Sepsis is a lethal condition characterized by multiple organ dysfunction due to disrupted host responses to severe infections.[1] Affected patients often have a Sequential Organ Failure Assessment (SOFA) score ≥2.[2] Patients with a SOFA score < 2 and at least one of the following were considered as “suspected sepsis”: (1) quick SOFA (qSOFA) score ≥2; (2) SOFA score = 1; or (3) National Early Warning Score (NEWS) 4-6.[3] Compared with studies on fluid resuscitation in sepsis patients, there are few studies on fluid management in patients with suspected sepsis. Therefore, we conducted a retrospective cohort study to evaluate the relationship between fluid management and disease progression in suspected sepsis patients.
METHODS
Study design and patient selection
The present work is a retrospective cohort study on suspected sepsis patients included in the Multi-parameter Intelligent Monitoring in Intensive Care IV (MIMIC-IV1.0) database. Adult patients (>18 years old) diagnosed with suspected sepsis and admitted to the intensive care unit (ICU) were included. Patients with a length of ICU stay of less than 48 h or with missing information regarding fluid management were excluded from the present study. In patients with multiple ICU admissions during one hospital stay, only the first admission was counted.
Database access and data collection
The MIMIC-IV database was reviewed to retrieve the following information: demographics, clinical comorbidities, SOFA score, qSOFA score, Simplified Acute Physiology Score II (SAPS II), NEWS, first-day laboratory results, first-day vital signs, fluid intake, fluid output, fluid balance, urine output, blood product transfusion, in-hospital mortality, acute kidney injury (AKI), and length of hospital and ICU stay. The worst SOFA, qSOFA, and NEWS values collected within 24 h after admission were used to identify patients with or without sepsis. Patients with the worst SOFA score < 2 were selected for further screening for suspected sepsis based on qSOFA, SOFA, and NEWS scores as described before.
Definitions and diagnosis
Suspected infection was defined as the acquisition of a body fluid culture temporally contiguous to the antibiotic administration after ICU admission.[4] The comorbidities analyzed in this study were determined based on the International Classification of Diseases 9 or 10 coding algorithms for Charlson comorbidity.[5] Fluid management data were extracted from tables called “input events” and “output events” in the MIMIC-IV database.
Group assignment and outcome measurements
First, patients were assigned to either the stable group or the deterioration group according to the highest SOFA score between 24 h and 72 h after ICU admission to identify the association between fluid management and disease deterioration. Specifically, patients with the highest SOFA score≥2 (within 72 h after admission to the ICU) were included in the deterioration group; otherwise, they were included in the stable group. Then, patients were assigned into different groups according to their levels of fluid management.
The primary outcome was disease deterioration into sepsis within 72 h after ICU admission. The secondary outcomes included in-hospital mortality, length of ICU stay, length of hospital stay, and incidence of AKI.
Statistical analysis
All statistical analyses were conducted using the R software package (version 4.1.2, R Foundation for Statistical Computing, Austria). A two-sided P<0.05 was considered statistically significant. Continuous data are presented as the mean with standard deviation (SD) or median with interquartile range (IQR). Categorical data are presented as proportions. Two groups were compared with Student’s t-test, Wilcoxon rank-sum test, and Pearson’s Chi-square test, as appropriate. Univariable logistic regression was used to identify covariates associated with disease deterioration. In multivariate logistic regression, a stepwise backward elimination method was used to keep the variables with a P≤0.2 until all variables that remained in the model were clinically and statistically significant. The fit of this final model was tested by the partial likelihood ratio test.[6] Patients were assigned into different groups according to fluid management (the cutoff values of fluid management were chosen as the inflection point of restricted cubic spline [RCS] functions). Propensity score matching (PSM) was used to adjust the baseline characteristic differences between different volumes of fluid management. After PSM, standardized mean differences (SMDs) were used to evaluate the balance of characteristics between the two groups (variables used to balance characteristics between groups and SMDs are shown in supplementary Table 1). A variable with SMD greater than 0.1 would be viewed as imbalanced.
RESULTS
A total of 1,285 patients were included in the analysis. The flowchart of patient enrollment is shown in supplementary Figure 1. Patients were assigned into two groups, with 745 (58%) in the stable group and 540 (42%) in the deterioration group. Differences in the baseline characteristics and clinical outcomes between the two groups are shown in supplementary Table 2.
