INTRODUCTION
Sepsis has an in-hospital mortality of 25.8%, and has been one of the leading causes of death in critically ill patients.[1,2] Inflammation-associated anemia is mainly due to the involvement of inflammatory mediators, which is commonly observed in patients with sepsis. It often develops in patients with long-term infection, inflammatory disease, or malignancy, accompanied by low serum iron despite adequate systemic iron store, decreased serum transferrin, and mild decrease in size and hemoglobin content of erythrocytes.[3] Interleukin (IL)-6 is a key inflammatory cytokine inducing an abrupt increase in hepcidin synthesis, thereby decreasing the serum iron in sepsis patients.[3] The survival of sepsis patients is closely associated with parameters that reflect inflammation-related iron metabolism disorders and anemia. Jiang et al[3] have observed significantly decreased serum iron, hemoglobin, and soluble transferrin receptor (sTfR)/log ferritin, with remarkably increased serum erythropoietin, hepcidin, ferritin, and IL-6 in sepsis patients during the first week of intensive care unit (ICU) admission. More importantly, the 28-day mortality was closely associated with the changes in serum hepcidin, ferritin, and IL-6.[3]
At present, effective treatment for severe inflammation-related anemia and iron metabolism disorders in sepsis patients remains unclear. Moreover, conventional blood transfusion and iron supplement are not routinely recommended in the current clinical practice. Blood transfusion has been reported to be associated with higher mortality and failure of anemia correction in critically ill patients.[4] Although enteral iron supplementation can reduce the blood transfusion rate in critically ill patients with baseline iron deficiency, iron remains as a toxic compound that may induce oxidative stress and bacterial growth.[5]
Continuous renal replacement therapy (CRRT) has been traditionally used as an important treatment strategy for renal failure.[6,7] CRRT can non-selectively remove inflammatory mediators, rebalance acid-base disorders, regulate immune stability, and maintain a stable environment through the mechanisms of convection and ultrafiltration.[8] Therefore, CRRT is applicable for eliminating inflammatory mediators in sepsis.[9] However, the effects of CRRT on inflammation-related anemia and iron metabolism disorders in sepsis remain unclear. The present prospective study aimed to determine whether CRRT would affect the parameters of inflammation-related anemia, iron metabolism, and prognosis in sepsis patients with acute kidney injury (AKI).
METHODS
Study design and patients
Sepsis patients with AKI, who were admitted to the emergency ICU at the First Affiliated Hospital of Dalian Medical University from October 2015 to December 2017, were prospectively enrolled. All patients met the Sepsis-2 (severe sepsis between October 2015 and February 2016) or Sepsis-3 (sepsis after the publishment of the Sepsis-3 criteria on February 23, 2016) criteria, and had sepsis-induced AKI with rapid increase in creatinine, or oliguria with fluid overload and/or hyperkalemia.[6,7] AKI was diagnosed according to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines.[10]
The exclusion criteria were as follows: <18 years old; pregnant patients; patients with stomach cancer and acute bleeding; patients with blood system diseases (e.g., leukemia, myeloma, red blood cell diseases including polycythemia vera, abnormal mean red blood cell volume, abnormal mean hemoglobin content, or abnormal mean hemoglobin concentration); patients with chronic renal insufficiency requiring hemodialysis; patients with liver cirrhosis; patients with prior blood transfusion within one week before admission or during hospitalization; patients with anemia, who were treated with iron and erythropoietin within three months before admission.
All enrolled sepsis patients were randomized into the CRRT (patients who received CRRT) and control (patients who received conventional treatment without CRRT) groups using computer-generated, pre-randomized sealed envelopes. The patients and their caregivers were not blinded to the allocation of treatment.
The present study was performed in accordance with the Declaration of Helsinki adopted by the World Medical Association (2013 edition). The study protocol was approved by the Medical Ethics Committee of the First Affiliated Hospital of Dalian Medical University (PJ-KS-KY-2020-138). A written informed consent was obtained from each participant or legal guardian on admission. The present study was registered to the Chinese Clinical Trial Registry (ChiCTR2100048817, http://www.chictr.org.cn), and followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Treatments
Patients in the control group received conventional treatment according to the Surviving Sepsis Campaign guidelines.[11] The patients whose renal function deteriorated also received delayed CRRT, and they were included in the CRRT group. Patients in the CRRT group received CRRT within 24 h after the diagnosis of sepsis-induced AKI, in addition to conventional treatment. The CRRT protocol was provided in the supplementary Figure 1.
Data collection
The following clinical data were collected on admission: gender, age, infection sites, comorbidities, number of post-operative patients, number of patients who used vasopressors, number of patients who received mechanical ventilation, laboratory results, and 28-day mortality. Furthermore, the hemoglobin, red blood cell distribution width (RDW), reticulocytes, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration were measured using an automatic blood cell analyzer (XN-2000; Sysmex, Japan). The Sequential Organ Failure Assessment (SOFA) score was assessed on ICU admission.
Peripheral venous blood (5 mL) was drawn on days 1 (before CRRT), 3 and 7 of ICU admission. Then, the collected blood was centrifuged (TGL-20MS [BIORIDGE, China], 4 °C, 2,000×g) for 10 min, and stored at -80 °C for the subsequent analysis. Afterwards, the serum IL-6, hepcidin (Uscn Life Sciences, China), erythropoietin, ferritin (Abcam, Cambridge, USA), and sTfR (R&D Systems, USA) were detected by enzyme-linked immunosorbent assay (ELISA).
