INTRODUCTION
Catestatin, an active peptide derived from chromogranin A (CgA), was initially discovered as an endogenous antagonist of the nicotinic cholinergic receptor, which inhibited catecholamine release from both chromaffin cells and noradrenergic neurons. Subsequently, catestatin was found to act as a potent vasodilator by stimulating histamine excretion from mast cells.[1] Catestatin is a multifunctional neuroendocrine peptide that is closely related to many cardiovascular diseases.[2⇓-4] In metabolic syndromes, serum catestatin levels are decreased compared to control patients.[5]Catestatin is responsible for hepatic/plasma lipid and insulin regulation, improving insulin sensitivity, reducing hypertension, and attenuating obesity in murine models. Lower catestatin level is a significant risk factor for hypertensive adult patients.[6]Plasma levels of catestatin are higher in patients with heart failure than those in the healthy control group.[7]Catestatin also reflects myocardial fibrosis and sympathetic overactivity during acute worsening of heart failure or acutely decompensated heart failure.[8,9] The predictive value of plasma catestatin in chronic heart failure patients was also reported in a study conducted on 202 patients with chronic heart failure, in which catestatin was an independent and strong risk factor for all-cause mortality and cardiac death.[10] For coronary artery disease (CAD), conflicting results have been recently reported with increased or decreased plasma levels of catestatin in patients with CAD. An early study showed that catestatin levels increased after acute myocardial infarction (AMI).[11] Increased circulating catestatin levels have also been correlated with the occurrence of malignant arrhythmia,[12] adverse events (death from cardiovascular causes, readmission with ACS or CHF),[13] and left ventricular remodeling after AMI.[11,14]In agreement with these results, we previously found that plasma catestatin levels decreased in patients with AMI and unstable angina compared to patients without a diagnosis of CAD, and there were no associations between catestatin and major adverse cardiac events (MACEs).[15]Recent studies have reported that circulating levels of catestatin significantly decreased in patients with CAD compared with the healthy control group.[16,17] Furthermore, serum catestatin concentration was inversely associated with the severity of atherosclerosis.[17]
Studies focusing on the pathophysiological mechanisms report that catestatin suppresses plaque progression and preserves vascular elasticity by suppressing lipopolysaccharide (LPS)- or tumor necrosis factor-α (TNF-α)-induced expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in human embryonic cells (ECs), suppressing the inflammation in human macrophages, the proliferation of human vascular smooth muscle cells (VSMCs), and the expression of collagen-1 and collagen-3, and by increasing elastin expression.[18,19] Catestatin can be used to defend against oxidative stress-induced apoptosis by activating the beta 2 adrenergic receptor and protein kinase B (PKB/Akt) pathway in the ischemic-reperfused myocardium.[20] In cultured ECs, catestatin suppressed TNF-α-induced expression of inflammatory cytokines and adhesion molecules by activating angiotensin-converting enzyme 2 (ACE2).[17]These multiple effects establish catestatin as a master regulator of cardiovascular functions.[3] Catestatin might be used as a new biological marker for cardiovascular diseases; however, further studies are needed.
There have been no studies about the predictive value of catestatin for AMI in elderly patients. Our previous study found that catestatin levels were not associated with MACEs during a follow-up period of two years.[15]However, outstanding questions still remain such as whether catestatin can predict MACEs if the follow-up period is longer or whether the predictive value of catestatin differs between young and elderly patients with AMI.
The present study aims to investigate the associations between plasma catestatin levels and long-term outcomes of AMI and the differences in the prognostic value of catestatin in young and elderly patients with AMI.
METHODS
Subjects
Patients with AMI who underwent emergency percutaneous coronary intervention (PCI) for the first time within 12 h after symptom onset between July 2012 and December 2013 at Peking University Third Hospital were enrolled in this series. AMI was diagnosed based on the universal definition of MI[21,22]: troponin elevation in conjunction with ischemic symptoms, ischemic electrocardiogram changes, and imaging evidence. AMI included ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI), according to the universal definition of myocardial infarction put forth by the European Society of Cardiology and the American Heart Association.[23]
The exclusion criteria were as follows: patients whose symptom onset lasted more than 12 h; patients who underwent thrombolysis, unsuccessful PCI (Thrombolysis in Myocardial Infarction [TIMI] score from 0 to 2 at the end of the procedure), or emergency surgery; patients with rheumatic heart disease, severe valvular heart disease, chronic congestive heart failure, cardiomyopathy, deep venous thrombosis, acute pulmonary embolism, infection, systemic inflammatory, tumor disease, documented renal failure, or clinical evidence of renal impairment.