Relationship between baseline variables and disease progression
The association between the variables and disease progression was explored by RCS function, as shown in supplementary Figure 2. Platelet count, blood urine nitrogen, systolic blood pressure, fluid intake within 48 h, urine output within 48 h and fluid balance within 48 h had linearity Wald tests of P<0.05 and were transformed using the linear spline functions, with the relative cutoff values shown in supplementary Table 3.
Factors associated with disease progression
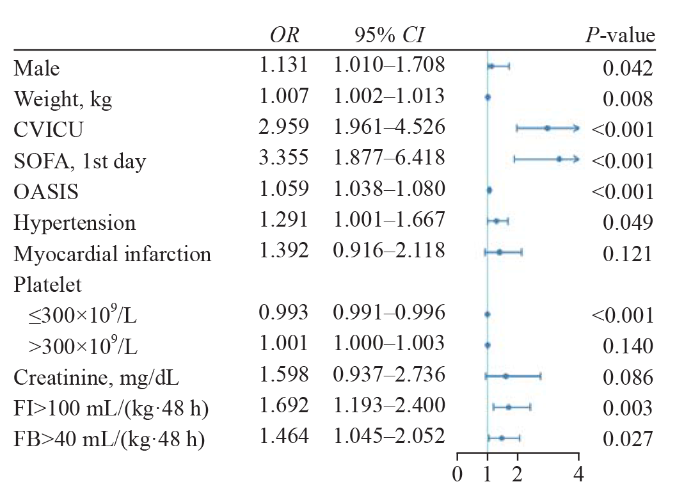
Univariate logistic regression was used to identify factors associated with disease deterioration (supplementary Table 4). The results from multivariate logistic regression showed that within 24 h after ICU admission, fluid intake and fluid balance were not significantly associated with disease progression after adjusting for confounding factors (supplementary Table 5). Within 48 h after ICU admission, compared with low fluid intake (≤100 mL/[kg·48 h]), high fluid intake (>100 mL/[kg·48 h]) was significantly associated with disease progression (adjusted odds ratio [OR] 1.692, 95% confidence interval [95% CI] 1.913-2.400, P=0.003). Compared with low fluid balance (≤40 mL/[kg·48 h]), high fluid balance (>40 mL/[kg·48 h]) was significantly associated with disease progression (OR 1.464, 95% CI 1.045-2.052, P=0.027) (Figure 1 and supplementary Table 6).
Figure 1.
Figure 1.
Indicators associated with disease deterioration using multivariable logistic regression. CVICU: cardiovascular intensive care unit; FB: fluid balance; FI: fluid intake; OASIS: Oxford Acute Severity of Illness Score; SOFA: Sequential Organ Failure Assessment.
Fluid intake and clinical outcomes before and after PSM
According to the fluid intake within 48 h after ICU admission, the patients were further assigned into two groups: the low fluid intake group (≤100 mL/[kg·48 h], n=772) and the high fluid intake group (>100 mL/[kg·48 h], n=513). The characteristics of these two groups are reflected in supplementary Table 7. After the 1:1 PSM, each group had 376 patients. The high fluid intake group still had significantly higher incidences of disease progression (50.0% vs. 35.4%, P<0.001) and longer hospital stay (8.1 d vs. 7.1 d, P=0.001) than the low fluid intake group. No differences were detected in in-hospital mortality, the incidence of AKI, or the length of ICU stay between groups (supplementary Table 8).
Fluid balance and clinical outcomes before and after PSM
According to the fluid balance within 48 h after ICU admission, the patients were assigned into two groups: the low fluid balance group (≤40 mL/[kg·48 h], n=734) and the high fluid balance group (>40 mL/[kg·48 h], n=551). The characteristics of these two groups can be found in supplementary Table 9. After PSM, patients in the high fluid balance group had a significantly higher incidence of disease deterioration (51.9% vs. 34.0%, P<0.001), AKI (57.9% vs. 47.0%, P=0.001), and in-hospital mortality (6.3% vs. 2.7%, P=0.015), as well as a longer hospital stay (8.3 d vs. 7.3 d, P<0.001) than those in the low fluid balance group (supplementary Table 10).