Statistical analysis
The data were analyzed using SPSS 23.0 (IBM, USA). Continuous variables were expressed as mean±standard deviation (SD) for normal distribution, or median (interquartile range) for skewed distribution. The categorical variables were compared using Pearson’s Chi-square test or Fisher’s exact test. Continuous variables were compared using Mann-Whitney U-test or t-test. A P-value of <0.05 was considered statistically significant.
RESULTS
Baseline characteristics
A total of 179 consecutive sepsis patients with AKI were initially enrolled for the present study. Among these patients, 99 patients were randomized into the CRRT (n=49) and control (n=50) groups and 80 patients were excluded from the study (supplementary Figure 2). After randomization, 10 patients in the CRRT group abandoned the therapy due to financial problems or other causes. Finally, 39 patients in the CRRT group and 50 patients in the control group were included for the present study. The number (15 vs. 74) and proportion (16.9% vs. 83.1%) of included patients meeting the criterion for Sepsis-2 were lower due to the short study duration (4 months), when compared with those meeting the criterion for Sepsis-3 with a long study duration (22 months). The proportion (16.7% vs. 17.1%) of patients in the control group who met the criterion of Sepsis-2 was also similar to that of patients in the CRRT group who met the criterion of Sepsis-3 (P>0.05). Moreover, the proportion of patients with AKI stage 1, 2 and 3 did not significantly differ between the control (32/50, 18/50, and 0/50, respectively) and CRRT (24/39, 15/39, and 0/39, respectively) groups (P>0.05). Two patients in the stage 2 in the control group, who received delayed CRRT within 24 h of ICU admission due to renal function deterioration, were included in the CRRT group, but both of them died.
There were no significant differences in baseline characteristics, including age, male gender, infection sites, comorbidities, number of postoperative patients, number of patients who used vasopressors, number of patients who received mechanical ventilation, length of mechanical ventilation, PaO2/FiO2, procalcitonin, lactate, creatinine, urine output, potassium, reticulocytes, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, or SOFA score, between the two groups (Table 1 and supplementary Table 2). The parameters for the CRRT are summarized in supplementary Table 1. The median survival for hemofiltration was 33.5 h, which was determined by early recovery of renal function or failure to maintain the function of CRRT. The latter was mainly due to elevated transmembrane pressure in three patients with secondary sepsis after surgery, who received no heparinization during CRRT. None of the patients in the CRRT group suffered from bleeding due to anticoagulant use.
Table 1. Baseline characteristics
| Parameters | Control group (n=50) | CRRT group (n=39) | P-valuea |
|---|---|---|---|
| Age, years | 76.0 (68.2, 85.1) | 74.5 (65.0, 83.3) | 0.489 |
| Male gender, n (%) | 29 (58.0) | 18 (46.2) | 0.292 |
| Infection sites, n (%) | |||
| Lung | 21 (42.0) | 12 (30.8) | 0.377 |
| Abdomen | 13 (26.0) | 17 (43.6) | 0.114 |
| Biliary system | 9 (18.0) | 6 (15.4) | 0.784 |
| Soft skin tissue | 9 (18.0) | 4 (10.2) | 0.375 |
| Comorbidities, n (%) | |||
| Hypertension | 28 (56.0) | 20 (51.3) | 0.675 |
| Diabetes mellitus | 20 (40.0) | 11 (28.2) | 0.271 |
| Stroke Acute myocardial infarction Deep venous thrombosis | 1 (2.0) 2 (4.0) 4 (8.0) | 0 (0) 0 (0) 1 (2.6) | 1.000 0.502 0.380 |
| Post-operative patients, n (%) | 7 (14.0) | 3 (7.7) | 0.503 |
| Patients using vasopressors, n (%) | 27 (54.0) | 20 (51.5) | 0.833 |
| Patients with mechanical ventilation, n (%) | 26 (52.0) | 17 (43.6) | 0.523 |
| Length of mechanical ventilation, h | 102.0 (75.8, 136.0) | 117.0 (79.0, 151.0) | 0.386 |
| PaO2/FiO2, mmHg | 216.5 (175.8, 245.0) | 217.0 (192.0, 244.0) | 0.579 |
| Serum procalcitonin, ng/mL | 7.32 (2.45, 10.23) | 5.00 (1.16, 16.90) | 0.993 |
| Serum lactate, mmol/L | 4.05 (2.28, 5.53) | 3.10 (1.80, 4.90) | 0.101 |
| Serum creatinine, µmol/L | 242.0 (181.8, 261.3) | 247.0 (209.0, 265.0) | 0.721 |
| Urine output, mL/(kg·h) | 0.32 (0.28, 0.49) | 0.37 (0.29, 0.48) | 0.735 |
| Serum potassium, mmol/L | 4.16 (3.84, 4.58) | 4.36 (3.96, 4.65) | 0.186 |
| Reticulocytes, ×109/L | 66.0 (43.3, 81.3) | 50.0 (38.0, 65.0) | 0.083 |
| Mean corpuscular volume, fL | 88.5 (82.0, 93.0) | 88.0 (85.0, 93.0) | 0.967 |
| Mean corpuscular hemoglobin, pg | 32.0 (30.0, 34.0) | 32.0 (29.0, 34.0) | 0.800 |
| MCHC, g/L | 334.5 (320.8, 347.0) | 333.0 (314.0, 348.0) | 0.628 |
The values are expressed as median (interquartile range) or number (percentage). a: the P-values refer to the differences between the CRRT and control groups. The normal value range of procalcitonin, lactate, creatinine, potassium, reticulocytes, mean corpuscular volume, mean corpuscular hemoglobin and MCHC detected using an automatic blood cell analyzer was 0-0.05 ng/mL, 0.7-2.1 mmol/L, 58-110 µmol/L, 3.5-5.2 mmol/L, (24-84) ×109/L, 82-100 fL, 27-34 pg and 316-354 g/L, respectively. CRRT: continuous renal replacement therapy; MCHC: mean corpuscular hemoglobin concentration; PaO2/FiO2: arterial partial pressure of oxygen/fraction of inspiration oxygen.
