPBP), tralopyril and capsaicin. This study aims at extending the available information on the toxicity of these three emerging AF biocides to key marine invertebrates. Here we investigate the toxicity of tralopyril and capsaicin to the early life stages of the mussel Mytilus galloprovincialis and the sea urchin Paracentrotus lividus and also of tralopyril, capsaicin and TPBP to the early life stages of the copepod Tisbe battagliai. The EC50 that causes abnormal development of mussel's D-veliger larvae and impairs the growth of sea urchin pluteus larvae are respectively 3.1 and 3.0 μg/L for tralopyril and 3,868 and 5,248 μg/L for capsaicin. Regarding the copepod T. battagliai, the LC50 was 0.9 μg/L for tralopyril, 1,252 μg/L for capsaicin and 14 μg/L for TPBP. The results obtained for the three substances are compared to a reference AF biocide, tributyltin (TBT), and their ecological risk evaluated. These compounds pose a lower environmental risk than TBT but still, our results suggest that tralopyril and TPBP may represent a considerable threat to the ecosystems.
Economic impact of biofouling on a naval surface ship
DOI:10.1080/08927014.2010.542809
PMID:21161774
[Cited within: 1]

In the present study, the overall economic impact of hull fouling on a mid-sized naval surface ship (Arleigh Burke-class destroyer DDG-51) has been analyzed. A range of costs associated with hull fouling was examined, including expenditures for fuel, hull coatings, hull coating application and removal, and hull cleaning. The results indicate that the primary cost associated with fouling is due to increased fuel consumption attributable to increased frictional drag. The costs related to hull cleaning and painting are much lower than the fuel costs. The overall cost associated with hull fouling for the Navy's present coating, cleaning, and fouling level is estimated to be $56M per year for the entire DDG-51 class or $1B over 15 years. The results of this study provide guidance as to the amount of money that can be reasonably spent for research, development, acquisition, and implementation of new technologies or management strategies to combat hull fouling.
AC 303,630—an insecticide/acaricide from a novel class of chemistry
A comprehensive review of the current knowledge of chlorfenapyr: synthesis, mode of action, resistance, and environmental toxicology
AC 303,630—summary of 1988-89 field trial results
Survival after chlorfenapyr intoxication by non-gastrointestinal route
Toxicity from intra-abdominal injection of chlorfenapyr
Delayed hyperthermia from chlorfenapyr overdose
Chlorfenapyr: a new insecticide with novel mode of action can control pyrethroid resistant malaria vectors
Bioaccumulation, metabolism and the toxic effects of chlorfenapyr in zebrafish (Danio rerio)
Tralopyril induces developmental toxicity in zebrafish embryo (Danio rerio) by disrupting the thyroid system and metabolism
AIDA directly connects sympathetic innervation to adaptive thermogenesis by UCP1
DOI:10.1038/s41556-021-00642-9
PMID:33664495
[Cited within: 1]

The sympathetic nervous system-catecholamine-uncoupling protein 1 (UCP1) axis plays an essential role in non-shivering adaptive thermogenesis. However, whether there exists a direct effector that physically connects catecholamine signalling to UCP1 in response to acute cold is unknown. Here we report that outer mitochondrial membrane-located AIDA is phosphorylated at S161 by the catecholamine-activated protein kinase A (PKA). Phosphorylated AIDA translocates to the intermembrane space, where it binds to and activates the uncoupling activity of UCP1 by promoting cysteine oxidation of UCP1. Adipocyte-specific depletion of AIDA abrogates UCP1-dependent thermogenesis, resulting in hypothermia during acute cold exposure. Re-expression of S161A-AIDA, unlike wild-type AIDA, fails to restore the acute cold response in Aida-knockout mice. The PKA-AIDA-UCP1 axis is highly conserved in mammals, including hibernators. Denervation of the sympathetic postganglionic fibres abolishes cold-induced AIDA-dependent thermogenesis. These findings uncover a direct mechanistic link between sympathetic input and UCP1-mediated adaptive thermogenesis.
A fatal case of chlorfenapyr poisoning and a review of the literature
Cytotoxic and genotoxic effects of abamectin, chlorfenapyr, and imidacloprid on CHOK1 cells
Who is the real killer? Chlorfenapyr or detergent micelle-chlorfenapyr complex?
DOI:10.1080/00498254.2016.1236300
PMID:27616623
[Cited within: 2]

