Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is indicated in patients with severe cardiogenic shock. During extracorporeal membrane oxygenation (ECMO) use, systemic anticoagulation is generally used to ensure the normal functioning of ECMO. Hemorrhage and thrombosis are two important complications of ECMO. However, despite adequate anticoagulation, some patients still experience thrombosis, such as inferior vena cava thrombosis (IVCT), after the removal of ECMO.[1,2] A meta-analysis showed that the incidence of venous thrombosis during ECMO treatment is approximately 10%,[3] but IVCT is rarely reported. We report two cases of IVCT after the removal of VA-ECMO cannulas.
CASE
Case 1
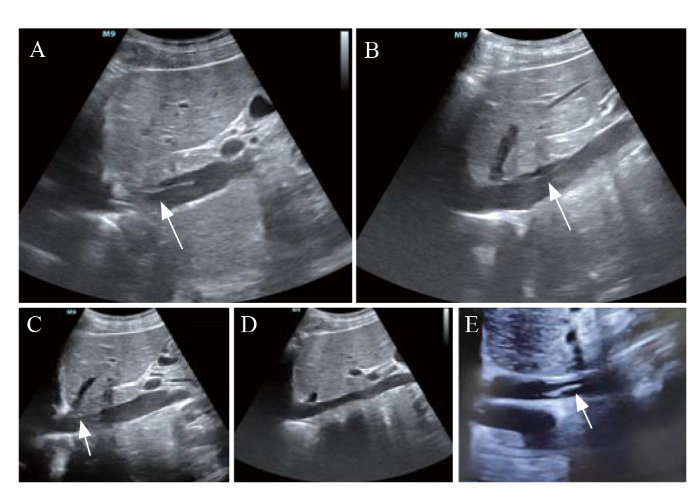
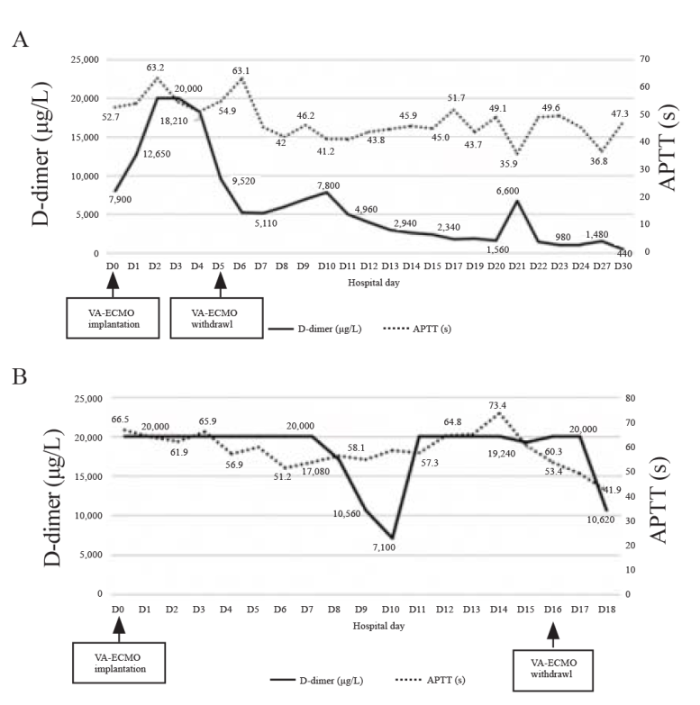
A 30-year-old woman fell into coma after being trapped in a fire. She had severe smoke inhalation injury and cardiac arrest. Transthoracic echocardiography (TTE) showed left ventricular ejection fraction (LVEF) < 10%, and she was treated with VA-ECMO because of circulation instability, with an estimated no-flow time of several minutes and low-flow time of 30 min. During VA-ECMO operation, the patient was anticoagulated with heparin to a target activated partial thromboplastin time (APTT) of 50-70 s and activated clotting time (ACT) of 160-200 s. ECMO was successfully weaned after 6 d due to the recovery of cardiac function. Then, heparin was stopped, and subcutaneous low-molecular-weight heparin (LMWH) was administered at an initial dose of 40 mg once daily to prevent deep venous thrombosis. TTE after ECMO removal showed cable floating objects in the inferior vena cava (IVC) (Figure 1A) with a tail swing, which was considered to be IVCT. After comprehensive evaluation of the risk of bleeding and thrombosis, the maintenance dose of LMWH was adjusted to 20 mg twice daily subcutaneously. Daily TTE examinations showed that the thrombosis gradually decreased (Figures 1 B and C), and after two weeks of anticoagulant therapy, the thrombosis finally disappeared (Figure 1D). The D-dimer level steadily decreased during LMWH treatment (Figure 2A). However, the patient was clinically diagnosed with brain death and declared clinically dead.