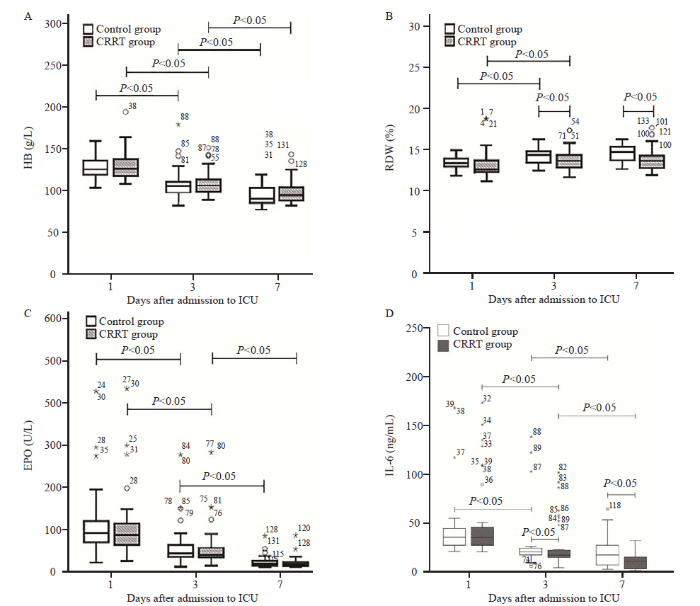
Effect of CRRT on hemoglobin, RDW and serum erythropoietin
There were no significant differences in hemoglobin, RDW, and serum erythropoietin on admission between the two groups (all P>0.05). Furthermore, the hemoglobin and erythropoietin gradually decreased in both groups during the first week (all P<0.05; Figures 1A and 1C), but these did not significantly differ between the two groups. Moreover, the RDW was higher in both groups on days 3 and 7, when compared to that on day 1, but there was no significant difference in RDW between days 3 and 7 (both P>0.05; Figure 1B). In addition, on days 3 and 7, the RDW was significantly lower in the CRRT group, when compared to the control group (both P<0.05).
Figure 1.
Figure 1.
Comparison of parameters correlated to anemia in patients with sepsis between the CRRT and control groups. The normal range of HB and RDW detected by an automatic blood cell analyzer was 130-175 g/L and <15%, respectively. The asterisks, circles, and numbers beside these denote the outliers, and were defined in the usual manner as points above (and below) 1.5 times the interquartile range. CRRT: continuous renal replacement therapy; ICU: intensive care unit; HB: hemoglobin; RDW: red blood cell distribution width; EPO: erythropoietin; IL-6: interleukin-6.
Effect of CRRT on serum IL-6
On admission, the serum IL-6 did not significantly differ between the two groups (P>0.05, Figure 1D). Furthermore, a gradual decrease in serum IL-6 was observed during the first week in both groups (all P<0.05). However, on days 3 and 7, the serum IL-6 were lower in the CRRT group, when compared to the control group (both P<0.05).
Effect of CRRT on parameters related to iron metabolism
There were no significant differences in serum iron, hepcidin, ferritin or sTfR on admission between the two groups (all P>0.05; Figure 2). The serum iron in the control group initially slightly decreased, and subsequently slightly increased in the first week (all P>0.05; Figure 2A), while the serum iron in the CRRT group was higher on day 7, when compared to that on day 1. In addition, on day 7, the serum iron was higher in the CRRT group, when compared to the control group (P<0.05). Furthermore, the serum hepcidin in both groups were lower on days 3 and 7, when compared to those on day 1 (all P<0.05; Figure 2(B)). On days 3 and 7, the serum hepcidin was lower in the CRRT group, when compared to the control group (both P<0.05). However, the serum ferritin in both groups was higher on days 3 and 7, when compared to that on day 1 (all P<0.05; Figure 2(C)), while on days 3 and 7, the serum ferritin was significantly lower in the CRRT group, when compared to the control group (both P<0.05). There were no significant differences in serum sTfR between the two groups, or among the different time points (all P>0.05; Figure 2D).
Figure 2.
Figure 2.