1. Chlorfenapyr [4-bromo-2-(4-chlorophenyl)-1-(ethoxymethl)-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile] is a commonly employed pesticide throughout the world. The mechanism of chlorfenapyr action is to uncouple oxidative phosphorylation in the mitochondria. The characteristic features of chlorfenapyr intoxication are high fever, rhabdomyolysis and neurologic symptoms that gradually get worse until death. 2. In recent years, suicide attempt cases using commercial chlorfenapyr pesticide were reported. Even small doses of commercial chlorfenapyr pesticide intoxication caused human fatality. However, world health organization (WHO) has classified chlorfenapyr as class 2-moderately hazardous chemical. Animal studies using technical grade (94.5%; AC 7504-59A) chlorfenapyr in 0.5% carboxy methyl cellulose as the vehicle, single dose through oral route in male rats were well tolerated. 3. We planned a therapeutic strategy for suicidal chlorfenapyr intoxication, therefore we evaluated the three different toxic doses of chlorfenapyr (10% chlorfenapyr and 90% detergent) through oral route in male rats for human extrapolation. The major difference between the technical grade chlorfenapyr and commercial grade chlorfenapyr was the vehicle. In the technical grade chlorfenapyr study, 0.5% carboxy methyl cellulose was used as a vehicle, whereas in the present study 90% detergent acted as a vehicle. The LD50 of commercial grade chlorfenapyr-40.63 mg/kg bw, which was approximately tenfold decrease than technical grade chlorfenapyr, LD50 - 441 mg/kg bw. 4. The combination of chlorfenapyr and detergent, a deadly cocktail to form micelle complex that can greatly influence bioavailability by attaching to biological membranes in vivo. To conclude, the enhanced bioavailability of chlorfenapyr by the detergent causes the fatality in suicidal attempts using chlorfenapyr.
A case of chlorfenapyr intoxication with central nervous system involvement
Chlorfenapyr-induced toxic leukoencephalopathy with radiologic reversibility: a case report and literature review
A patient fatality following the ingestion of a small amount of chlorfenapyr
DOI:10.4103/0974-2700.136874
PMID:25114438
[Cited within: 4]

Chlorfenapyr has been used worldwide for agricultural pest control since 1995. Despite its widespread use, acute human poisoning data are insufficient; only a small number of fatalities from chlorfenapyr poisoning have been reported. The signs and symptoms of chlorfenapyr toxicity include nausea, vomiting, fever, rhabdomyolysis, among others. In addition, central nervous system effects in association with delayed toxicity have also been observed. Here, we detail a fatality resulting from delayed chlorfenapyr toxicity following the ingestion of a small amount of pesticide.
Experience in the treatment of chlorfenapyr poisoning
DOI:10.2131/jts.48.221
PMID:37005280
[Cited within: 2]

