INTRODUCTION
Chronic obstructive pulmonary disease (COPD), a chronic lung disorder characterized by inflammation of the airway, lung parenchyma, and pulmonary vessels, is one of the most common respiratory diseases in China.[1,2] COPD is a complex pathological process mediated by numerous inflammatory pathways,[3,4] and is also one of the main causes of morbidity and mortality in adults.[5] Patients with COPD typically have immune deficiency, and the programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) axis is closely related to the inflammatory response, impaired immunity, and increased risk of acute exacerbation of COPD. Blocking the PD-1/PD-L1 axis has a potential anti-inflammatory effect on improving the clinical outcome for this disease.[6] The purpose of this study is to observe the expression of PD-1 and PD-L1 and the morphological changes of the lung tissue in COPD model mice, and to explore the potential therapeutic effect of Maxingloushi decoction. This study might provide a novel theoretical basis for a further preclinical and clinical investigation into Maxingloushi decoction as a potential treatment for COPD.
METHODS
Animal preparation
BALB/C mice of specific pathogen-free (SPF) grade (36 males, weight 20-25g) were purchased from SBF Biotechnology Co., Ltd, Beijing, China (production license for laboratory animals: SCXK [Beijing] 2019-0010). Mice were fed ad libitum and continuously maintained in separate cages at 20-25 ℃, with a relative humidity of 60% and 12-hour light:12-hour dark cycles. Drinking water and cages were sterilized by a high-pressure sterilizer, feed and padding were irradiated, and padding was changed every day. Studies commenced after 7 days of adaptation to our vivarium. All procedures complied with specified ethical requirements of the Beijing Experimental Animal Management Committee.
Drug preparation
The ingredients of Maxingloushi decoction include Ephedra (6 g), Almond (10 g), Scutellaria baicalensis (9 g), Gypsum (30 g), Trichosanthes kirilowii maxim (15 g), and Licorice (6 g). The crude drug quantity was 76 g, which was provided by the free decoction granule pharmacy of Dongzhimen Hospital, Beijing University of Chinese Medicine. PD-1 inhibitor (SR0987, 10 mg/kg) was purchased from MedChemExpress Co., Ltd. (Monmouth Junction, USA).
Primary reagents and instruments
Reagents and instruments fundamental to the current study included isoflurane (reward), lipopolysaccharide (LPS, Sigma-Aldrich, USA), a portable small animal anesthesia machine (ZS-MV-IV; Beijing Zhongshidichuang Biotechnology Development Co. Ltd., China), electronic balance scale (Vante1002; BioPharm, USA), scanner (3D HISHTECH Panaramic 250; Budapest, Hungary), pathological slicer (RM2235; Leica Biosystems, Germany), Nikon Ci-S inverted microscope and imaging system (DS-U3; Nikon, Japan), automatic biological tissue staining instrument (RS-18 Ⅲ; Hubei Hongye Medical Instrument Co., Ltd., China), LED optical fiber cold light source (202026330; Osway, China), dehydrator (JT-12J; Wuhan Junjie Electronics Co., Ltd., China), embedding machine (JB-P7; Wuhan Junjie Electronics Co., Ltd., China).