Comparison of parameters correlated to iron metabolism in sepsis patients between the CRRT and control groups. The asterisks, circles, and numbers beside these denote the outliers, and were defined in the usual manner as points above (and below) 1.5 times the interquartile range. CRRT: continuous renal replacement therapy; ICU: intensive care unit; sTfR: soluble transferrin receptor.
Effect of CRRT on serum creatinine, hemodynamics, renal function, SOFA scores and outcome
The serum creatinine and SOFA scores in both groups gradually decreased from day 1 to day 7 (all P<0.05). However, patients in the CRRT group had lower serum creatinine levels and SOFA scores on day 7 after admission to the ICU, improved hemodynamic parameters on days 3 and 7 after admission to the ICU, and shorter ICU stays, when compared to patients in the control group (all P<0.05; supplementary Table 2). There was no significant difference in 28-day mortality (38.0% vs. 28.2%) (supplementary Table 2) between the control and CRRT groups.
DISCUSSION
The present study evaluating the effect of CRRT on inflammation-related anemia, iron metabolism, and the prognosis of sepsis patients with AKI had three main findings. First, the CRRT did not significantly alter the hemoglobin, erythropoietin or sTfR levels in sepsis patients with AKI. Second, patients with sepsis had significantly higher serum levels of IL-6, hepcidin, ferritin and RDW, but these parameters decreased after CRRT. Third, CRRT was associated with the increase in serum iron and improvement in SOFA scores but not the 28-day mortality.
The incidence of sepsis-related anemia reached as high as 90% within three days after ICU admission. The anemia was mainly caused by a variety of mechanisms, including infection, iatrogenic blood loss, dilution during fluid resuscitation, decrease in serum iron, inhibition of erythropoietin production, shortened red blood cell life, and malnutrition.[12] For sepsis patients, inflammation upregulates the generation of hepcidin which in turn inhibit serum iron, but these changes exert a measurable effect on hemoglobin levels only from seven days after the diagnosis of sepsis.[13] Therefore, the effect of decreased hepcidin by CRRT on hemoglobin production may be insignificant.
RDW is typically used as a part of the complete blood count to quantify size changes of circulating red blood cells.[14] For patients with sepsis, a significant increase in RDW was observed on admission, which may serve as a useful predictor of mortality as evidenced by a previous study.[3] In the present study, we found that sepsis patients had lower RDWs following CRRT. However, the mechanism by which CRRT affects RDW remains unknown. Furthermore, it was observed that RDW was strongly correlated with IL-6.[3,14] Moreover, pro-inflammation cytokines, such as IL-6, can impair the maturation of red blood cells, thereby accelerating the entrance of immature red blood cells into circulation.[14] Therefore, we speculated that the decrease in RDW after CRRT, at least in part, may be correlated to the clearance of IL-6.
It was previously identified that serum erythropoietin is elevated with a tendency to gradually decrease in patients with sepsis.[3] In the present study, no significant change in serum erythropoietin levels was observed in sepsis patients after CRRT. This phenomenon could be explained by the fact that erythropoietin has a molecular weight of 34 kDa, which makes it difficult to be filtered by the membrane during CRRT.[15] In addition, the effects of CRRT on erythropoietin production are complicated. Erythropoietin production is usually elevated in response to anemia and hypoxemia, and this is inhibited by impaired renal function and elevated pro-inflammatory cytokines (such as IL-6 and TNF-α) in sepsis patients.[3] Furthermore, CRRT may ameliorate the hypoxemia and acute lung injury, thereby decreasing erythropoietin production.[16] Meanwhile, CRRT may remove pro-inflammatory cytokines, thereby increasing erythropoietin production. Considering the inconsistent effects of CRRT on erythropoietin production, it was not surprising to observe that the CRRT failed to affect the serum erythropoietin in sepsis patients in the present study.
Serum IL-6, hepcidin, iron, ferritin and sTfR are parameters of inflammation-related iron metabolism. A previous study found that these parameters, except for serum iron, significantly increased in patients with sepsis, and that serum IL-6 was positively correlated with serum hepcidin and ferritin.[3] Hepcidin, as a liver-producing peptide hormone, plays a central role in regulating serum iron.[13] This inhibits iron absorption from the duodenum, and its release from hepatocytes and circulating macrophages, resulting in a low level of serum iron.[17] IL-6 can induce the upregulation of hepcidin through the STAT-3 signaling pathway, thereby causing alterations in iron metabolism. These alterations were observed to be significantly associated with anemia and 28-day mortality.[3,5,18] Notably, the present study revealed that CRRT significantly reduced the serum IL-6, hepcidin and ferritin, rather than the serum sTfR.