The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of the Peking University Third Hospital. Written informed consent was obtained before coronary angiography.
Assays for plasma catestatin
Blood samples for catestatin measurements were obtained from the antecubital vein without stasis in all patients before coronary angiography. Blood samples were collected in chilled ethylene diamine tetra acetate (EDTA) vacutainers containing 2,500 U/mL aprotinin, immediately centrifuged at 3,000 r/min for 10 min at 4 °C, and stored at -80 °C until analysis. Plasma catestatin levels were measured using a catestatin ELISA kit (Phoenix Pharmaceuticals, USA), according to the manufacturer's instructions. Measurements were obtained by trained technicians in the clinical laboratory of the hospital.
Medical data
The clinical and laboratory data of all subjects were collected from medical records. Clinical data included risk factors for coronary heart disease, such as age, sex, hypertension, dyslipidemia, diabetes mellitus (DM), smoking, and body mass index. Left ventricular ejection fraction (LVEF) was assessed by echocardiography using Simpson's method during the first 24 h after admission to the cardiac care unit. Other laboratory indicators, such as routine blood tests and lipid and glucose levels, were also assessed.
MACEs
All patients were followed-up every three months through clinical interviews or telephone calls to collect information regarding MACEs after discharge. Patients were followed-up until the first MACE within four years. If no MACEs occurred, the patients were followed up for four years after discharge. MACEs included cardiovascular death, recurrent AMI, re-hospitalization for heart failure, and revascularization. All MACEs were confirmed using the medical records.
Statistical analysis
Descriptive statistics were used for all baseline variables, with means and standard deviations for normally distributed variables; medians and interquartile ranges for non-normally distributed variables; and rates and proportions for categorical variables. Student's t-test and Pearson's Chi-square test were used to compare differences between the groups. Kaplan-Meier hazard analyses were plotted for AMI patients with low and high catestatin levels according to the median level (0.86 ng/mL). The association between catestatin and MACEs was assessed using Cox proportional hazards regression, in which confounders such as sex, hypertension, dyslipidemia, DM, smoking, and LVEF were also included. All analyses were carried out among young and elderly patients with a cut-off age of 60 years. All tests of significance were two-tailed. Statistical significance was defined as P<0.05. Statistical analysis was performed using SPSS (Version 19.0).
RESULTS
The final study included 165 patients, including 113 with NSTEMI and 52 with STEMI. There were 24 MACEs (16 readmissions for revascularization, three recurrent myocardial infarctions, and five rehospitalizations for heart failure) among 165 patients, including 102 young patients (<60 years old) and 63 elderly patients (≥60 years old). The MACEs group had lower catestatin levels than the non-MACEs group (0.74±0.49 ng/mL vs. 1.10±0.79 ng/mL, P=0.033) and were older (59.0±11.4 years old vs. 53.2±12.8 years old, P=0.036). The rate of MACEs was significantly higher in the elderly group than in the young group (23.8% [15/16] vs. 8.8% [9/102], P=0.008). The proportion of females was higher in the elderly group than in the young group (P=0.001). Elderly patients were more likely to have hypertension (P=0.009), but were less likely to smoke (P=0.001). The rates of dyslipidemia and DM were not significantly different between the young and elderly groups. The counts of white blood cell (WBC) and platelet (PLT) were lower in the elderly group (P=0.005, P=0.009). The catestatin levels in the elderly group were not significantly higher than those in the young group (P=0.069). There were no significant differences among the other indicators. The details were shown in supplementary Table 1.