Figure 1.
Figure 1.
The subxiphoid view of TTE of case 1 (A-D) and case 2 (E). A: IVCT detected by TTE after ECMO withdrawal (arrow); B: one week after ECMO withdrawal, the IVCT decreased in size through anticoagulation therapy (arrow); C: ten days after ECMO withdrawal, the IVCT almost disappeared with anticoagulation therapy (arrow); D: the IVCT disappeared after two weeks of anticoagulation therapy; E: IVCT of case 2 after ECMO withdrawal (arrow). TTE: transthoracic echocardiography; ECMO: extracorporeal membrane oxygenation; IVCT: inferior vena cava thrombosis.
Figure 2.
Figure 2.
The D-dimer and APTT of case 1 (A) and case 2 (B). VA-ECMO: veno-arterial extracorporeal membrane oxygenation; APTT: activated partial thromboplastin time.
Case 2
A 49-year-old man with severe triple vessel disease was treated with extracorporeal cardiopulmonary resuscitation (ECPR); then, he was transferred to our hospital and received percutaneous coronary intervention (PCI). His LVEF reached 30% after one week of PCI, and VA-ECMO was successfully removed. Routine TTE evaluation after ECMO withdrawal revealed short-segment strip thrombosis in the IVC (Figure 1E). Because the patient continued to receive continuous renal replacement therapy, heparin was used to maintain his APTT between 50 s and 70 s (Figure 2B), and this was the treatment for the IVCT. The patient was transferred back to the local hospital two days after ECMO withdrawal. At discharge, his overall condition was better than that at admission, but IVCT still existed, possibly due to an insufficient anticoagulant course.
DISCUSSION
Some patients have been found to have IVCT during or after ECMO use. The incidence of IVCT secondary to ECMO catheterization is difficult to determine. However, similar to ECMO catheterization, the incidence of filter-related IVCT is approximately 2%.[4] The symptoms of IVCT may be varied; some patients are asymptomatic,[1] some patients develop inferior cava syndrome,[5] and some patients even develop fatal pulmonary embolism.[6] The mortality of ECMO catheter-related thrombosis (CRT) depends more on the severity of the primary disease. IVCT is difficult to detect during ECMO use and is usually inadvertently discovered by ultrasound examination or CT scan after ECMO withdrawal.[1,7] Possible risk factors for cannula-associated IVCT include blood loss, hypovolemia, persistent tube shaking, slow blood flow,[8] low-intensity anticoagulation or non-heparin anticoagulation, prolonged ECMO use, and previous infection.[9] Moreover, the larger the diameter of the catheter is, the higher the risk of thrombosis.[10] In the two patients reported here, thrombosis was detected by TTE after ECMO removal, which may be related to the vascular endothelium injury during ECMO catheterization.
There are no therapeutic guidelines pertaining to IVCT. Shi[11] reviewed the etiology and treatment of acute IVCT and detailed the various treatment options, including systemic anticoagulation and endovascular strategies. The two patients with asymptomatic IVCT in this report were given standard anticoagulant therapy in accordance with the guidelines to avoid further thrombotic progression.[12] Other treatments for ECMO catheter-related thrombosis, such as thrombolysis and vascular interventional therapy, have not been recommended and are rarely reported.[13] Elhassan et al[6] reported a case of fatal ECMO catheter-related thrombosis in which thrombectomy was performed after the evaluation of the thrombus size and mobility and after individualized patient-based assessment of embolism risk, which provides a reference for the acute management of this condition.