Establishment of COPD model
The COPD model was prepared by fumigation combined with LPS.[7] The mice were placed in a plexiglass poison box (120 cm × 80 cm × 80 cm) and subjected to passive cigarette smoke (CS) twice per day (each lasting for 2 hours, with an interval of at least 4 hours), equivalent to 9-10 cigarettes per 2 hours for 6 days within one week. Total nicotine content and tar of the cigarettes used were recorded. On the 1st and 14th days, LPS (7.5 µg per mouse, dissolved in 50 µL normal saline) was injected into the mice, and smoking was suspended on the day of LPS induction. The success of the model preparation was judged by the following criteria: (1) general state and weight changes of mice; (2) lymphocyte staining count in bronchoalveolar lavage fluid (BLF); (3) histopathological changes of the lung tissue.[8]
Grouping and intervention methods
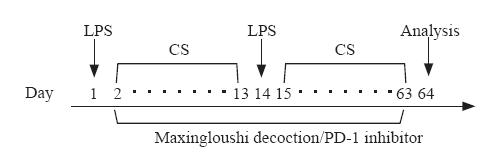
The experimental design is shown in Figure 1. Thirty-six BALB /C mice were randomly (random number) divided into four groups: normal group (group A, n=6), COPD model group (group B, n=10), Maxingloushi decoction + COPD group (group C, n=10) and PD-1 inhibitor + COPD group (group D, n=10). We referred to the human and mouse dose conversion table: the conversion coefficient was 9.13. According to the formula, the equivalent dose of mice was 0.115 g/10 g, and the concentration was calculated according to the formula. Maxingloushi decoction (0.384 g/mL) was administered by gavage after the final smoke inhalation step every day for 62 days, and the gavage dose was 0.1 mL/10 g body weight. The mice in the group D were intraperitoneally injected (PD-1 inhibitor 10 g/0.1 mL[9]) after the last fumigation every day. The dosage was calculated according to the weight of mice. The first dose of PD-1 inhibitor was 10 mg/kg, and then 5 mg/kg thereafter, and administered once every 4 days, for a total of 15 times. The group A was given normal saline by gavage. Blood was taken after inhalation of anesthesia, and mice were quickly executed via cervical dislocation. No mice died from any treatment in any of the groups (Figure 1).
Figure 1.
Figure 1.
Experimental design for COPD model and interventions.COPD:chronic obstructive pulmonary disease; LPS: lipopolysaccharide; CS: cigarette smoke.
Pathological examination of the lung tissue
The left lobe of the lung was selected. H&E staining and pathological scoring of inflammatory cell infiltrate and alveolar morphology were carried out.
Immunohistochemistry (IHC) staining of the lung tissue and semi-quantitative analysis
Immuno-fluorescence optical density (IOD) analysis was used to detect the expression of PD-1 and PD-L1. The middle lobe of the right lung was selected for analysis. There were three sections in each group. Images of three 400× visual fields were randomly selected from each section. Each photo was analyzed using Image Pro Plus 6.0 (Media Cybernetics, USA) to obtain the cumulative IOD value of each photo.
Enzyme-linked immunosorbent assay (ELISA)
The expression of PD-1 and PD-L1 in mouse plasma and BLF were detected by ELISA.
Statistical methods
The SPSS 17.0 software was used to analyze the data. Students’ t-test was applied for comparisons for variables between groups that conformed to normal Gaussian distribution. A P-value <0.05 was considered statistically significant.
RESULTS
Lung tissue morphology
The lung tissue structure of the group A was typical without obvious inflammatory cell infiltration (e.g., neutrophils) in the tissue (Figure 2A). The lung tissue of the group B exhibited moderately abnormal lung tissue structure characterized by partial alveolar atrophy and collapse, and alveolar wall thickening. A cell nodule was seen in the tissue, and a large number of inflammatory cells were observed around the bronchus (Figure 2C). In the groups C and D, the lung tissue structure had less alveolar derangement and inflammatory cell infiltration (Figures 2 E and G).
Figure 2.
Figure 2.
H&E staining and IHC staining of lung tissue (left×20, right×40). IHC: immunohistochemistry. Group A: normal group; group B: COPD model group; group C: Maxingloushi decoction + COPD group; group D: PD-1 inhibitor + COPD group.