DISCUSSION
In this study, we report that fluid management within 48 h after ICU admission is significantly associated with clinical outcomes in suspected sepsis patients. Specifically, after PSM, we found that the high fluid balance group was more likely to have disease deterioration, AKI, and higher in-hospital mortality, as well as a longer hospital stay when compared with the low fluid balance group.
Suspected sepsis could be considered a transitional stage from a non-complicated infection to sepsis.[7] During suspected sepsis, the clinical presentations of organ dysfunction and blood perfusion impairment caused by the infection may not occur, but the underlying pathophysiological changes can progress. This critical transition stage has not attracted adequate attention from physicians.
Fluid therapy is an essential treatment for critically ill patients[8] and one of the most popular research topics in sepsis. However, excess fluid administration can cause fluid overload, which is associated with unfavorable conditions.[9,10] There is no widely accepted definition of fluid overload for critically ill patients. Our study found that fluid management (within 48 h after ICU admission) was associated with disease deterioration and clinical outcomes. The exact causal relationship between fluid management and clinical outcomes is not clear. There exists a possibility that fluid overload can exacerbate clinical conditions and push patients from suspected sepsis into sepsis. It can also be interpreted that patients with more severe conditions were more likely to transition from suspected sepsis to sepsis, and they needed greater fluid administration. Nevertheless, our results might indicate the potential clinical application of fluid balance evaluation to predict the outcomes of suspected sepsis patients.
In the early years of clinical practice, the resuscitation targets of sepsis were to rapidly achieve adequate oxygen delivery by augmenting cardiac output to meet the demand of the patients, which paid little attention to the potential damage from excessive fluid administration.[11] Most physicians considered a state of “constant dehydration” or “in need of fluid” in severely infected patients. These severe septic patients commonly received a large volume of intravenous fluid. In our clinical practice, we have noticed a potential association between unfavorable outcomes and fluid overload. Therefore, we tested whether a “U” shaped model can explain the relationship between the infused fluid volume and clinical outcomes in septic patients. However, in the present study, we did not detect such a pattern using RCS function analysis. Our results showed an elevated risk of disease deterioration with increased fluid intake. The same relationship was also observed between fluid balance and disease deterioration (at 24 h and 48 h, supplementary Figure 2). Therefore, fluid interventions, even those initially considered to be essential to save lives, may cause adverse reactions,[12] such as edema,[13] dilutional coagulopathy,[14] and multiple organ dysfunction.[15] It was reported that even the “physiological” range of central venous pressure could be more harmful than beneficial in critically ill patients.[16]
Nevertheless, we believe that there is no “one size fits all” during fluid resuscitation in patients with sepsis. “No harm” should be the first criterion guiding clinical practice. Our results might prompt clinicians to pay closer attention to the volume of fluid infusion during the resuscitation of suspected sepsis patients.
Limitations
Our study lacked a standardized definition of suspected sepsis. The study population represented patients admitted to the ICU, which could limit the generalizability of the results. To obtain adequate information for analysis, we also excluded patients with a length of ICU stay of less than 48 h. This could exclude patients who had rapid deterioration and death or improvement and discharge from the ICU within 48 h. Future prospective clinical trials are needed to confirm our results and further investigate the relationship between the volume of fluid resuscitations and patient outcomes. Although we identified the associations between fluid management and short-term outcomes in suspected sepsis patients, the causal relationships between fluid management and clinical outcomes need further investigation.
CONCLUSION
In suspected sepsis patients admitted to the ICU, a high volume of fluid intake may be associated with disease deterioration and longer hospitalization. A higher volume of fluid balance may be associated with increased risks of disease deterioration, AKI, in-hospital mortality, and longer hospitalization.
Funding: None.
Ethical approval: The right of this database was approved by the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA) and consent was obtained for the original data collection. Patients’ information in the MIMIC-IV database was anonymized; therefore, informed consent was not required.
Conflicts of interest: The authors have no financial or other conflicts of interest regarding this article.
Contributors: MB and ZHW contributed equally to this work. MB proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
All the supplementary files in this paper are available at http://wjem.com.cn.