For patients with ECMO support, dynamic ultrasound evaluation during ECMO use and after withdrawal is necessary.[14] Ultrasound also plays an important role in thrombosis monitoring of other invasive catheters. Wu et al[15] recently published a large multicenter prospective study of ultrasound monitoring of CRT and found that the incidence of CRT was 16.9%, which highlighted the importance of ultrasound in thrombosis monitoring. The two cases of IVCT reported here were detected during daily ultrasound evaluation and were monitored daily during anticoagulant therapy. Ultrasound is noninvasive, inexpensive, and reproducible. Trained operators can perform real-time bedside ultrasound, which not only enable fine management under ultrasound guidance but also facilitate timely detection of thrombosis and early treatment.
CONCLUSION
IVCT may occur after ECMO catheterization for various reasons. Anticoagulant therapy is an effective and primary treatment for ECMO catheter-related IVCT. Ultrasound can be used for the detection and follow-up of IVCT during ECMO use or after ECMO withdrawal.
Funding: None.
Ethical approval: This study was approved by Ethical Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine.
Conflicts of interests: The authors declare no conflicts of interest.
Contributors: XC proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study.
Reference
Echocardiographic image of a cannula in the inferior vena cava after decannulation of venoarterial extracorporeal membrane oxygenation
Inferior vena cava thrombosis during extracorporeal membrane oxygenation: a case report and review of the literature
A meta-analysis of complications and mortality of extracorporeal membrane oxygenation
PMID:23944202
[Cited within: 1]

To comprehensively assess published peer-reviewed studies related to extracorporeal membrane oxygenation (ECMO), focusing on outcomes and complications of ECMO in adult patients.Systematic review and meta-analysis.MEDLINE/PubMed was searched for articles on complications and mortality occurring during or after ECMO.Included studies had more than 100 patients receiving ECMO and reported in detail fatal or nonfatal complications occurring during or after ECMO. Primary outcome was mortality at the longest follow-up available; secondary outcomes were fatal and non-fatal complications.Twelve studies were included (1763 patients), mostly reporting on venoarterial ECMO. Criteria for applying ECMO were variable, but usually comprised acute respiratory failure, cardiogenic shock or both. After a median follow-up of 30 days (1st-3rd quartile, 30-68 days), overall mortality was 54% (95% CI, 47%-61%), with 45% (95% CI, 42%-48%) of fatal events occurring during ECMO and 13% (95% CI, 11%-15%) after it. The most common complications associated with ECMO were: renal failure requiring continuous venovenous haemofiltration (occurring in 52%), bacterial pneumonia (33%), any bleeding (33%), oxygenator dysfunction requiring replacement (29%), sepsis (26%), haemolysis (18%), liver dysfunction (16%), leg ischaemia (10%), venous thrombosis (10%), central nervous system complications (8%), gastrointestinal bleeding (7%), aspiration pneumonia (5%), and disseminated intravascular coagulation (5%).Even with conditions usually associated with a high chance of death, almost 50% of patients receiving ECMO survive up to discharge. Complications are frequent and most often comprise renal failure, pneumonia or sepsis, and bleeding.
Inferior vena cava thrombosis risk in 1582 patients with inferior vena cava filters
DOI:10.1148/radiol.211169 PMID:35133197 [Cited within: 1]
Long-segment caval thrombus after removal of ECMO cannula
DOI:10.1007/s00134-015-3781-6 PMID:25860445 [Cited within: 1]
Pulmonary thromboembolism from extracorporeal life support (ECLS) cannula-associated thrombus
DOI:S0952-8180(19)30954-7 PMID:31430646 [Cited within: 2]
Prevalence and risk factors for thrombotic complications following venovenous extracorporeal membrane oxygenation: a CT scan study
DOI:10.1097/CCM.0000000000004129
PMID:31939787
[Cited within: 1]

The aims of this study were to: 1) analyze the cannula-associated deep vein thrombosis frequency after venovenous extracorporeal membrane oxygenation using a CT scan and 2) identify the associated risk factors for cannula-associated deep vein thrombosis.Retrospective observational analysis at a single center.Tertiary referral university teaching hospital.Patients under venovenous extracorporeal membrane oxygenation with a femorofemoral or femorojugular cannulation admitted for acute respiratory distress syndrome or primary graft dysfunction after pulmonary transplantation. CT scan was performed within 4 days after decannulation.None.We included 105 of 228 patients screened. Bacterial pneumonia was the main indication of venovenous extracorporeal membrane oxygenation (46.7%). CT scans were performed at a median of 2 days (1-3 d) after decannulation. Cannula-associated deep vein thrombosis was found in 75 patients (71.4%) despite it having a mean activated partial thromboplastin time ratio of 1.60 ± 0.31. Femorofemoral cannulation induced femoral cannula-associated deep vein thrombosis more frequently than femorojugular cannulation (69.2% vs 63.1%, respectively; p = 0.04). Seventeen of the 105 patients (16.2%) had a pulmonary embolism. Multivariate logistic regression analysis showed that higher the percentage of thrombocytopenia less than 100 G/L during extracorporeal membrane oxygenation period, lower the risk for developing cannula-associated deep vein thrombosis (hazard ratio, 0.98; 95% CI, 0.98-1.00; p = 0.02).Cannula-associated deep vein thrombosis after venovenous extracorporeal membrane oxygenation is a frequent complication. This plead for a systematic vascular axis imaging after venovenous extracorporeal membrane oxygenation. Thrombocytopenia is associated with a reduction in the occurrence of thrombotic events.