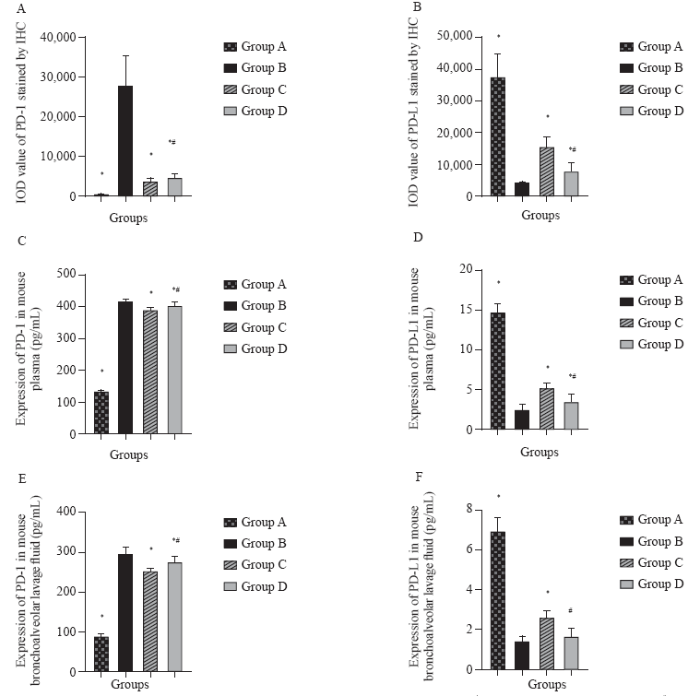
The IOD values of PD-1 and PD-L1 detected by IHC in the lung tissue
IHC staining results of the lung tissue showed that COPD could induce the high expression of PD-1 and inhibit the expression of PD-L1 in the lung tissue of mice. The groups C and D significantly reduced the expression of PD-1 induced by smoking combined with LPS in the lung tissue and promoted the expression of PD-L1 (Table 1, Figure 2). The expression of PD-1 in the group B was significantly higher than that in groups A and D (P<0.05), and the expression of PD-L1 in the group B was significantly lower than that in groups A, C, and D (P<0.05). PD-1 expression was greatly diminished by Maxingloushi decoction intervention (P<0.05). In addition, the expression of PD-1 in the group C was significantly lower than that in the group D (P<0.05) (Figure 3A). Importantly, the expression of PD-L1 in the group C was significantly higher than that in the group D (P<0.05) (Figure 3B). These results similarly suggested higher efficacy of Maxingloushi decoction compared with that of PD-1 inhibitor treatment.
Table 1 IOD value of analysis of PD-1 and PD-L1 stained by IHC (mean±SD)
| Groups | n | PD-1 | PD-L1 |
|---|---|---|---|
| Group A | 9 | 386.26±85.41* | 37,193.11±7,382.04* |
| Group B | 9 | 27,748.31±7,532.47 | 4,159.88±389.25 |
| Group C | 9 | 3,484.48±922.72*# | 15,582.37±2,804.94*# |
| Group D | 9 | 4,527.86±1,122.84* | 7,807.50±2,660.21* |
Compared with group B, *P<0.05; compared with group D, #P<0.05. Group A: normal group; group B: COPD model group; group C: Maxingloushi decoction + COPD group; group D: PD-1 inhibitor + COPD group; n: the total number of section images; IOD: immuno-fluorescence optical density; PD-1: programmed death-1; PD-L1: programmed death-ligand 1.
Figure 3.
Figure 3.
Expression of PD-1 and PD-L1 in plasma and BLF. Compared with group B, *P<0.05; compared with group C, #P<0.05. Group A: normal group; group B: COPD model group; group C: Maxingloushi decoction + COPD group; group D: PD-1 inhibitor + COPD group; IOD: immuno-fluorescence optical density; IHC: immunohistochemistry; PD-1: programmed death-1; PD-L1: programmed death-ligand 1.
