Dear editor,
According to the World Health Organization, ischemic heart disease and stroke were the top 2 leading causes of death in 2019.[1] In the past few years, intravenous recombinant tissue-type plasminogen activator (rt-PA) has been a significantly effective treatment to increase survival and reduce mortality in acute ischemic stroke.[2,3] However, as the number of patients who receive alteplase is increasing, a rare but potentially life-threatening adverse complication of alteplase administration is becoming more common—orolingual angioedema (OA).[4,5,6] OA is acute swelling of the tongue, lips or face and may be life-threatening, as it can increase the risk of upper airway obstruction. Some studies have found that angiotensin-converting enzyme (ACE) inhibitors could increase the risk of OA caused by rt-PA intravenous thrombolysis, which is related to an anaphylactoid reaction.[5,6,7] However, it has also presented in acute stroke patients not taking these medications. The rt-PA-related OA has rarely been reported in Asian countries, especially in China. Herein, we report a case of an elderly Chinese man who experienced OA after thrombolysis in emergency conditions, which might have been caused both by acute hypersensitivity and anaphylactoid reactions. We suggest that increased attention should be given during or after alteplase therapy.
CASE
A 77-year-old Chinese man with known hypertension and a previous transient ischemic attack presented to the emergency department of our hospital at 2 hours after the acute onset of dysarthria and right-sided weakness. He was only taking irbesartan. On arrival at the hospital, his blood pressure was 120/70 mmHg (1 mmHg=0.133 kPa), heart rate was 92 beats per minute, and the National Institutes of Health Stroke Scale (NIHSS) score was 3. His complete blood count, serum glucose, coagulation parameters, and electrocardiography were normal. A brain computed tomography (CT) scan was negative for any acute hemorrhage. A brain CT angiography indicated no evidence of artery embolism. This patient met the criteria to treat acute ischemic stroke with intravenous alteplase. He was given 0.9 mg/kg alteplase, including a 10% bolus initially, and the remaining drug was infused over 60 minutes. Treatment with intravenous alteplase was initiated at 144 minutes after stroke onset.
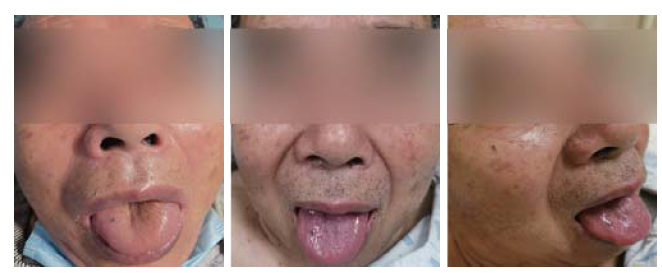
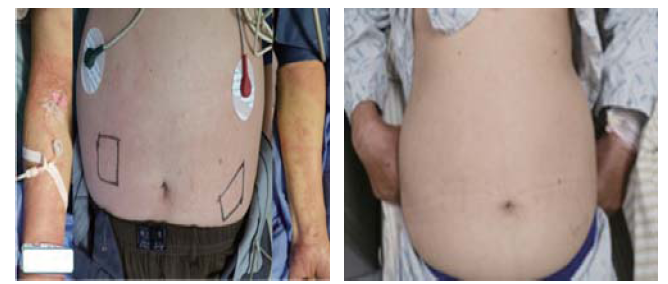
At 37 minutes after treatment initiation, the patient presented some discomfort and itching. An urticarial rash was noted in areas of his abdomen and arms. Then, his tongue, lips, and periorbital region developed extensive bilateral swelling (Figures 1 and 2), and his blood pressure dropped to 89/62 mmHg. Alteplase infusion was discontinued after he received a total of 37 mg of alteplase. He was immediately administered intravenous methylprednisolone (40 mg), crystalloid, and oral loratadine (8.8 mg). The patient’s blood pressure rose to 103/78 mmHg 15 minutes later. No dyspnea or bronchospasm was observed. We tested his blood and found that his serum levels of IgE were elevated to 103.65 U/mL (normal level ≤60 U/mL) after 6 hours. Magnetic resonance imaging/angiography demonstrated mild intracranial atherosclerosis of the cerebral arteries. His OA and urticarial rash resolved completely within 24 hours (Figures 1 and 2). His neurologic deficits improved, and the NIHSS score dropped to 0. After two weeks, the patient was discharged without sequelae.