The decrease in serum IL-6 after CRRT was consistent with that in previous findings, in which inflammatory mediators, such as serum IL-6 and TNF-α, can be cleared by hemofiltration in animal and human experiments.[6,8,11] The mechanism by which CRRT clears the serum IL-6 may be the adsorption, rather than the convection, because IL-6 has a molecular weight of 26 kDa, making it difficult to be filtered by membrane during CRRT.[19] Furthermore, CRRT can effectively adsorb lipopolysaccharide, which is an activator of cytokines production. The rapid removal of lipopolysaccharide during CRRT can lead to the decrease in production of IL-6.[19] Similarly, hepcidin, with a molecular weight of approximately 10 kDa, can be effectively removed through the membrane during CRRT.[20] In addition, the reduction in serum hepcidin and ferritin in sepsis patients after CRRT may be associated with the reduction in serum IL-6.[18] This is because the clearance of IL-6 during CRRT can relieve the upregulation of hepcidin through the IL-6-hepcidin axis, and block the induction of pro-inflammatory cytokines to ferritin production.[18] Considering that hepcidin plays a vital role in decreasing serum iron,[13] it was speculated that the elevated serum iron after CRRT may be mainly associated with the decrease in serum hepcidin. The serum sTfR levels appeared to be unaffected by the CRRT in sepsis patients in the present study. This was mainly because sTfR has a large molecular weight of 85 kDa, resulting in a very low clearance rate during CRRT. Furthermore, sTfR reflects the degree of iron availability for cells, but this is not affected by inflammation. Therefore, the clearance of IL-6 during CRRT does not affect sTfR production.
Undoubtedly, the correction of iron metabolism disorders may only partially contribute to the improvement in SOFA scores after CRRT in the present study. Increasing evidence has revealed that CRRT can exert multiple effects on sepsis patients, including the correction of acidemia, electrolyte imbalance and extracellular volume expansion, the removal of inflammatory mediators, and the improvement of AKI, and these are all associated with the improvement in SOFA scores.[6,7,9,18] However, the 28-day mortality of sepsis patients did not improve after CRRT in the present study, which is inconsistent with the meta-analysis results published by Putzu et al.[7] There are several explanations for this result. First, the relatively small sample size of the present study may not be adequate to reach a statistically significant result. Second, iron metabolism disorders only partially contribute to the severity of sepsis, and these may be insufficient to affect the outcome. Other complications and comorbidities may also influence the mortality.
Limitation
There were several limitations in the present study. First, the differences in the subgroups of gender and septic shock were not assessed. However, the gender distribution and number of patients who used vasopressors on admission were similar between the two groups. Second, the pre-dilution might have reduced the removal of target cytokines. Third, the replacement of extracorporeal circuits every 48 h was not a sound strategy, but the efficacy of the AN69ST membrane might have been reduced due to the prolonged use. Fourth, the loss rate of follow-up was close to 20% in the CRRT group, which might introduce a bias. Fifth, the current study did not involve the exploration of potential mechanisms. Finally, the sample size was relatively small, and subgroups of septic patients with different etiologies such as urinary tract infection were not all included. Hence, future studies with large sample sizes are warranted.
CONCLUSIONS
CRRT might have beneficial effects on the improvement in inflammation-related iron metabolism and disease severity during the first week of ICU admission but not anemia and 28-day mortality in sepsis patients with AKI.
Funding: This study was funded by the Shenzhen Key Medical Discipline Construction Fund (SZXK046) and the National Nature Science Foundation of China (81571869).
Ethics approval: The study protocol was approved by the Medical Ethics Committee of the First Affiliated Hospital of Dalian Medical University (PJ-KS-KY-2020-138), Dalian, China. A written informed consent was obtained from each participant or legal guardian at the time of initial admission.
Conflicts of interest: The authors declare no conflicts of interest.
Contributors: MMA and CXL contributed equally to this work as co-first authors. GP: concept and design; MMA: statistical analysis; PG: funding; MMA and CXL: drafting of the article; MMA and CXL: data collection. All authors revised the article and approved the final version of the article.
The supplementary files in this paper are available at http://wjem.com.cn.
Reference
Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit
DOI:10.1016/S2213-2600(14)70061-X URL [Cited within: 1]
Comparison of different versions of the quick sequential organ failure assessment for predicting in-hospital mortality of sepsis patients: A retrospective observational study
DOI:10.5847/wjem.j.1920-8642.2022.027
PMID:35237364
[Cited within: 1]

The quick sequential organ failure assessment (qSOFA) is recommended to identify sepsis and predict sepsis mortality. However, some studies have recently shown its poor performance in sepsis mortality prediction. To enhance its effectiveness, researchers have developed various revised versions of the qSOFA by adding other parameters, such as the lactate-enhanced qSOFA (LqSOFA), the procalcitonin-enhanced qSOFA (PqSOFA), and the modified qSOFA (MqSOFA). This study aimed to compare the performance of these versions of the qSOFA in predicting sepsis mortality in the emergency department (ED).This retrospective study analyzed data obtained from an electronic register system of adult patients with sepsis between January 1 and December 31, 2019. Receiver operating characteristic (ROC) curve analyses were performed to determine the area under the curve (AUC), with sensitivity, specificity, and positive and negative predictive values calculated for the various scores.Among the 936 enrolled cases, there were 835 survivors and 101 deaths. The AUCs of the LqSOFA, MqSOFA, PqSOFA, and qSOFA were 0.740, 0.731, 0.712, and 0.705, respectively. The sensitivity of the LqSOFA, MqSOFA, PqSOFA, and qSOFA were 64.36%, 51.40%, 71.29%, and 39.60%, respectively. The specificity of the four scores were 70.78%, 80.96%, 61.68%, and 91.62%, respectively. The LqSOFA and MqSOFA were superior to the qSOFA in predicting in-hospital mortality.Among patients with sepsis in the ED, the performance of the PqSOFA was similar to that of the qSOFA and the values of the LqSOFA and MqSOFA in predicting in-hospital mortality were greater compared to qSOFA. As the added parameter of the MqSOFA was more convenient compared to the LqSOFA, the MqSOFA could be used as a candidate for the revised qSOFA to increase the performance of the early prediction of sepsis mortality.Copyright: © World Journal of Emergency Medicine.