Expression of PD-1 and PD-L1 in mouse plasma and BLF
The expression of PD-1 in plasma and BLF in the group B was significantly higher than that in the group A (P<0.05). Treatment with Maxingloushi decoction led to decreased PD-1 in plasma and BLF as compared with the group B (P<0.05). The expresion of PD-1 in plasma and BLF in the group B was significantly higher than that in the group D (P<0.05). Importantly, the expresion of PD-1 in plasma and BLF in the group C was lower than that in the group D (P<0.05) (Table 2, Figures 3 C and E). Expresion of PD-L1 in plasma and BLF in the group B was significantly lower than that in the group A (P<0.05). Treatment with Maxingloushi decoction led to an increase in PD-L1 in plasma and BLF as compared with the group B (P<0.05). Plasma PD-L1 expression in the group B was lower than that in the group D (P<0.05). BLF PD-L1 expression in the group B was also lower than that in the group D, however the difference was not statistically significant (P>0.05). The expression of PD-L1 in plasma and BLF was substantially higher in the group C than in the group D (P<0.05) (Figures 3 D and F). These findings indicated a potentially higher efficacy of Maxingloushi decoction than PD-1 inhibitor treatment.
Table 2 Expression of PD-1 and PD-L1 in mouse plasma and bronchoalveolar lavage fluid (mean±SD, pg/mL)
| Groups | n | PD-1 (plasma) | PD-L1 (plasma) | PD-1 (bronchoalveolar lavage fluid) | PD-L1 (bronchoalveolar lavage fluid) |
|---|---|---|---|---|---|
| Group A | 6 | 132.65±4.43* | 14.76±1.13* | 88.24±5.90* | 6.92±0.69* |
| Group B | 10 | 416.56±8.80 | 2.52±0.62 | 295.20±18.12 | 1.43±0.25 |
| Group C | 10 | 388.50±11.87*# | 5.15±0.73*# | 252.33±6.24*# | 2.58±0.37*# |
| Group D | 10 | 401.83±10.95* | 3.52±0.90* | 275.21±14.15* | 1.66±0.42 |
Compared with group D, #P<0.05; compared with group B, *P<0.05. Group A: normal group; group B: COPD model group; group C: Maxingloushi decoction + COPD group; group D: PD-1 inhibitor + COPD group; n: the total number of mice; PD-1: programmed death-1; PD-L1: programmed death-ligand 1.
DISCUSSION
COPD is a chronic lung condition characterized by persistent airway inflammation and irreversible airflow restriction. At present, the immune imbalance is considered to be a major pathogenic mechanism of local and systemic inflammatory damage caused by COPD.[10] PD-1 and PD-L1 play an important role in immune imbalance with COPD.[11] Maxingloushi decoction is a traditional Chinese medicine preparation established by Professor Wu Wei-ping.[12] Maxingloushi decoction might have therapeutic effects on the treatment of COPD.[13,14]
PD-1, also known as CD279, is a transmembrane protein isolated from T cells undergoing programmed death.[6] It belongs to the immunoglobulin superfamily and is mainly expressed on the surface of T cells, B cells, and bone marrow cells. PD-1 is a signaling pathway factor that mediates self-tolerance by down-regulating the immune system and inhibiting T cell activation.[15] PD-1 is a negative costimulatory molecule that has been associated with immune response deficiency and a report suggest that it increases the risk of an acute attack of COPD.[16] PD-1 has been reported to be elevated in patients with COPD.[17] PD-L1 (also known as B7-H1, or CD274) is a surface molecule expressed on the plasma membrane of T and B lymphocytes, DC cells, and macrophages, as well as on a variety of non-immune cells including pancreatic, epithelial, and vascular epidermal cells. PD-L1 expression has also been reported in a variety of human tissues and organs. [6] PD-1 combined with PD-L1 can inhibit T and B cell function, inhibit the proliferation of T cells,[11] and reduce cytokines such as IL-2, IL-10, and IFN- γ. As a part of a vital immune-suppressive signaling pathway, PD-1 produces inhibitory signals, mainly by down-regulating the response of the human immune system to cells and by inhibiting the inflammatory activities of T cells to regulate the immune system and promote its own tolerance, thus inhibiting the immune system.