Reference
Intestinal microcirculation dysfunction in sepsis: pathophysiology, clinical monitoring, and therapeutic interventions
DOI:10.5847/wjem.j.1920-8642.2022.031
PMID:36119779
[Cited within: 1]

Intestinal microcirculation dysfunction is an important factor that causes poor prognosis in sepsis patients and is an important pathophysiological basis for the occurrence and development of sepsis.PubMed, Web of Science, and China National Knowledge Infrastructure (CNKI) were searched from inception to August 1, 2021. The search was limited to the English language only. Two reviewers independently identified studies related to intestinal microcirculation dysfunction in sepsis. Exclusion criteria were duplicate articles according to multiple search criteria.Fifty articles were included, and most of them were animal studies. These studies reported pathogenesis, including endothelial dysfunction, leukocyte recruitment and adhesion, microthrombus formation, microcirculation hypoperfusion, and redistribution of intestinal wall blood flow. The monitoring methods of intestinal microcirculation were also diverse, including handheld microscopes, intravital microscopy (IVM), laser Doppler blood flow instruments, laser speckle contrast imaging, tissue reflectance spectrophotometry, biochemical markers of intestinal ischemia, and histopathological examination. In view of the related pathogenesis of intestinal microcirculation disorder in sepsis, existing studies also have different opinions on its treatment.Limited by monitoring, there are few clinical studies on intestinal microcirculation dysfunction in sepsis. Related research mainly focuses on basic research, but some progress has also been made. Therefore, this review may provide a reference for future research on intestinal microcirculation dysfunction in sepsis.Copyright: © World Journal of Emergency Medicine.
Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021
DOI:10.1007/s00134-021-06506-y PMID:34599691 [Cited within: 1]
New understanding of prevention and treatment of adult sepsis in emergency department - interpretation of early care of adults with suspected sepsis in the emergency department and out-of-hospital environment: a consensus-based task issued by American College of Emergency Physicians
A comparative analysis of sepsis identification methods in an electronic database
Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data
DOI:10.1097/01.mlr.0000182534.19832.83
PMID:16224307
[Cited within: 1]

Implementation of the International Statistical Classification of Disease and Related Health Problems, 10th Revision (ICD-10) coding system presents challenges for using administrative data. Recognizing this, we conducted a multistep process to develop ICD-10 coding algorithms to define Charlson and Elixhauser comorbidities in administrative data and assess the performance of the resulting algorithms.ICD-10 coding algorithms were developed by "translation" of the ICD-9-CM codes constituting Deyo's (for Charlson comorbidities) and Elixhauser's coding algorithms and by physicians' assessment of the face-validity of selected ICD-10 codes. The process of carefully developing ICD-10 algorithms also produced modified and enhanced ICD-9-CM coding algorithms for the Charlson and Elixhauser comorbidities. We then used data on in-patients aged 18 years and older in ICD-9-CM and ICD-10 administrative hospital discharge data from a Canadian health region to assess the comorbidity frequencies and mortality prediction achieved by the original ICD-9-CM algorithms, the enhanced ICD-9-CM algorithms, and the new ICD-10 coding algorithms.Among 56,585 patients in the ICD-9-CM data and 58,805 patients in the ICD-10 data, frequencies of the 17 Charlson comorbidities and the 30 Elixhauser comorbidities remained generally similar across algorithms. The new ICD-10 and enhanced ICD-9-CM coding algorithms either matched or outperformed the original Deyo and Elixhauser ICD-9-CM coding algorithms in predicting in-hospital mortality. The C-statistic was 0.842 for Deyo's ICD-9-CM coding algorithm, 0.860 for the ICD-10 coding algorithm, and 0.859 for the enhanced ICD-9-CM coding algorithm, 0.868 for the original Elixhauser ICD-9-CM coding algorithm, 0.870 for the ICD-10 coding algorithm and 0.878 for the enhanced ICD-9-CM coding algorithm.These newly developed ICD-10 and ICD-9-CM comorbidity coding algorithms produce similar estimates of comorbidity prevalence in administrative data, and may outperform existing ICD-9-CM coding algorithms.
Variable selection with stepwise and best subset approaches
DOI:10.21037/atm.2016.03.35
PMID:27162786
[Cited within: 1]

While purposeful selection is performed partly by software and partly by hand, the stepwise and best subset approaches are automatically performed by software. Two R functions stepAIC() and bestglm() are well designed for stepwise and best subset regression, respectively. The stepAIC() function begins with a full or null model, and methods for stepwise regression can be specified in the direction argument with character values "forward", "backward" and "both". The bestglm() function begins with a data frame containing explanatory variables and response variables. The response variable should be in the last column. Varieties of goodness-of-fit criteria can be specified in the IC argument. The Bayesian information criterion (BIC) usually results in more parsimonious model than the Akaike information criterion.