Inflammatory anemia-associated parameters are related to 28-day mortality in patients with sepsis admitted to the ICU: a preliminary observational study
DOI:10.1186/s13613-019-0542-7
PMID:31183575
[Cited within: 10]

Anemia is one of the most common complications of sepsis. Sepsis-related anemia is associated mainly with inflammation. We aimed to observe the changes in the inflammatory anemia-associated parameters of patients with sepsis in the early stage of intensive care unit (ICU) admission and to evaluate their association with 28-day mortality.A total of 198 patients with sepsis were divided into survivor (n = 110) and non-survivor (n = 88) groups on the basis of 28-day survival. Healthy volunteers (n = 20) were enrolled as a control group. Plasma levels of iron, ferritin, erythropoietin (EPO), soluble transferrin receptor (sTfR), hepcidin, interleukin-6 (IL-6), hemoglobin and the red blood cell distribution width (RDW) were measured on days 1, 3 and 7 of ICU admission. Clinical data and laboratory findings were collected, and the Sequential Organ Failure Assessment (SOFA) score was calculated.Patients with sepsis showed significant decreases in hemoglobin, plasma iron and sTfR/log ferritin and significant increases in plasma EPO, sTfR, hepcidin, ferritin and IL-6 on days 1, 3 and 7 of ICU admission compared with healthy volunteers. Hemoglobin was correlated negatively with plasma IL-6 and hepcidin. In patients with sepsis, non-survivors had significantly lower plasma iron, EPO and sTfR/log ferritin, but higher plasma hepcidin, ferritin and IL-6 than survivors on days 1, 3 and 7 of ICU admission. Plasma EPO, hepcidin, ferritin, IL-6, sTfR/log ferritin, the RDW and SOFA score were associated significantly with 28-day mortality but to a varying extent. In particular, in predicting 28-day mortality, plasma hepcidin had an area under the receiver operating curve of 0.808 and 87.3% specificity, which was the highest among the inflammatory anemia-associated parameters tested.Inflammatory anemia-associated parameters changed significantly in patients with sepsis in the first week of ICU admission. Plasma EPO, hepcidin, ferritin, IL-6, sTfR/log ferritin, the RDW and SOFA score were associated significantly with 28-day mortality. Plasma hepcidin might have a superior predictive value, with high specificity, compared with other inflammatory anemia-associated parameters for 28-day mortality of sepsis patients in the ICU.
Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Iron deficiency in critically ill patients: highlighting the role of hepcidin
DOI:10.1186/cc9992 URL [Cited within: 2]
CRRT for sepsis-induced acute kidney injury
DOI:10.1097/MCC.0000000000000544
PMID:30239411
[Cited within: 4]

Sepsis-induced acute kidney injury (SI-AKI) represents the first cause of AKI in ICUs, and renal replacement therapy (RRT) is frequently applied in advanced AKI stages. The debate between 'rescue' indications for RRT start in patients with severe AKI (acidosis, hyperkalemia, uremia, oliguria/anuria, volume overload) and a proactive RRT initiation is still ongoing. In addition, current SI-AKI pathophysiologic theory has identified the toxic effects of soluble middle-molecules released during sepsis and inflammation (pathogen and damaged associated molecular patterns).The purpose of the present review is to summarize the recent literature on RRT for patients with SI-AKI. Supportive or replacement measures for severe stages of renal dysfunction and blood purification techniques for sepsis syndrome will be reviewed.Anticipated RRT for SI-AKI does not seem to improve survival or renal recovery. There is no clinical advantage by delivering continuous RRT at high doses for blood purification purposes. Similarly, specific applications with dedicated devices and membranes have yielded no clinical benefit in these patients, so far.In the present review, the recent insights and results from large randomized and nonrandomized trials in the area of RRT applied both as supportive measures for kidney failure and blood purification techniques are described.
Blood purification with continuous veno-venous hemofiltration in patients with sepsis or ARDS: a systematic review and meta-analysis
DOI:10.23736/S0375-9393.17.11946-2
PMID:28607338
[Cited within: 4]

Severe inflammatory conditions, as severe sepsis/septic shock and acute respiratory distress syndrome (ARDS), are related to high morbidity and mortality. We performed a meta-analysis of randomized trials to assess if blood purification with continuous veno-venous hemofiltration (CVVH) reduces mortality in these settings.Online databases were searched for pertinent studies up to March 2017. We included randomized-controlled trials on the use of CVVH as blood purification technique in comparison to conventional therapy in adult patients with severe sepsis/septic shock or ARDS but no acute kidney injury needing renal replacement therapy.Eleven studies and 679 patients were included in the analysis. Patients who received CVVH had significantly lower mortality compared to conventional therapy (96 of 351 [27.35%] patients in the CVVH group vs. 129 of 328 [39.33%] in the conventional therapy group, OR=0.58 [95% CI: 0.42, 0.81], P=0.002, I2=10%, number needed to treat: 8) at longest follow-up available.Overall, low-quality evidence indicates that blood purification with CVVH might be associated with a significant reduction in mortality when performed in patients with sepsis or ARDS. The evidence is still insufficient to support a definitive conclusion of benefit. Further high-quality randomized controlled trials, adequately powered for mortality, are needed to clarify the impact of CVVH on these conditions.