Table 1. The differences of baseline clinical characteristics between MACE and non-MACE group respectively among young and elderly patients
| Variables | Young patients (<60 years old) | Elderly patients (≥60 years old) | |||||
|---|---|---|---|---|---|---|---|
| Non-MACE (n=93) | MACE (n=9) | P | Non-MACE (n=48) | MACE (n=15) | P | ||
| Male | 79 (84.9) | 7 (77.8) | 0.572 | 28 (58.3) | 11 (73.3) | 0.296 | |
| Hypertension | 54 (58.1) | 7 (77.8) | 0.249 | 41 (85.4) | 9 (60.0) | 0.034 | |
| Dyslipidemia | 57 (61.3) | 5 (55.6) | 0.737 | 26 (54.2) | 10 (66.7) | 0.393 | |
| DM | 27 (29.0) | 3 (33.3) | 0.787 | 15 (31.3) | 3 (20.2) | 0.400 | |
| Smoke | 62 (66.7) | 6 (66.7) | 0.990 | 23 (47.9) | 9 (60.0) | 0.330 | |
| BMI, kg/m2 | 26.4±2.6 | 26.1±3.4 | 0.802 | 25.8±3.9 | 27.1±2.8 | 0.346 | |
| Age, years old | 45.4±7.1 | 46.2±5.8 | 0.745 | 68.1±6.5 | 66.7±6.3 | 0.484 | |
| WBC, ×109/L | 8.73±3.32 | 9.52±2.93 | 0.517 | 6.72±2.69 | 8.07±2.88 | 0.149 | |
| HB, g/L | 145.45±13.74 | 131.63±17.33 | 0.009* | 130.61±14.07 | 161.00±74.46 | 0.017* | |
| PLT, ×109/L | 217.76±47.02 | 210.75±77.17 | 0.707 | 182.24±70.31 | 205.31±57.50 | 0.307 | |
| UA, μmol/L | 366.24±171.85 | 325.38±96.28 | 0.511 | 340.19±89.81 | 356.21±67.89 | 0.550 | |
| TCHO, mmol/L | 4.66±1.17 | 4.76±0.98 | 0.800 | 4.33±0.96 | 4.77±0.88 | 0.146 | |
| TG, mmol/L | 2.29±1.44 | 2.12±1.02 | 0.733 | 1.90±2.15 | 2.08±0.96 | 0.762 | |
| HDL-C, mmol/L | 0.93±0.21 | 0.93±0.18 | 0.997 | 1.04±0.25 | 0.94±0.28 | 0.203 | |
| LDL-C, mmol/L | 2.95±1.12 | 2.96±0.86 | 0.964 | 2.53±0.72 | 2.96±0.62 | 0.050 | |
| GLU, mmol/L | 5.97±2.42 | 6.31±2.67 | 0.702 | 5.20±0.83 | 5.82±2.44 | 0.238 | |
| HBA1C, % | 6.52±1.46 | 7.13±1.96 | 0.342 | 6.38±1.22 | 6.24±1.02 | 0.768 | |
| LVEF, % | 64.56±10.58 | 53.67±10.27 | 0.004* | 65.44±11.29 | 62.33±11.15 | 0.358 | |
| Cr, μmol/L | 86.21±18.80 | 76.56±17.40 | 0.142 | 84.94±15.92 | 92.67±21.04 | 0.135 | |
| CK-MB, U/L | 58.52±19.67 | 153.25±75.63 | 0.014* | 56.28±19.52 | 50.85±14.66 | 0.879 | |
| NT-proBNP, pg/mL | 890.36±117.88 | 1309.29±440.22 | 0.552 | 1302.17±511.18 | 513.70±325.02 | 0.642 | |
| Catestatin, ng/mL | 0.98±0.78 | 0.69±0.49 | 0.275 | 1.31±0.77 | 0.76±0.50 | 0.012* | |
Data are expressed as means ± standard deviations or n (%). MACE: major adverse cardiovascular events; DM: diabetes mellitus; BMI: body mass index; WBC: white blood cell; HB: hemoglobin ; PLT: platelet ; UA: uric acid; TCHO: total cholesterol; TG: triglyceride; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; GLU: blood glucose; HBA1C: glycosylated hemoglobin A1C; LVEF:left ventricular ejection fraction; Cr: creatinine; CK-MB: creatine kinase-myocardial band isoenzyme; NT-proBNP: N-terminal pro-B-type natriuretic peptide. *P<0.05.
As shown in Table 1, the young patients with MACEs had lower hemoglobin (P=0.009) and lower LVEF (P=0.004). The catestatin levels did not significantly differ between the MACEs group and the non-MACEs group in the young group, while they were significantly lower in the elderly group (0.76±0.50 ng/mL vs. 1.31±0.77 ng/mL, P=0.012). Other baseline characteristics were similar between the MACEs and non-MACEs groups.
