Dear editor,
A 21-year-old male student was admitted to the emergency department of our hospital due to chest distress, dyspnea for 1.5 hours, and loss of consciousness for one minute. Before admission, the patient had been advised rest for two months because of left ankle sprain, leading to less activity. At admission, the patient was unconscious, with facial cyanosis, and his limbs were cold. The heart rate (HR), blood pressure (BP), respiratory rate (RR), and pulse oxygen saturation (SPO2) were 146 beats/minute, 88/74 mmHg (1 mmHg=0.133 kPa), 27 breaths/minute, and 72%, respectively. Tracheal intubation was immediately performed and blood pressure was maintained at 80/65 mmHg with norepinephrine at a dosage of 2.08 mg/(kg·minute). However, the patient’s condition worsened rapidly with two successive cardiac arrests. The return of spontaneous circulation (ROSC) was achieved after external chest compression (the first set of chest compression lasted for >10 minutes and defibrillation was performed twice; the second set of chest compression lasted for few minutes). Laboratory data were obtained in succession. The arterial blood gas showed pH 6.866, partial pressure of carbon dioxide (PCO2) 73.4 mmHg, partial pressure of oxygen (PO2) 422 mmHg, standard bicarbonate (SB) 9.2 mmol/L, base excess (BE) -21.9 mmHg, lactic acid 14.8 mmol/L, and oxygen saturation 100%. General coagulation tests showed normal fibrinogen, activated partial thromboplastin time (APTT), thrombin time, prolonged prothrombin time (PT, 15.9 seconds), international normalized ratio (INR, 1.35), and increased D-dimer (DD, 15.43 mg/L fibrinogen equivalent units [FEU]). The level of troponin I was 1.4 mg/L. The electrocardiogram showed sinus tachycardia and SITIIIQIII. Bedside transthoracic echocardiography showed that the range of left ventricular ejection fraction was 70%, and pulmonary artery systolic pressure was 34 mmHg, with decreased systolic function and mild tricuspid regurgitation, without obvious enlargement of the right ventricle. Compression venous ultrasonography showed that deep veins of both lower extremities were filled without interruption. Therefore, the patient was considered to be at a high risk for acute pulmonary embolism (PE). Because of the extremely unstable hemodynamics, computed tomography pulmonary angiography (CTPA) was not performed immediately. The patient received veno-arterial extracorporeal membrane oxygenation (VA-ECMO) insertion. Then, arterial perfusion cannula (Edwards Lifesciences, 18 Fr) was placed into the right femoral artery, and femoral venous cannula (Edwards Lifesciences, 22 Fr) was placed into the left femoral vein. Heparin was administered as an anticoagulant at 0.5 mg/kg for initiation of ECMO and subsequently at 2-20 mg/hour to maintain APTT in the range of 45-70 seconds. After ECMO, the norepinephrine dose decreased rapidly. In addition, transthoracic echocardiography showed dilatation of the right heart, mild pulmonary hypertension (pulmonary artery systolic pressure of 44 mmHg), and normal left ventricular function. Combined with ECMO, CTPA examination showed embolization of the lower-left pulmonary artery (Figure 1A), the middle and inferior lobe arteries of the right lung (Figure 1B), and their branches. After 38 hours of ECMO support, norepinephrine was discontinued and hemodynamics was stabilized. Subsequently, the ECMO was successfully removed after running for 63 hours, and 800 mL of red blood cells (RBCs) were administered because of blood loss. Heparin was continued for seven days as the anticoagulation therapy before changing to low-molecular-weight heparin (LMWH) (enoxaparin, Sanofi, 0.4 mL for q12h). On day 9 after admission, the reexamination of CTPA showed a significant reduction in embolism as compared to the previous level. Moreover, transthoracic echocardiography showed normal cardiac atrioventricular size, mild tricuspid regurgitation, normal left ventricular diastolic and systolic functions, and pulmonary artery systolic pressure of 20 mmHg. After 14 days in the intensive care unit, the patient was transferred to the general ward due to improvement in his condition and the treatment was replaced with rivaroxaban for anticoagulation at 15 mg bid for three weeks, which was subsequently adjusted to 20 mg qd. Finally, he was discharged from the hospital on September 30, 2019. On December 27, 2019, the reexamination of CTPA did not show any signs of vascular stenosis (Figures 1C and D). The echocardiogram showed mild tricuspid regurgitation and pulmonary artery systolic pressure of 27 mmHg. Laboratory indexes of thrombophilia revealed that lupus anticoagulant, protein C, protein S, and antithrombin III were normal, and no variation was found in the whole exon sequencing of the patient. Hence, after three months, anticoagulation therapy was discontinued. The follow-up for five months showed no chest distress or dyspnea, but decreased activity endurance in the patient.