Clinical review: extracorporeal blood purification in severe sepsis
DOI:10.1186/cc1889
PMID:12720560
[Cited within: 2]

Sepsis and septic shock are the leading causes of acute renal failure, multiple organ system dysfunction, and death in the intensive care unit. The pathogenesis of sepsis is complex and comprises a mosaic of interconnected pathways. Several attempts to improve patient outcomes by targeting specific components of this network have been unsuccessful. For these reasons, the ideal immunomodulating strategy would be one that restores immunologic stability rather than blindly inhibiting or stimulating one or another component of this complex network. Hence, the recent focus of immunomodulatory therapy in sepsis has shifted to nonspecific methods of influencing the entire inflammatory response without suppressing it. Here, we discuss the various modalities of extracorporeal blood purification, the existing evidence, and future prospects.
Continuous haemofiltration in the intensive care unit
DOI:10.1186/cc718
PMID:11123877
[Cited within: 2]

Continuous renal replacement therapy (CRRT) was first described in 1977 for the treatment of diuretic-unresponsive fluid overload in the intensive care unit (ICU). Since that time this treatment has undergone a remarkable technical and conceptual evolution. It is now available in most tertiary ICUs around the world and has almost completely replaced intermittent haemodialysis (IHD) in some countries. Specially made machines are now available, and venovenous therapies that use blood pumps have replaced simpler techniques. Although, it remains controversial whether CRRT decreases mortality when compared with IHD, much evidence suggests that it is physiologically superior. The use of CRRT has also spurred renewed interest in the broader concept of blood purification, particularly in septic states. Experimental evidence suggests that this is a promising approach to the management of septic shock in critically ill patients. The evolution and use of CRRT is likely to continue and grow over the next decade.
Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1)
DOI:10.1186/cc11454 URL [Cited within: 1]
Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016
DOI:10.1007/s00134-017-4683-6
PMID:28101605
[Cited within: 2]

To provide an update to "Surviving Sepsis Campaign Guidelines for Management of Sepsis and Septic Shock: 2012".A consensus committee of 55 international experts representing 25 international organizations was convened. Nominal groups were assembled at key international meetings (for those committee members attending the conference). A formal conflict-of-interest (COI) policy was developed at the onset of the process and enforced throughout. A stand-alone meeting was held for all panel members in December 2015. Teleconferences and electronic-based discussion among subgroups and among the entire committee served as an integral part of the development.The panel consisted of five sections: hemodynamics, infection, adjunctive therapies, metabolic, and ventilation. Population, intervention, comparison, and outcomes (PICO) questions were reviewed and updated as needed, and evidence profiles were generated. Each subgroup generated a list of questions, searched for best available evidence, and then followed the principles of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system to assess the quality of evidence from high to very low, and to formulate recommendations as strong or weak, or best practice statement when applicable.The Surviving Sepsis Guideline panel provided 93 statements on early management and resuscitation of patients with sepsis or septic shock. Overall, 32 were strong recommendations, 39 were weak recommendations, and 18 were best-practice statements. No recommendation was provided for four questions.Substantial agreement exists among a large cohort of international experts regarding many strong recommendations for the best care of patients with sepsis. Although a significant number of aspects of care have relatively weak support, evidence-based recommendations regarding the acute management of sepsis and septic shock are the foundation of improved outcomes for these critically ill patients with high mortality.
Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness
DOI:10.1053/jcrc.2001.21795
PMID:11230723
[Cited within: 1]

The purpose of this article was to determine the prevalence of iron, vitamin B12, and folate deficiency and to evaluate the erythropoietin (EPO) response to anemia in a cohort of long-term intensive care unit (ICU) patients.All patients admitted to three academic medical center multidisciplinary ICUs were screened for eligibility into a randomized trial of EPO for the treatment of ICU anemia. On their second or third ICU day, patients enrolled in this trial had EPO levels drawn and were screened for iron, B12, and folate deficiency. Weekly EPO levels were obtained throughout patients' ICU stay.A total of 184 patients were screened for iron, B12, and folate deficiency. Sixteen patients (9%) were iron deficient by study criteria, 4 (2%) were B12 deficient, and 4 (2%) were folate deficient. Mean hemoglobin and reticulocyte percents of the remaining 160 patients were 10.3 +/- 1.2 g/dL and 1.66 +/- 1.09%, respectively. In most patients, serum iron and total iron binding capacity levels were very low, whereas ferritin levels were very high. Mean and median day 2 EPO levels were 35.2 +/- 35.6 mIU/mL and 22.7 mIU/mL, respectively (normal = 4.2-27.8). Serial EPO levels in most persistently anemic patients remained within the normal range.In this cohort, screening for iron, B12, and folate deficiency identified potentially correctable abnormalities in more than 13% of patients and should be considered in those who are anticipated to have long ICU stays. Even at an early point of critical illness, most patients had iron studies consistent with anemia of chronic disease (ACD), as well as a blunted EPO response that may contribute to this ACD-like anemia of critical illness.