Sudden dysfunction of veno-venous extracorporeal membrane oxygenation caused by intermittent cannula obstruction: the key role of echocardiography
DOI:10.1007/s00134-017-4704-5 PMID:28184952 [Cited within: 1]
Venous or arterial thromboses after venoarterial extracorporeal membrane oxygenation support: frequency and risk factors
DOI:10.1016/j.healun.2020.12.007 URL [Cited within: 1]
Incidence and risk factors for cannula-related venous thrombosis after venovenous extracorporeal membrane oxygenation in adult patients with acute respiratory failure
DOI:10.1097/CCM.0000000000003650
URL
[Cited within: 1]

Venovenous extracorporeal membrane oxygenation is indicated in patients with severe refractory acute respiratory failure. Venous thrombosis due to indwelling catheters is a frequent complication. The aim of this study was to analyze the incidence of cannula-related thrombosis and its risk factors after venovenous extracorporeal membrane oxygenation.
Etiology and treatment of acute inferior vena cava thrombosis
DOI:S0049-3848(16)30490-X
PMID:27865097
[Cited within: 1]

Inferior vena cava thrombosis (IVCT) is a rare but severe disease that is associated with a high rate of mortality. IVCT can be categorized into primary versus secondary thrombosis dependent upon the underlying pathophysiology. The diagnosis includes both clinical probability assessment as well as the imaging evaluation. The optimal therapeutic strategy remains the target of continued research. Although anticoagulation therapy remains fundamental in treating IVCT, its inherent limitations have led to the use of minimally invasive, endovascular treatment options, including transcatheter thrombolysis, mechanical thrombectomy or a combination of these techniques. This review focuses on the etiology, diagnostic assessment, and endovascular treatment options for IVCT.Copyright © 2016. Published by Elsevier Ltd.
Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-based Clinical Practice Guidelines
DOI:10.1378/chest.11-2301 URL [Cited within: 1]
Vein thrombosis after ECMO decannulation, a frequent and sometimes missed complication
DOI:S0167-5273(16)31843-5 PMID:27552576 [Cited within: 1]
Venous thrombosis in and after extracorporeal membrane oxygenation: detection and follow-up by color Doppler sonography
PMID:9369503
[Cited within: 1]

The purpose of our study was to evaluate thrombosis of venous vessels during and after extracorporeal membrane oxygenation (ECMO) using color Doppler sonography. We prospectively performed serial color Doppler sonography investigations in 30 ECMO patients [age: newborn to 3 years, male:female = 20:10, venoarterial (VA) ECMO = 18, venovenous (VV) ECMO = 12]. During ECMO obstruction and/or thrombosis of the superior vena cava (SVC) was observed in 2 neonates on VA ECMO. Furthermore, a thrombotic clot from an initially open duct of Arantii with partial portal vein thrombosis, reaching into the inferior vena cava (IVC), occurred despite adequate heparinization. After ECMO, late septic SVC thrombus occurred in one neonate. IVC thrombus was observed in two pediatric VV ECMO patients. The overall incidence of venous clots was 20 % (6 of 30). Routine color Doppler sonography monitoring of vessels in children on and after ECMO was found to be useful for early detection of venous thrombosis. It enabled consequent administration of appropriate therapy as well as follow-up after decannulation and reconstruction.
Daily point-of-care ultrasound-assessment of central venous catheter-related thrombosis in critically ill patients: a prospective multicenter study