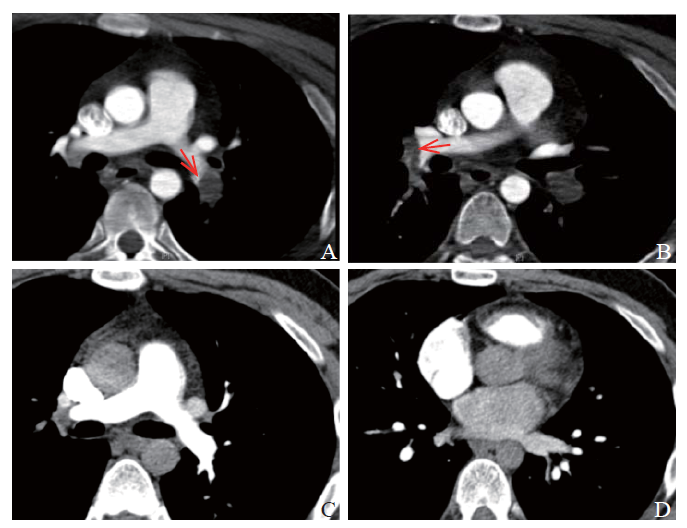
Figure 1.
Figure 1.
Results of computed tomography pulmonary angiography. A: embolization of the lower-left pulmonary artery (red arrow); B: embolization of the middle and inferior lobe arteries of the right lung (red arrow); C and D: no sign of pulmonary embolism.
DISCUSSION
High-risk PE with cardiac arrest is a critical condition. Thrombolytic therapy can improve pulmonary perfusion, reverse the right ventricular dysfunction,[1] and prevent the escalation of therapy in patients with acute PE.[2] Most guidelines recommend that thrombolysis is beneficial for patients with acute PE and hypotension, and is a widely accepted indication.[3,4] However, thrombolytic therapy is associated with increased risk of hemorrhage.[5] A previous study evaluated 104 patients who received alteplase for acute PE, and major bleeding occurred in 19.2% (20/104) of patients.[6] Another study also showed that the use of thrombolytics was associated with the risk of bleeding.[7] Prolonged external chest compression and catecholamine administration for systemic arterial hypotension were independent predictors of hemorrhage.[3,6] However, severe high-risk PE often requires high doses of catecholamine and even results in cardiac arrest followed by prolonged external chest compression. Clinicians are often hesitant to administer thrombolytic therapy, even in the highest-risk PE patients, because of the concern of major bleeding.
Despite the high risk of bleeding, thrombolytic therapy is recommended for patients with high-risk PE, especially those with severe shock and cardiac arrest. However, clinicians can wait when ECMO is working.[8] After full anticoagulation, the body’s fibrinolysis mechanism dissolves the fibrin and the clot. In patients unresponsive to anticoagulation, ECMO can serve as a bridge to other definitive advanced therapies (for example, thrombolysis orthromboembolectomy) to reduce the thrombus burden. Thus, most bleeding complications of thrombolysis can be avoided. Strikingly, ECMO needs anticoagulation similar to the thrombolysis strategy, in addition to the risk of bleeding. In the 13 studies published after 2009, 8% of major bleeding complications were observed if patients received anticoagulation for a low target APTT (<60 seconds).[9] The incidence rate of severe bleeding events per 100 extracorporeal life support (ECLS) days was 19 events for patients with veno-arterial (VA) ECLS in a study among adult patients who had ECLS between March 1, 2010, and August 15, 2013.[10] ECMO in most high-risk PE patients can be withdrawn in 3-6 days.[11,12] The risk associated with ECMO is relatively limited.