Inflammation-induced hepcidin-25 is associated with the development of anemia in septic patients: an observational study
DOI:10.1186/cc9408 URL [Cited within: 3]
Determinants of red cell distribution width (RDW) in cardiorenal patients: RDW is not related to erythropoietin resistance
DOI:10.1016/j.cardfail.2011.04.009
PMID:21807323
[Cited within: 3]

Studies have shown that red cell distribution width (RDW) is related to outcome in chronic heart failure (CHF). The pathophysiological process is unknown. We studied the relationship between RDW and erythropoietin (EPO) resistance, and related factors such as erythropoietic activity, functional iron availability and hepcidin.In the Mechanisms of Erythropoietin Action in the Cardiorenal Syndrome (EPOCARES) study, which investigates the role of EPO in 54 iron-supplemented anemic patients with CHF and chronic kidney disease (CKD) (n = 35 treated with 50 IU/kg/wk Epopoetin beta, n = 19 control), RDW was not associated with EPO resistance. We defined EPO resistance by EPO levels (r = 0.12, P =.42), the observed/predicted log EPO ratio (r = 0.12, P = .42), the increase in reticulocytes after 2 weeks of EPO treatment (r = -0.18, P =.31), and the increase of hemoglobin after 6 months of EPO treatment (r = 0.26, P =.35). However, RDW was negatively correlated with functional iron availability (reticulocyte hemoglobin content, r = -0.48, P <.001, and transferrin saturation, r = -0.39, P =.005) and positively with erythropoietic activity (soluble transferrin receptor, r = 0.48, P <.001, immature reticulocyte fraction, r = 0.36, P =.01) and positively with interleukin-6 (r = 0.48, P <.001). No correlation existed between hepcidin-25 and RDW.EPO resistance was not associated with RDW. RDW was associated with functional iron availability, erythropoietic activity, and interleukin-6 in anemic patients with CHF and CKD.Copyright © 2011 Elsevier Inc. All rights reserved.
Erythropoietin in critical illness and trauma
DOI:S0749-0704(18)30785-1
PMID:30784609
[Cited within: 1]

Erythropoietin (EPO) is a 34kD pleiotropic cytokine that was first identified as being essential for red blood cell (RBC) production. It is now recognized however that EPO is produced by many tissues. It plays a key role in the modulation of the response to injury, inflammation, and tissue hypoxia via the inhibition of apoptosis. Large clinical trials in the critically ill failed to demonstrate a role for EPO as an RBC transfusion sparing agent; however, improved clinical outcomes, attributable to EPO role in tissue protection are observed in critically ill trauma patients. Further research to confirm or refute these observations is required.Crown Copyright © 2018. Published by Elsevier Inc. All rights reserved.
Continuous venovenous hemofiltration decreases mortality and ameliorates acute lung injury in canine model of severe salt water drowning
DOI:10.1186/s13049-016-0224-5 URL [Cited within: 1]
Hepcidin and iron homeostasis
DOI:10.1016/j.bbamcr.2012.01.014
PMID:22306005
[Cited within: 1]

Despite fluctuations in dietary iron intake and intermittent losses through bleeding, the plasma iron concentrations in humans remain stable at 10-30 μM. While most of the iron entering blood plasma comes from recycling, appropriate amount of iron is absorbed from the diet to compensate for losses and maintain nontoxic amounts in stores. Plasma iron concentration and iron distribution are similarly regulated in laboratory rodents. The hepatic peptide hepcidin was identified as the systemic iron-regulatory hormone. In the efferent arc, hepcidin regulates intestinal iron absorption, plasma iron concentrations, and tissue iron distribution by inducing degradation of its receptor, the cellular iron exporter ferroportin. Ferroportin exports iron into plasma from absorptive enterocytes, from macrophages that recycle the iron of senescent erythrocytes, and from hepatocytes that store iron. In the more complex and less well understood afferent arc, hepatic hepcidin synthesis is transcriptionally regulated by extracellular and intracellular iron concentrations through a molecular complex of bone morphogenetic protein receptors and their iron-specific ligands, modulators and iron sensors. Through as yet undefined pathways, hepcidin is also homeostatically regulated by the iron requirements of erythroid precursors for hemoglobin synthesis. In accordance with the role of hepcidin-mediated iron redistribution in host defense, hepcidin production is regulated by inflammation as well. Increased hepcidin concentrations in plasma are pathogenic in iron-restrictive anemias including anemias associated with inflammation, chronic kidney disease and some cancers. Hepcidin deficiency causes iron overload in hereditary hemochromatosis and ineffective erythropoiesis. Hepcidin, ferroportin and their regulators represent potential targets for the diagnosis and treatment of iron disorders and anemias. This article is part of a Special Issue entitled: Cell Biology of Metals.Copyright © 2012 Elsevier B.V. All rights reserved.
Bench-to-bedside review: iron metabolism in critically ill patients
DOI:10.1186/cc2862 URL [Cited within: 4]
Mediator removal with CRRT: complement and cytokines
DOI:10.1016/S0272-6386(97)90541-2 URL [Cited within: 2]
Reduction of serum hepcidin by hemodialysis in pediatric and adult patients
DOI:10.2215/CJN.08161109 URL [Cited within: 1]