In China, the extensive use of the pesticide chlorfenapyr has led to an increase in chlorfenapyr poisoning. However, there are limited reports on chlorfenapyr poisoning, and most of them are fatal cases. This study retrospectively analyzed four patients admitted to the emergency room after chlorfenapyr intake and detected different concentrations of chlorfenapyr in their plasma. Among them, one patient died and three patients survived. Case 1 suffered respiratory and circulatory failure with a deep coma shortly after oral administration of 100 mL of a the chlorfenapyr-containing mixture and died 30 min after admission. Case 2 experienced transient nausea and vomiting after oral administration of chlorfenapyr (50 mL). The patient had normal laboratory results and was discharged with no further treatment. Case 3 developed nausea and vomiting and a light coma after taking 30 mL of chlorfenapyr orally. He underwent blood perfusion and plasma exchange in the intensive care unit (ICU) and was discharged with recovery. A two-week follow-up visit, however, revealed hyperhidrosis. Case 4 (advanced age with severe underlying disease) developed a light coma after oral intake of 30 mL of chlorfenapyr. Subsequently, pulmonary infection and gastrointestinal bleeding were developed. The patient experienced blood perfusion and mechanical ventilation in the ICU and finally survived after treatment. The present study provides the basic information, plasma concentration of toxins, onset of poisoning and treatment process of the four patients mentioned above, providing novel insights into the clinical diagnosis and treatment of chlorfenapyr poisoning.
A fatal case of chlorfenapyr poisoning following dermal exposure
A fatal case of chlorfenapyr poisoning and the therapeutic implications of serum chlorfenapyr and tralopyril levels
A case of acute myocardial injury combined with sinus arrest induced by imidacloprid poisoning
Fatality from acute chlorfenapyr poisoning
DOI:10.3109/15563651003750074 PMID:20586574 [Cited within: 2]
A case of survival after chlorfenapyr intoxication with acute pancreatitis
PMID:27752575
[Cited within: 1]

Chlorfenapyr is a moderately hazardous insecticide. There have been previous reports of chlorfenapyr intoxication, but none have reported patient survival or an association with pancreatitis. A 61-year-old woman was brought to the emergency department with vomiting after ingesting 10 mL chlorfenapyr in a suicide attempt 1 hour before. The patient was treated with gastric lavage and activated charcoal, then transferred to the intensive care unit. Initial laboratory data were unremarkable except for elevated amylase/lipase levels (134/222 U/L), which were even higher 7 days later and remained elevated for 2 weeks. Abdominal computed tomography showed diffuse pancreatic swelling. The patient improved with conservative care and was discharged to home 19 days after admission. This is the first reported case of survival after chlorfenapyr intoxication. We recommend early aggressive management in the emergency department and close monitoring in the intensive care unit to detect and treat potentially fatal deterioration after chlorfenapyr intoxication.
Determination of market, field samples, and dietary risk assessment of chlorfenapyr and tralopyril in 16 crops
Evaluation of a commercial immunoassay for the detection of chlorfenapyr in agricultural samples by comparison with gas chromatography and mass spectrometric detection
PMID:15941050
[Cited within: 1]

A commercially available enzyme-linked immunosorbent assay (ELISA) kit with a high affinity monoclonal antibody was applied to residual analysis of insecticide chlorfenapyr in agricultural samples, and drawn a parallel between the ELISA and gas chromatography (GC) with mass spectrometry (MS). For standards prepared in water containing 5% (v/v) methanol, the sensitivity (I50 value), the dynamic range, and the limit of detection of the ELISA kit were 2.3, 1 - 10, and 0.1 ng/g, respectively. The used monoclonal antibody in the ELISA kit had a high selectivity. The ELISA kit was applied to the determination of chlorfenapyr in two kinds of fruits (apple and peach). The examination of the influence of these matrices on the reliability of the assay performance indicated that the ELISA could determine it in these samples near the regulation values in Japan simply by diluting the methanolic extract or by concentrating it, without any clean-up procedures. Recovery and precision of the proposed ELISA method were assessed by fortifying fruit samples with chlorfenapyr ranging from 0.05 to 1.5 microg/g. Mean recoveries were 94.2 and 90.3% for apple and peach, and coefficients of variation were below 16% in most cases. The results obtained from the proposed ELISA method correlate well the reference GC/MS method for both fruit samples (r > 0.98). These considerations make the ELISA kit very useful analytical tool for monitoring and regulatory programs, without the need of complex and expensive instrumentation.
Autonomic dysfunction and white matter microstructural changes in drug-naïve patients with Parkinson’s disease
Parkinsonism-hyperpyrexia syndrome: a case series and literature review
Neuroleptic malignant syndrome associated with acute organophosphate poisoning: case report
Dyskinesia-hyperpyrexia syndrome triggered by overdose of istradefylline: a case report
Parkinsonism-hyperpyrexia syndrome and dyskinesia-hyperpyrexia syndrome in Parkinson’s disease: two cases and literature review
A review on ingested cyanide: risks, clinical presentation, diagnostics, and treatment challenges
DOI:10.1007/s13181-018-0688-y
PMID:30539383
[Cited within: 1]