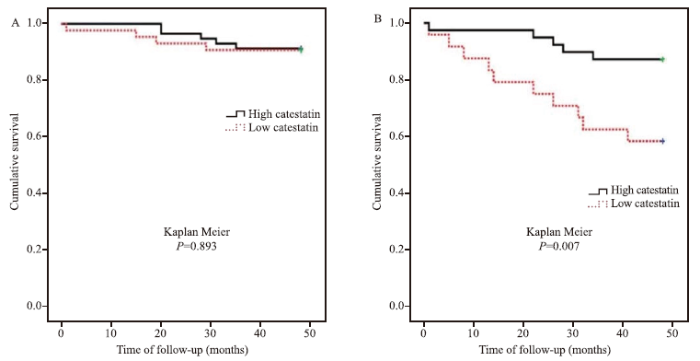
MACEs were significantly different between low and high levels of catestatin (Kaplan Meier, P=0.007) in the elderly group, but not in the young group (Kaplan Meier, P=0.893) (Figure 1). In the Cox proportional hazards regression analysis to explore the predictors of MACEs among elderly patients, high catestatin was one of the independent factors (hazard ratio [HR] 0.19, 95% confidence interval [CI] 0.06-0.62, P=0.006) after adjustment for other risk factors (Table 2). However, in the young patients, LVEF, but not catestatin, was one of the independent factors (HR 0.93, 95% CI 0.88-0.98, P=0.004) (Table 3).
Figure 1.
Figure 1.
The associations between catestatin and major adverse cardiovascular events (MACEs) among young and elderly patients. A: the MACEs were not significantly different between low and high levels of catestatin among young patients (Kaplan Meier, P=0.893); B: elderly patients with lower catestatin levels were more likely to have MACEs (Kaplan Meier, P=0.007).
Table 2. The Cox regression model for MACEs among elderly patients
| Variables | ꞵ | HR | 95%CI | P |
|---|---|---|---|---|
| Gender | -0.190 | 0.83 | 0.23-3.01 | 0.774 |
| Hypertension | -0.822 | 0.44 | 0.15-1.33 | 0.146 |
| Dyslipidemia | 0.659 | 1.93 | 0.60-6.28 | 0.273 |
| DM | -0.949 | 0.39 | 0.10-1.57 | 0.184 |
| Smoke | 0.446 | 1.56 | 0.49-5.01 | 0.454 |
| LVEF | -0.025 | 0.98 | 0.93-1.03 | 0.351 |
| Catestatin | -1.655 | 0.19 | 0.06-0.62 | 0.006* |
HR: hazard ratio; CI: confidence interval; DM: diabetes mellitus; LVEF: left ventricular ejection fraction. *P<0.05.
Table 3. The Cox regression model for MACEs among among young patients
| Variables | ꞵ | HR | 95%CI | P |
|---|---|---|---|---|
| Gender | 0.851 | 2.34 | 0.26-21.44 | 0.451 |
| Hypertension | 0.918 | 2.50 | 0.50-12.65 | 0.267 |
| Dyslipidemia | -0.342 | 0.71 | 0.16-3.17 | 0.654 |
| DM | -0.590 | 0.55 | 0.11-2.82 | 0.477 |
| Smoke | 0.382 | 1.47 | 0.23-9.55 | 0.690 |
| LVEF | -0.075 | 0.93 | 0.88-0.98 | 0.004* |
| Catestatin | -0.366 | 0.69 | 0.15-3.30 | 0.645 |
HR: hazard ratio; CI: confidence interval; DM: diabetes mellitus; LVEF: left ventricular ejection fraction. *P<0.05.
DISCUSSION
In the present study, patients with lower plasma catestatin were more likely to have MACEs. Plasma catestatin levels were an independent factor for MACEs after adjusting for confounders. These findings were significant among elderly patients but not among young patients. The prognostic value of catestatin for MACEs among patients with AMI differed between the young and elderly groups.
Our findings are consistent with those of previous studies.[15,17,24] Chen et al[17] reported that serum catestatin concentration was lower in patients with CAD and that decreased circulating catestatin levels were inversely correlated with disease severity. Low plasma catestatin is a valuable prognostic parameter in predicting death from all causes and unplanned hospitalization in patients with heart failure with reduced ejection fraction at the two-year follow-up.[24] In our previous study,[15] patients with CAD also had lower catestatin levels. However, catestatin did not predict MACEs at the two-year follow-up. In the present study, when patients were divided into two groups (young or elderly patients), we found that catestatin was an independent factor for MACEs after adjusting for confounders among elderly patients during the four-year follow-up. Compared with low catestatin levels, high levels of catestatin reduced the MACE risk (HR 0.19).