DISCUSSION
Apart from intracranial hemorrhage, allergic reactions and other adverse effects of rt-PA have gradually received increasing attention from clinicians. The incidence of rt-PA-related OA has been reported to be 0.89%-7.90% in acute ischemic stroke patients. However, in Asians, the rt-PA-related OA incidence is only 0.89%.[8,9,10] There is only one large sample size report in China that recruited 1,223 patients at one center over one year and found that 1.14% of patients developed rt-PA-related OA.[7] Most OA is mild and reversible within 24 hours; however, in a few severe cases, it can rapidly progress and become life-threatening. Patients need rapid establishment of emergent airway management with intubation or cricothyrotomy and to be transferred to the ICU.
Currently, the underlying pathophysiology and influential factors for rt-PA-related OA are unclear. Although alteplase is an endogenous protein and relatively safe, it may also provoke immunogenic reactions, which have been found to be anti-alteplase IgE antibodies in one case of rt-PA-related OA. We also found that serum IgE levels in this patient were significantly elevated 6 hours later without ischemic lesions in the insula and frontal lobe. Compared with previous reports of similar cases, the interval between the patient’s symptom onset and alteplase infusion was within the typical range of allergic-type I reactions.[11] Although anaphylactic-type reactions to alteplase seem to be extremely rare, we hypothesize that our patient suffered an anaphylactic reaction.
Figure 1.
Figure 1.
Orolingual angioedema following thrombolysis for ischemic stroke after 37 minutes (left) and the vanishing of the symptoms of angioedema after 24 hours (middle, right).
Figure 2.
Figure 2.
Urticarial rash following thrombolysis for ischemic stroke after 37 minutes (left) and the vanishing after 24 hours (right).
Furthermore, rt-PA-related OA might be an anaphylactoid reaction, which is attributed to the activation of the vasoactive mediators histamine and plasmin of the bradykinin and complement pathways.[11] Concurrent use of an ACE inhibitor and rt-PA is proposed to cause further bradykinin accumulation by blocking plasma kininases, which may increase the risk of OA. In fact, angiotensin receptor blockers (ARBs) could also increase bradykinin levels.[12] According to the empirical clinical evidence, our treatment with antihistamines and corticosteroids is based on the treatment of angioedema and anaphylactic shock. We avoided to use epinephrine, which could increase the risk of intracerebral hemorrhage and a sudden increase in blood pressure.
CONCLUSIONS
OA should be considered a possible life-threatening anaphylactoid reaction after rt-PA treatment despite a little-known adverse event in China. Although the incidence of anaphylactic reactions of rt-PA is extremely rare, the occurrence cannot be ruled out. The prompt and appropriate management of our ischemic stroke patient improved the neurological outcome. Emergency physicians should be aware of the risk that rt-PA-related OA may occur in intravenous thrombolysis patients who use ARBs.
Funding: None.
Ethical approval: The study was approved by the Ethics Committee of Jiangsu Province Hospital.
Conflicts of interests: The authors have no competing interests relevant to the present study.
Contributors: YW wrote the study. All authors read and approved the final manuscript.