Pre-sepsis: A necessary concept to complete the Sepsis-3 picture?
DOI:S0883-9441(17)31693-3 PMID:29121586 [Cited within: 1]
Hypertonic saline in critical illness - A systematic review
DOI:S0883-9441(17)30226-5
PMID:28746899
[Cited within: 1]

The optimal approach to fluid management in critically ill patients is highly debated. Fluid resuscitation using hypertonic saline was used in the past for more than thirty years, but has recently disappeared from clinical practice. Here we provide an overview on the currently available literature on effects of hypertonic saline infusion for fluid resuscitation in the critically ill.Systematic analysis of reports of clinical trials comparing effects of hypertonic saline as resuscitation fluid to other available crystalloid solutions. A literature search of MEDLINE and the Cochrane Controlled Clinical trials register (CENTRAL) was conducted to identify suitable studies.The applied search strategy produced 2284 potential publications. After eliminating doubles, 855 titles and abstracts were screened and 40 references retrieved for full text analysis. At total of 25 scientific studies meet the prespecified inclusion criteria for this study.Fluid resuscitation using hypertonic saline results in volume expansion and less total infusion volume. This may be of interest in oedematous patients with intravascular volume depletion. When such strategies are employed, renal effects may differ markedly according to prior intravascular volume status. Hypertonic saline induced changes in serum osmolality and electrolytes return to baseline within a limited period in time. Sparse evidence indicates that resuscitation with hypertonic saline results in less perioperative complications, ICU days and mortality in selected patients. In conclusion, the use of hypertonic saline may have beneficial features in selected critically ill patients when carefully chosen. Further clinical studies assessing relevant clinical outcomes are warranted.Copyright © 2017 Elsevier Inc. All rights reserved.
Cumulative fluid accumulation is associated with the development of acute kidney injury and non-recovery of renal function: a retrospective analysis
DOI:10.1186/s13054-019-2673-5
[Cited within: 1]

Acute kidney injury (AKI) is common in patients in the intensive care unit (ICU) and may be present on admission or develop during ICU stay. Our objectives were (a) to identify factors independently associated with the development of new AKI during early stay in the ICU and (b) to determine the risk factors for non-recovery of AKI.
Fluid overload and mortality in adult critical care patients - a systematic review and meta-analysis of observational studies
DOI:10.1097/CCM.0000000000004617
PMID:33009098
[Cited within: 1]

Fluid administration in combination with the increase in vasopermeability induced by critical illness often results in significant fluid overload in critically ill patients. Recent research indicates that mortality is increased in patients who have received large volumes of fluids. We have systematically reviewed and synthesized the evidence on fluid overload and mortality in critically ill patients and have performed a meta-analysis of available data from observational studies.A systematic search was performed on PubMed, EmBase, and the Cochrane Library databases.All studies were eligible that investigated the impact of fluid overload (defined by weight gain > 5%) or positive cumulative fluid balance on mortality in adult critical care patients. We excluded animal studies and trials in pediatric populations (age < 16 years old), pregnant women, noncritically ill patients, very specific subpopulations of critically ill patients, and on early goal-directed therapy. Randomized controlled trials were only evaluated in the section on systematic review. Assessment followed the Cochrane/meta-analysis of observational trials in epidemiology guidelines for systematic reviews.A total of 31 observational and three randomized controlled trials including 31,076 ICU patients met the inclusion criteria. Only observational studies were included in the meta-analysis. Fluid overload and cumulative fluid balance were both associated with pooled mortality: after 3 days of ICU stay, adjusted relative risk for fluid overload was 8.83 (95% CI, 4.03-19.33), and for cumulative fluid balance 2.15 (95% CI, 1.51-3.07), at any time point, adjusted relative risk for fluid overload was 2.79 (95% CI, 1.55-5.00) and 1.39 (95% CI, 1.15-1.69) for cumulative fluid balance. Fluid overload was associated with mortality in patients with both acute kidney injury (adjusted relative risk, 2.38; 95% CI, 1.75-2.98) and surgery (adjusted relative risk, 6.17; 95% CI, 4.81-7.97). Cumulative fluid balance was linked to mortality in patients with sepsis (adjusted relative risk, 1.66; 95% CI, 1.39-1.98), acute kidney injury (adjusted relative risk, 2.63; 95% CI, 1.30-5.30), and respiratory failure (adjusted relative risk, 1.19; 95% CI, 1.03-1.43). The risk of mortality increased by a factor of 1.19 (95% CI, 1.11-1.28) per liter increase in positive fluid balance.This systematic review and meta-analysis of observational studies reporting adjusted risk estimates suggests that fluid overload and positive cumulative fluid balance are associated with increased mortality in a general population and defined subgroups of critically ill patients.