These data show that catestatin is a master regulator of cardiovascular function through multiple mechanisms. (1) Catestatin exerts vasodilatory effects through inhibiting catecholamine secretion, stimulating histamine release,[3] and increasing nitric oxide (NO) release from vascular ECs.[25] Catestatin infusion directly dilates blood vessels and reduces blood pressure.[26] (2) Catestatin exerts anti-atherosclerotic effects. (3) Catestatin exerts protective effects against ischemia/reperfusion-induced myocardial dysfunction via the NO-dependent pathway in rats.[27]Catestatin also exerts direct protective effects on rat cardiomyocytes undergoing ischemia/reperfusion by stimulating the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/glycogen synthase kinase 3β (GSK3β) pathway.[28] (4) Catastatin reduces inflammation through its role in tissue homeostasis by regulating immune cell infiltration and macrophage differentiation. Catestatin has been shown to reduce inflammation in various mouse models of diabetes, colitis, and atherosclerosis where catestatin treatment resulted in less infiltration of immune cells into affected tissues.[29] (5) Catestatin plays an important role in angiogenesis by stimulating the migration and proliferation of ECs and contributing to ischemia-induced angiogenesis.[30] Catestatin is associated with the development of coronary collateral circulation and chronic myocardial ischemia.[31] A growing body of evidence suggests that catestatin may be an interesting therapeutic target for CAD treatment and/or diagnosis.
In the present study, age was an important factor affecting the predictive value of catestatin. Among elderly patients, high levels of catestatin can reduce the risk of MACEs, but not in young patients. Pathophysiological states can change with age, which may explain the conflicting results regarding reports of increased or decreased plasma levels of catestatin in patients with CAD. Other factors may also have contributed to the variance in the results of different studies. Catestatin changes with the time of onset of ischemia; [13]therefore, the time point of the sample would lead to differences in catestatin levels. The types of CAD and concomitant diseases could also affect catestatin levels.
This study provides new insights into catestatin at different ages. However, there are some limitations to this study that need to be addressed. First, catestatin levels were measured at only one time point; therefore, dynamic changes in each patient were not obtained. Other indicators such as CgA and norepinephrine were not assessed in this study. Second, we did not examine the mechanism underlying the association between catestatin use and MACEs. Third, the sample size of this study was small. Prospective, large-sample trials are needed to investigate the associations and mechanisms between plasma catestatin and MACEs.
CONCLUSIONS
Elderly patients with lower plasma catestatin levels were more likely to have MACEs. Catestatin may be a novel marker for the long-term prognosis of AMI, especially in elderly patients. More efforts to investigate the key roles of catestatin bioactivity and to detect its levels at different time points in various ages are necessary to thoroughly assess its pathophysiological function and value as a prognostic marker.
Funding: This work was supported by the National Natural Science Foundation of China (81400319).
Ethical approval: The study protocol conformed to the ethical guidelines of the Declaration of Helsinki. The written informed consent to participate in the study were obtained from all participants. The study was approved by the Institutional Review Board of Peking University Third Hospital.
Conflicts of interests: The authors have no conflicts of interest to declare.
Contributors: WXX and YYF are the co-first authors. WXX, YYF, and LJG designed and carried out the study, wrote and revised the manuscript; YS and XL collected and analyzed data; HL analyzed the data and revised the manuscript. All authors reviewed the manuscript.
Reference
Catestatin: a multifunctional peptide from chromogranin A.