Reference
Toward “zero” cardiovascular events in Asia: the HOPE Asia Network
DOI:10.1016/j.jacasi.2021.03.003 URL [Cited within: 1]
Tissue plasminogen activator for acute ischemic stroke
DOI:10.1056/NEJM199512143332401 URL [Cited within: 1]
Canadian Alteplase for Stroke Effectiveness Study I. Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study
DOI:10.1503/cmaj.1041561 URL [Cited within: 1]
Effectiveness of an educational program on improving healthcare providers' knowledge of acute stroke: a randomized block design study
DOI:10.5847/wjem.j.1920-8642.2021.02.002
PMID:33728000
[Cited within: 1]

Stroke is a time-sensitive neurological disease and a life-threatening medical condition. Providing timely management for stroke patients is a crucial issue in healthcare settings. The primary objective of this study is to evaluate the effectiveness of an evidence-based educational program on healthcare providers' (HCPs) overall knowledge of stroke.A randomized block design with post-test only was used. A total of 189 HCPs (physicians, registered nurses, and paramedics) involved with treating stroke patients in the emergency were recruited. Participants were randomly assigned to either the intervention or waiting list control group. A one-session, stroke educational program was offered to the HCPs followed by a post-test designed to assess knowledge about stroke.A significant main effect on the profession type was found, with physicians having higher mean scores of stroke knowledge compared with nurses and paramedics ( [2, 183]=48.55, <0.001). The implemented educational program had a positive effect on increasing the level of stroke knowledge among HCPs ( [1, 183]=43.31, <0.001). The utilization of any evidence-based assessment tools for patients with suspected stroke was denied by 36% of the total sample.The implemented intervention can increase HCP's knowledge regarding stroke. Stroke education should be considered as one of the essential requirements for professional development for all HCPs in the emergency.Copyright: © World Journal of Emergency Medicine.
Life-threatening orolingual angioedema during thrombolysis in acute ischemic stroke
PMID:16184341
[Cited within: 2]

Orolingual angioedema can occur during thrombolysis with alteplase in stroke patients. However, data about its frequency, severity and the significance of concurrent use of angiotensin-converting-enzyme inhibitors (ACEi) are sparse.(1), to alert to the potentially life-threatening complication of orolingual angioedema. (2), to present CT-scans of the tongue which exclude lingual hematoma. (3), to estimate the frequency of orolingual angioedema. (4), to evaluate the risk associated with the concurrent use of ACEi.Single center, databank-based observational study on 120 consecutive patients with i. v. alteplase for acute stroke. Meta-analysis of all stroke studies on alteplase-associated angioedema, which provided detailed information about the use of ACE-inhibitors. Across studies, the Peto odds ratio of orolingual angioedema for "concurrent use of ACEi" was calculated.Orolingual angioedema occurred in 2 of 120 patients (1.7%, 95% CI 0.2-5.9 %). Angioedema was mild in one, but rapidly progressive in another patient. Impending asphyxia prompted immediate intubation. CT showed orolingual swelling but no bleeding. One of 19 (5%) patients taking ACEi had orolingual angioedema, compared to 1 of 101 (1%) patients without ACEi. Medline search identified one further study about the occurrence of alteplase-associated angioedema in stroke patients stratified to the use of ACEi. Peto odds ratio of 37 (95 % CI 8-171) indicated an increased risk of alteplasetriggered angioedema for patients with ACEi (p <0.001).Orolingual angioedema is a potentially life-threatening complication of alteplase treatment in stroke patients, especially in those with ACEi. Orolingual hematoma as differential diagnosis can be excluded by CT-scan.
Orolingual angioedema after alteplase therapy of acute ischaemic stroke: incidence and risk of prior angiotensin-converting enzyme inhibitor use
DOI:10.1111/ene.12472
PMID:24909847
[Cited within: 2]

Orolingual angioedema (OA) is an uncommon but potentially life-threatening complication of treatment with recombinant tissue plasminogen activator (rt-PA; alteplase) during acute ischaemic stroke. This study aimed to determine the incidence of rt-PA-related OA in an Asian stroke population and the risk of pre-stroke anti-hypertensive drug use for development of this complication.A multi-center stroke registry was used to identify the pre-stroke medications of acute ischaemic stroke patients receiving intravenous rt-PA from January 2002 to December 2013. The clinical manifestations of rt-PA-related OA were recorded and the incidence of this complication was determined. The risks of pre-stroke use of different anti-hypertensive agents for the occurrence of rt-PA-related OA were determined from this study and from a meta-analysis.A total of 559 patients received intravenous rt-PA over a 12-year period. Five patients (two males) developed OA after rt-PA administration. The incidence of OA amongst these patients was 0.89% (95% confidence interval 0.29%-2.09%), which was lower than that obtained by meta-analysis (1.9%). Amongst pre-stroke anti-hypertensive medications, angiotensin-converting enzyme (ACE) inhibitors were found in this study to have the highest relative risk for rt-PA-related OA (17.1; 95% confidence interval 3.0-96.9). Meta-analysis also revealed that pre-stroke use of ACE inhibitors was associated with a high relative risk of OA after intravenous rt-PA (12.9; 95% confidence interval 4.5-37.0).The incidence of rt-PA-related OA in the Asian population is lower than that in the Caucasian population. Pre-stroke use of ACE inhibitors significantly increases the risk of this complication.© 2014 The Author(s) European Journal of Neurology © 2014 EAN.