Early goal-directed therapy in the treatment of severe sepsis and septic shock
DOI:10.1056/NEJMoa010307 URL [Cited within: 1]
The dark sides of fluid administration in the critically ill patient
DOI:10.1007/s00134-017-4989-4 PMID:29128963 [Cited within: 1]
Role of the glycocalyx in fluid management: small things matter
DOI:10.1016/j.bpa.2014.06.003
PMID:25208958
[Cited within: 1]

Intravenous fluid therapy and perception of volume effects are often misunderstood. The pharmacokinetical difference between colloids and crystalloids depends on the condition of the vascular permeability barrier. Its functioning is still largely based on Starling's principle from 1896, realising that transport of fluid to and from the interstitial space follows the balance between opposing oncotic and hydrostatic pressures. In the past decade, the endothelial glycocalyx, located on the luminal side of healthy vasculature, has increasingly been taken into consideration around models of transvascular fluid filtration. While crystalloids can freely pass through the glycocalyx, colloids are held back in the vasculature by this structure. This is reflected by a markedly higher intravascular persistence of isooncotic colloids (80-100%) versus crystalloids (around 20%), at least as long as the glycocalyx is intact. Protecting this structure in surgical practice means limiting the surgical trauma and avoiding intravascular hypervolemia. Copyright © 2014. Published by Elsevier Ltd.
Management of severe peri-operative bleeding: guidelines from the European Society of Anaesthesiology and Intensive Care: second update 2022
DOI:10.1097/EJA.0000000000001803
PMID:36855941
[Cited within: 1]

Management of peri-operative bleeding is complex and involves multiple assessment tools and strategies to ensure optimal patient care with the goal of reducing morbidity and mortality. These updated guidelines from the European Society of Anaesthesiology and Intensive Care (ESAIC) aim to provide an evidence-based set of recommendations for healthcare professionals to help ensure improved clinical management.A systematic literature search from 2015 to 2021 of several electronic databases was performed without language restrictions. Grading of Recommendations, Assessment, Development and Evaluation (GRADE) was used to assess the methodological quality of the included studies and to formulate recommendations. A Delphi methodology was used to prepare a clinical practice guideline.These searches identified 137 999 articles. All articles were assessed, and the existing 2017 guidelines were revised to incorporate new evidence. Sixteen recommendations derived from the systematic literature search, and four clinical guidances retained from previous ESAIC guidelines were formulated. Using the Delphi process on 253 sentences of guidance, strong consensus (>90% agreement) was achieved in 97% and consensus (75 to 90% agreement) in 3%.Peri-operative bleeding management encompasses the patient's journey from the pre-operative state through the postoperative period. Along this journey, many features of the patient's pre-operative coagulation status, underlying comorbidities, general health and the procedures that they are undergoing need to be taken into account. Due to the many important aspects in peri-operative nontrauma bleeding management, guidance as to how best approach and treat each individual patient are key. Understanding which therapeutic approaches are most valuable at each timepoint can only enhance patient care, ensuring the best outcomes by reducing blood loss and, therefore, overall morbidity and mortality.All healthcare professionals involved in the management of patients at risk for surgical bleeding should be aware of the current therapeutic options and approaches that are available to them. These guidelines aim to provide specific guidance for bleeding management in a variety of clinical situations.Copyright © 2023 European Society of Anaesthesiology and Intensive Care. Unauthorized reproduction of this article is prohibited.
Reassessing the risk of hemodilutional anemia: some new pieces to an old puzzle
DOI:10.1007/s12630-010-9329-x URL [Cited within: 1]
Time to treatment and mortality during mandated emergency care for sepsis
DOI:10.1056/NEJMoa1703058 URL [Cited within: 1]