DOI:10.1016/j.regpep.2010.01.006 URL [Cited within: 1]
The emerging roles of chromogranins and derived polypeptides in atherosclerosis, diabetes, and coronary heart disease
DOI:10.3390/ijms22116118 URL [Cited within: 1]
Catestatin: a master regulator of cardiovascular functions
DOI:10.2174/0929867324666170425100416 URL [Cited within: 3]
Potential applications of catestatin in cardiovascular diseases
DOI:10.2217/bmm-2016-0086 URL [Cited within: 1]
Serum catestatin concentrations are decreased in obese children and adolescents
DOI:10.1111/pedi.12825
PMID:30714297
[Cited within: 1]

Catestatin is a chromogranin A-derived peptide with a wide spectrum of biological activities, such as inhibiting catecholamine release, decreasing blood pressure, stimulating histamine release, reducing beta-adrenergic stimulation, and regulating oxidative stress.The aims of our study were to determine serum catestatin concentrations in obese children and adolescents in regard to presence or absence of metabolic syndrome (MS) and to evaluate the possible relations between catestatin levels and other cardiovascular risk factors.Ninety-two obese subjects with a body mass index z score > 2, aged 10 to 18 years, and 39 healthy, normal weight controls were enrolled in the study.Serum catestatin concentrations were measured using an enzyme-linked immunosorbent assay.Significantly lower serum catestatin concentrations were recorded in the group of obese subjects compared with a control group (10.03 ± 5.05 vs 13.13 ± 6.25 ng/mL, P = 0.004). Further analyses revealed significantly lower catestatin concentrations in the subgroup of obese patients with MS (9.02 ± 4.3 vs 10.54 ± 5.36 vs 13.13 ± 6.25, P = 0.008). Serum catestatin concentrations were significantly negatively correlated with diastolic blood pressure (r = -0.253, P = 0.014), homeostatic model assessment of insulin resistance (r = -0.215, P = 0.037) and high sensitivity C-reactive protein (r = -0.208, P = 0.044).To the best of our knowledge, this study is the first to report catestatin concentrations in obese children and adolescents and their possible relations with MS and cardiovascular risk factors in a pediatric population. Obese subjects with MS have lower serum catestatin concentrations than obese subjects without MS and controls.© 2019 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Catestatin peptide of chromogranin A as a potential new target for several risk factors management in the course of metabolic syndrome
Plasma levels and diagnostic value of catestatin in patients with heart failure
DOI:10.1016/j.peptides.2013.05.003 URL [Cited within: 1]
Circulating sST2 and catestatin levels in patients with acute worsening of heart failure: a report from the CATSTAT-HF study
Catestatin in acutely decompensated heart failure patients: insights from the CATSTAT-HF study
The novel chromogranin A-derived serpinin and pyroglutaminated serpinin peptides are positive cardiac β-adrenergic-like inotropes
DOI:10.1096/fj.11-201111 URL [Cited within: 1]
Reduced serum levels of vasostatin-2, an anti-inflammatory peptide derived from chromogranin A, are associated with the presence and severity of coronary artery disease
DOI:10.1093/eurheartj/ehs122 URL [Cited within: 2]
Usefulness of catestatin to predict malignant arrhythmia in patients with acute myocardial infarction
DOI:10.1016/j.peptides.2014.02.016 URL [Cited within: 1]
Correlation of plasma catestatin level and the prognosis of patients with acute myocardial infarction
DOI:10.1371/journal.pone.0122993 URL [Cited within: 2]
Catestatin-A novel predictor of left ventricular remodeling after acute myocardial infarction
DOI:10.1038/srep44168 URL [Cited within: 1]
Plasma catestatin in patients with acute coronary syndrome
DOI:10.1159/000448987 URL [Cited within: 4]
Catestatin prevents macrophage-driven atherosclerosis but not arterial injury-induced neointimal hyperplasia
DOI:10.1160/TH17-05-0349 URL [Cited within: 1]
Decreased circulating catestatin levels are associated with coronary artery disease: the emerging anti-inflammatory role
DOI:10.1016/j.atherosclerosis.2018.12.025 URL [Cited within: 5]
Catestatin prevents macrophage-driven atherosclerosis but not arterial injury-induced neointimal hyperplasia
DOI:10.1160/TH17-05-0349 URL [Cited within: 1]
Inhibitory effects of vasostatin-1 against atherogenesis
DOI:10.1042/CS20180451 URL [Cited within: 1]
Catestatin in defense of oxidative-stress-induced apoptosis: a novel mechanism by activating the beta 2 adrenergic receptor and PKB/Akt pathway in ischemic-reperfused myocardium
DOI:10.1016/j.peptides.2019.170200 URL [Cited within: 1]
ESC guidelines on management of acute myocardial infarction in patients presenting with persistent ST-segment elevation
The 2012 ACCF/AHA focused update of the unstable angina/non-ST-elevation myocardial infarction (UA/NSTEMI) guideline: a critical appraisal
DOI:10.14797/mdcj-8-3-26
PMID:23227283
[Cited within: 1]

The American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) recently published the 2012 ACCF/AHA Focused Update of the Guidelines for the Management of Patients with Unstable Angina and Non-ST-Elevation Myocardial Infarction (Updating the 2007 Guideline and Replacing the 2011 Update).(1) These guidelines were developed in collaboration with multiple societies and represent an important landmark in the management of patients with unstable angina (UA) and non-ST-elevation myocardial infarction (NSTEMI). This paper provides a critical overview of some of the clinically relevant novel and modified recommendations proposed by the updated guideline.