Analysis of related factors of orolingual angioedema after rt-PA intravenous thrombolytic therapy
Orolingual angioedema during or after thrombolysis for cerebral ischemia
DOI:10.1161/STROKEAHA.116.013334
PMID:27197851
[Cited within: 1]

Orolingual angioedema (OLAE) is a life-threatening complication of intravenous thrombolysis. Our objective was to compare outcomes of patients with and without OLAE.We prospectively included consecutive patients who received intravenous thrombolysis for cerebral ischemia at Lille University Hospital. We examined tongue and lips every 15 minutes during thrombolysis and ≤30 minutes after. We evaluated the 3-month outcome with the modified Rankin scale (mRS) and compared outcomes of patients with and without OLAE.Of 923 consecutive patients, 20 (2.2%) developed OLAE. None of them needed oro-tracheal intubation. They were more likely to be under angiotensin-converting enzyme inhibitors (adjusted odds ratio [adjOR], 3.9; 95% confidence interval [CI], 1.6-9.7; P=0.005) to have total insular infarcts (OR, 5.0; 95% CI, 1.5-16.5; P=0.004) and tended to develop more symptomatic intracerebral hemorrhages. Results concerning angiotensin-converting enzyme inhibitors were not modified after adjustment for propensity scores (OR, 4.4; 95% CI, 1.6-11.9; P=0.004) or matched analysis based on propensity scores (OR, 3.4; 95% CI, 1.3-8.1; P=0.010). Patients with OLAE did not significantly differ at 3 months for the proportion of patients with mRS score of 0 to 1 (adjOR, 0.9; 95% CI, 0.3-2.1), mRS score of 0 to 2 (adjOR, 0.8; 95% CI, 0.1-1.8), and death (adjOR, 1.1; 95% CI, 0.3-3.8).OLAE occurs in 1 of 50 patients who receive intravenous thrombolysis, 1 of 10 in case of total insular infarct, and 1 of 6 if they are under angiotensin-converting enzyme inhibitors. Their long-term outcome does not differ from that of other patients.© 2016 American Heart Association, Inc.
Orolingual angiodema associated with alteplase treatment of acute stroke: a reappraisal
DOI:10.1016/j.jstrokecerebrovasdis.2014.07.045
PMID:25440357
[Cited within: 1]

Orolingual angioedema has been increasingly recognized as a potentially life-threatening complication associated with alteplase treatment of stroke. Concomitant treatment with an angiotension converting enzyme inhibitor (ACEi) and localization of infarction in the territory of middle cerebral artery seem to be associated with a higher risk of this complication.We report the cases of orolingual angioedema among the patients undergoing alteplase treatment in our Stroke Unit. Additionally, we reviewed the literature to evaluate the pathophysiology, clinical characteristics, and treatment options.In our Stroke Unit, among 236 patients given alteplase for acute stroke, 8 patients (3.4%) developed angioedema. The clinical picture varied from localized labial edema to extensive lingual edema with respiratory distress but in all cases it gradually resolved with symptomatic treatment. Seven patients had a hemispheric stroke (4 with lateralized angioedema, contralateral to the ischemic lesion), whereas the other 1 patient had a right superior cerebellar artery stroke (with lateralized angioedema, ipsilateral to the ischemic lesion). The National Institutes of Health Stroke Scale score at admission ranged from 6 to 24 (median 12.5). Five patients were taking an ACEi. Our results are similar to previously published data. In the literature, it appears that orolingual angioedema occurs in.2-5.1% of all stroke patients receiving Alteplase treatment.Orolingual angioedema is a potential complication of which treating physicians in stroke units need to be aware, even in those cases without history of ACEi treatment and without infarction in the territory of the middle cerebral artery. All patients who receive alteplase treatment should be monitored carefully.Copyright © 2015 National Stroke Association. Published by Elsevier Inc. All rights reserved.