Cyanide, a metabolic poison, is a rising chemial threat and ingestion is the most common route of exposure. Terrorist organizations have threatened to attack the USA and international food and water supplies. The toxicokinetics and toxicodynamics of oral cyanide are unique, resulting in high-dose exposures, severe symptoms, and slower onset of symptoms. There are no FDA-approved therapies tested for oral cyanide ingestions and no approved intramuscular or oral therapies, which would be valuable in mass casualty settings. The aim of this review is to evaluate the risks of oral cyanide and its unique toxicokinetics, as well as address the lack of available rapid diagnostics and treatments for mass casualty events. We will also review current strategies for developing new therapies. A review of the literature using the PRISMA checklist detected 7284 articles, screened 1091, and included 59 articles or other reports. Articles referenced in this review were specific to risk, clinical presentation, diagnostics, current treatments, and developing therapies. Current diagnostics of cyanide exposure can take hours or days, which can delay treatment. Moreover, current therapies for cyanide poisoning are administered intravenously and are not specifically tested for oral exposures, which can result in higher cyanide doses and unique toxicodynamics. New therapies developed for oral cyanide exposures that are easily delivered, safe, and can be administered quickly by first responders in a mass casualty event are needed. Current research is aimed at identifying an antidote that is safe, effective, easy to administer, and has a rapid onset of action.
Aluminum phosphide poisoning: an unsolved riddle
DOI:10.1002/jat.1692
PMID:21607993
[Cited within: 1]

Aluminum phosphide (ALP), a widely used insecticide and rodenticide, is also infamous for the mortality and morbidity it causes in ALP-poisoned individuals. The toxicity of metal phosphides is due to phosphine liberated when ingested phosphides come into contact with gut fluids. ALP poisoning is lethal, having a mortality rate in excess of 70%. Circulatory failure and severe hypotension are common features of ALP poisoning and frequent cause of death. Severe poisoning also has the potential to induce multi-organ failure. The exact site or mechanism of its action has not been proved in humans. Rather than targeting a single organ to cause gross damage, ALP seems to work at the cellular level, resulting in widespread damage leading to multiorgan dysfunction (MOD) and death. There has been proof in vitro that phosphine inhibits cytochrome c oxidase. However, it is unlikely that this interaction is the primary cause of its toxicity. Mitochondria could be the possible site of maximum damage in ALP poisoning, resulting in low ATP production followed by metabolic shutdown and MOD; also, owing to impairment in electron flow, there could be free radical generation and damage, again producing MOD. Evidence of reactive oxygen species-induced toxicity owing to ALP has been observed in insects and rats. A similar mechanism could also play a role in humans and contribute to the missing link in the pathogenesis of ALP toxicity. There is no specific antidote for ALP poisoning and supportive measures are all that are currently available.Copyright © 2011 John Wiley & Sons, Ltd.
The two faces of cyanide: an environmental toxin and a potential novel mammalian gasotransmitter
Multifaceted effects of subchronic exposure to chlorfenapyr in mice: implications from serum metabolomics, hepatic oxidative stress, and intestinal homeostasis
Study on the toxic effects of sodium pentachlorophenol (PCP-Na) on razor clam (Sinonovacula constricta)
Study on clearance of chlorfenapyr via blood purification (a case analysis)
The role of continuous renal replacement therapy in the treatment of poisoning
The crashing toxicology patient
Synergistic modes of interaction between the plant essential oils and the respiratory blocker chlorfenapyr