A novel prediction model of acute kidney injury based on combined blood variables in STEMI
DOI:10.1016/j.jacasi.2021.07.013 URL [Cited within: 1]
Catestatin as a new prognostic marker in stable patients with heart failure with reduced ejection fraction in two-year follow-up
Endothelium dependent cardiovascular effects of the chromogranin A-derived peptides vasostatin-1 and catestatin
PMID:22834796
[Cited within: 1]

The involvement of Chromogranin A (CgA) in the cardiovascular function regulation is attributed to its function as a prohormone. Several studies indicated that CgA-derived peptides, particularly Vasostatin-1 (VS-1) and Catestatin (CST), exert signaling effects in numerous organs/systems, including the cardiovascular system. This review focuses on the recently described signaling pathways activated by VS-1 and CST, giving insights into the mechanisms at the basis of their cardiac negative inotropic action, their vasodilator effects and their cardioprotective role observed in different experimental conditions. Accumulated evidences provided convincing support for VS-1 and CST as vasoactive peptides indirectly acting on cardiomyocytes through a Ca(2+)-independent/PI3-K-dependent NO release from endothelial cells. This pathway is supposed to be triggered by the interaction of these peptides with the plasma membrane. The premise of these studies grounds on the biochemical features of VS-1 and CST, which are structurally characterized by amphipathic properties and the ability to interact with mammalian and microbial membranes. On the other hand, recent data obtained in both isolated heart and isolated cardiomyocytes suggest that the VS-1 and CST-mediated cardioprotective effects are primarily direct on the myocardium, rather than endothelium-dependent. Anyway, both direct and indirect pathways seem to be characterized by the absence of specific membrane receptors on target cells, highlighting intriguing novelties in the topic of cell signaling, in particular respect to an hypothetical receptor-independent eNOS activation.
Direct vasoactive effects of the chromogranin A (CgA) peptide catestatin in humans in vivo
DOI:10.3109/10641960903265246 URL [Cited within: 1]
Chromofungin, CgA47-66-derived peptide, produces basal cardiac effects and postconditioning cardioprotective action during ischemia/reperfusion injury
DOI:10.1016/j.peptides.2015.06.013 URL [Cited within: 1]
Catestatin exerts direct protective effects on rat cardiomyocytes undergoing ischemia/reperfusion by stimulating PI3K-Akt-GSK3β pathway and preserving mitochondrial membrane potential
DOI:10.1371/journal.pone.0119790 URL [Cited within: 1]
Catestatin as a target for treatment of inflammatory diseases
DOI:10.3389/fimmu.2018.02199
PMID:30337922
[Cited within: 1]

It is increasingly clear that inflammatory diseases and cancers are influenced by cleavage products of the pro-hormone chromogranin A (CgA), such as the 21-amino acids long catestatin (CST). The goal of this review is to provide an overview of the anti-inflammatory effects of CST and its mechanism of action. We discuss evidence proving that CST and its precursor CgA are crucial for maintaining metabolic and immune homeostasis. CST could reduce inflammation in various mouse models for diabetes, colitis and atherosclerosis. In these mouse models, CST treatment resulted in less infiltration of immune cells in affected tissues, although in vitro monocyte migration was increased by CST. Both in vivo and in vitro, CST can shift macrophage differentiation from a pro- to an anti-inflammatory phenotype. Thus, the concept is emerging that CST plays a role in tissue homeostasis by regulating immune cell infiltration and macrophage differentiation. These findings warrant studying the effects of CST in humans and make it an interesting therapeutic target for treatment and/or diagnosis of various metabolic and immune diseases.
The neuropeptide catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism
DOI:10.1161/CIRCRESAHA.110.219493 URL [Cited within: 1]
Plasma catestatin: a useful biomarker for coronary collateral development with chronic myocardial ischemia
DOI:10.1371/journal.pone.0149062 URL [Cited within: 1]