[18] When PD-1 increases, the PD-L1 will increase correspondingly to bind to it, and the body will show impaired immune function. In our study, PD-1 significantly increased and PD-L1 significantly decreased in the group B, showing that the COPD model had immune dysfunction. Both groups C and D improved expression of PD-1 and PD-L1, actually a decrease in PD-1 levels and an increase in PD-L1 levels. Thus, Maxingloushi decoction and PD-1 inhibitor treatment could potentially improve the body’s immune function and play a two-way adjustment effect. It has been found that when the PD-1/PD-L1 pathway is blocked, the apoptosis of lymphocytes such as CD4+ and CD3+ in the cellular immune T lymphocyte subsets is reduced and the pathogen clearance ability is increased.[19]
Compared with PD-1 inhibitor treatment, Maxingloushi decoction significantly reduced the PD-1 expression induced by smoking combined with LPS in the lung tissues of mice, and promoted the PD-L1 expression according to the IHC detection analysis. Results showed that Maxingloushi decoction could regulate the PD-1/PD-L1 axis to alleviate the pulmonary immune dysfunction in COPD mice. Findings from the present study showed that COPD was associated with higher levels of PD-1 in the plasma and BLF as compared with normal and healthy mice, while the ligand PD-L1 levels were lower in COPD as compared with healthy mice. Histopathological analysis of the lung tissue confirmed that circulating levels of these markers were reflected in the tissue. The IOD value following IHC staining of the lung tissue in the group B was higher than that in the group D, and correspondingly the expression of ligand PD-L1 was lower in the group B than that in the group D. This suggests that impairment of immune function is an important part of COPD, leading to aggravated inflammation of the lung.
Our data further indicated that the increase of PD-1 played a negative role in regulating immune response. Maxingloushi decoction treatment can reduce the PD-1 expression, improve immune function, and play a positive role in bidirectional regulation. Another major finding of our study was that Maxingloushi decoction had a beneficial effect on lung inflammation and damage in COPD. Concurrent treatment of COPD mice with Maxingloushi decoction reduced the expression of PD-1 and increased the expression of ligand PD-L1, suggesting that Maxingloushi decoction may play a two-way regulating role in modulating the immune response and the inflammatory response of COPD mice and helping to restore immune balance by normalizing PD-1/PD-L1 ratios.
CONCLUSIONS
PD-1 and PD-L1 may play significant roles in COPD. Both Maxingloushi decoction and PD-1 inhibitor treatment can mitigate lung inflammation in COPD and normalize expression of PD-1 and PD-L1. The effect of Maxingloushi decoction may be superior to that of PD-1 inhibitor treatment. These findings support the prospect of traditional Chinese medicine in treating immune imbalance and inflammatory response in COPD, and also warrant further study of this therapeutic approach for this disease.
Funding: None.
Ethical approval: This research was approved by the Beijing Experimental Animal Management Committee.
Conflicts of interest: The authors declare that they have no competing interests.