Evidence of anaphylaxy after alteplase infusion
PMID:10229756
[Cited within: 1]

Although alteplase, a recombinant tissue plasminogen activator (tPA), is structurally identical to endogenous tPA and therefore should not induce allergy, single cases of acute hypersensitivity reactions have been reported. Until now, specific antibodies against alteplase were not detected in blood samples obtained in these patients.We report an anaphylactic reaction in a 70-year-old white female who was treated with intravenous alteplase for thrombolysis of acute ischemic stroke 160 minutes after onset of a right-sided hemiparesis. Thirty minutes after infusion of alteplase had been started, the patient suffered acute severe sinus tachycardia and hypotension, followed by cyanosis and loss of consciousness. The alteplase infusion was stopped, and following antiallergic therapy, tachycardia and hypotension resolved within 1 hour. The hemiparesis remained unaltered, but additional harm resulting from the hemodynamic complication was not observed. Serum samples analyzed with a radioimmunoprecipitation assay were negative for total antibodies to alteplase, but in a subsequent ELISA, both samples were positive for IgE antibodies to alteplase.The detection of specific IgE antibodies reactive with alteplase in this patient could provide the first evidence of an anaphylactic-type reaction to alteplase in man. Because previous exposure to alteplase can be excluded, the results suggest that this patient had preexisting antibodies that were cross-reactive with one or more epitopes of alteplase and therefore precipitated the anaphylactic-type reaction.
Losartan increases bradykinin levels in hypertensive humans
PMID:15655136
[Cited within: 2]

Studies in animals and humans indicate a role for kinins in the actions of angiotensin type 1 (AT1) receptor blockers. However, the effect of these compounds on kinin levels in humans is unknown.We measured angiotensin (Ang), bradykinin (BK), and kallidin peptides in subjects with essential hypertension administered placebo, losartan (50 mg OD), and eprosartan (600 mg OD) in randomized order in a double-blind, 3-period, 3-treatment, crossover trial. Peptides were measured in arterial blood using high-performance liquid chromatography-based radioimmunoassays. Losartan increased blood levels of BK-(1-9) and hydroxylated BK-(1-9) by approximately 2-fold and reduced the BK-(1-7)/BK-(1-9) ratio by 55%. There was a trend for eprosartan to produce similar changes in bradykinin levels. There were no changes in blood kallidin levels. Both losartan and eprosartan increased plasma levels of Ang I, Ang II, and Ang-(2-8), and eprosartan increased Ang-(3-8) levels. Ang-(1-7) and Ang-(1-9) levels were unchanged. There was an associated 30% to 35% reduction in Ang II/Ang I ratio and 63% to 69% reduction in Ang-(1-7)/Ang I ratio. Plasma ACE activity was unchanged.Losartan increases bradykinin levels. The reductions in BK-(1-7)/BK-(1-9), Ang II/Ang I, and Ang-(1-7)/Ang I ratios suggest that the increased bradykinin levels were the result of reduced metabolism by ACE and neutral endopeptidase. Increased bradykinin levels may represent a class effect of AT1 receptor blockers that contributes to their therapeutic actions and may also contribute to the angioedema that may accompany this therapy.
Angiotensin II type 2 receptor-mediated vasodilation. Focus on bradykinin, NO and endothelium-derived hyperpolarizing factor(s)
DOI:10.1016/j.vph.2005.01.005 URL [Cited within: 1]