Contributors: LL proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
Reference
Predictive role of interleukin-6 and CAT score in mechanical ventilation in patients with chronic obstructive pulmonary disease at the acute exacerbation stage in the emergency department
DOI:10.5847/wjem.j.1920-8642.2020.02.005 URL [Cited within: 1]
Expression of SIRT1, NF-κB, and MMP-9 in peripheral blood of COPD patients
Correlation between MCP-1, IL-17, IL-35 and lung function in patients with acute exacerbation of chronic obstructive pulmonary disease
The role of specific and non-specific immune cells in the pathogenesis of COPD
Progress in immunopathology of PD-1/PD-L1 axis and chronic obstructive pulmonary disease
Long non-coding RNA expression patterns in lung tissues of chronic cigarette smoke induced COPD mouse model
DOI:10.1038/s41598-018-25702-3 URL [Cited within: 1]
Tanshinone IIA sulfonate protects against cigarette smoke-induced COPD and down-regulation of CFTR in mice
DOI:10.1038/s41598-017-18745-5 URL [Cited within: 1]
Identification of insulin as a novel retinoic acid receptor-related orphan receptor α target gene
DOI:10.1016/j.febslet.2014.02.029 URL [Cited within: 1]
Imbalance between subpopulations of regulatory T cells in patients with acute exacerbation of COPD
DOI:10.1080/15412555.2017.1385055
PMID:29166179
[Cited within: 1]

Human regulatory T cells (Tregs) have been reported to be not significantly different in the peripheral blood of patients with chronic obstructive pulmonary disease (COPD) and healthy controls. Recent research has identified some new markers for Tregs and indicated that Tregs are composed of distinct subpopulations. The aim of the study was to describe the changing patterns of circulating Treg subpopulations in patients with acute exacerbation of COPD (AECOPD) and healthy controls, and to explore their potential roles in AECOPD pathogenesis. Blood samples were obtained from 30 never-smokers with normal lung function and 30 patients with COPD before and after they had an exacerbation. The proportions of Treg subpopulations were evaluated using flow cytometry. In the peripheral blood, decreased proportions of CD4CD25CD127 Tregs, CD4CD25CD45RA Tregs, and CD4CD25CD62L Tregs and an increased proportion of CD4CD25CD45RO Tregs were found in patients with stable COPD compared with non-smokers with normal lung function. The patients showed further changes in Treg subpopulations when they had an AECOPD, with an overall decrease in a suppressive subset, indicating that the immune negative regulatory population of Tregs did not play an effective role. Immune homeostasis favored inflammation, and a negative correlation between the circulating tumor necrosis factor-alpha and the proportions of CD4CD25CD62L cells (r = -0.698, p < 0.05) in patients with AECOPD was found. The imbalance between the suppressive subsets and the proinflammatory subset of Tregs and the decline of Treg subpopulations with immunosuppressive activity may play important roles in AECOPD progression.
Role of regulatory T cells in the pathogenesis of COPD
Effect of Maxingloushi decoction on acute AECOPD
Effect of Maxingloushi decoction combined with budesonide atomization inhalation on AECOPD and its effect on Th1/Th2 imbalance
Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection
DOI:10.4049/jimmunol.1302584
PMID:24752444
[Cited within: 1]

The detrimental impact of tobacco on human health is clearly recognized, and despite aggressive efforts to prevent smoking, close to one billion individuals worldwide continue to smoke. People with chronic obstructive pulmonary disease are susceptible to recurrent respiratory infections with pathogens, including nontypeable Haemophilus influenzae (NTHI), yet the reasons for this increased susceptibility are poorly understood. Because mortality rapidly increases with multiple exacerbations, development of protective immunity is critical to improving patient survival. Acute NTHI infection has been studied in the context of cigarette smoke exposure, but this is the first study, to our knowledge, to investigate chronic infection and the generation of adaptive immune responses to NTHI after chronic smoke exposure. After chronic NTHI infection, mice that had previously been exposed to cigarette smoke developed increased lung inflammation and compromised adaptive immunity relative to air-exposed controls. Importantly, NTHI-specific T cells from mice exposed to cigarette smoke produced lower levels of IFN-γ and IL-4, and B cells produced reduced levels of Abs against outer-membrane lipoprotein P6, with impaired IgG1, IgG2a, and IgA class switching. However, production of IL-17, which is associated with neutrophilic inflammation, was enhanced. Interestingly, cigarette smoke-exposed mice exhibited a similar defect in the generation of adaptive immunity after immunization with P6. Our study has conclusively demonstrated that cigarette smoke exposure has a profound suppressive effect on the generation of adaptive immune responses to NTHI and suggests the mechanism by which prior cigarette smoke exposure predisposes chronic obstructive pulmonary disease patients to recurrent infections, leading to exacerbations and contributing to mortality. Copyright © 2014 by The American Association of Immunologists, Inc.
Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation
Effects of interleukin-37 on regulatory T cell immune function in sepsis mice