Major bleeding events may have occurred if thrombolysis was conducted in our patient with cardiac arrest due to prolonged external chest compression and a high dose of catecholamines for systemic arterial hypotension. In a study of 10 cases, including nine patients with massive PE and cardiac arrest, all patients underwent thrombolysis before ECMO and developed bleeding complications (including puncture site, surgical wound, pelvic area, and hemothorax).[13] If thrombolysis failed, it would prolong the inadequate tissue perfusion time, increase the possibility of multiple organ dysfunction syndrome, and reduce the success rate of cerebral resuscitation.
For patients who choose ECMO treatment, the risk of cannulation-related injury and catheter-related blood stream infection will increase. In addition, the cost of ECMO is higher than that of thrombolytic therapy, which has to be considered in patients with limited economic resources.
CONCLUSIONS
Early ECMO therapy should be considered in high-risk PE patients complicated with cardiac arrest, if appropriate expertise and resources are available.
Funding: None.
Ethical approval: Not needed.
Conflicts of interests: The authors declare that they have no competing interests.
Contributors: ZRZ and XQZ contributed equally to this work. All authors contributed substantially to the writing and revision of this manuscript and approved of its contents.
Reference
Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion
PMID:8094768
[Cited within: 1]

Data from a non-randomised study have hinted that in patients with acute pulmonary embolism (PE), thrombolysis followed by heparin more rapidly reverses right-ventricular dysfunction and restores pulmonary tissue perfusion than does heparin alone. We have pursued this idea in a randomised protocol. 46 haemodynamically stable patients were randomised to recombinant tissue plasminogen activator (alteplase, rt-PA) 100 mg over 2 h followed by intravenous heparin and 55 to heparin alone. Right-ventricular wall motion was assessed qualitatively, and right-ventricular end diastolic area was estimated by planimetry from echocardiograms at baseline and at 3 and 24 hours. Pulmonary perfusion scans were obtained at baseline and 24 hours. In 39% of rt-PA patients but in only 17% of heparin alone patients right-ventricular wall motion at 24 hours had improved from baseline and in 2% and 17%, respectively, it worsened (p = 0.005). rt-PA patients also had a significant decrease in right-ventricular end-diastolic area during the 24 hours after randomisation and a significant absolute improvement in pulmonary perfusion (14.6% vs 1.5%). No clinical episodes of recurrent PE were noted among rt-PA patients, but there were 2 fatal and 3 non-fatal clinically suspected recurrent PEs within 14 days in patients randomised to heparin alone. rt-PA rapidly improves right-ventricular function and pulmonary perfusion among patients with PE and may lead to a lower rate of adverse clinical outcomes.
Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism
DOI:10.1056/NEJMoa021274 URL [Cited within: 1]
Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th Ed: American College of Chest Physicians Evidence-based Clinical Practice Guidelines
DOI:10.1378/chest.11-2301 URL [Cited within: 2]
Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report
DOI:S0012-3692(15)00335-9
PMID:26867832
[Cited within: 1]

We update recommendations on 12 topics that were in the 9th edition of these guidelines, and address 3 new topics.We generate strong (Grade 1) and weak (Grade 2) recommendations based on high- (Grade A), moderate- (Grade B), and low- (Grade C) quality evidence.For VTE and no cancer, as long-term anticoagulant therapy, we suggest dabigatran (Grade 2B), rivaroxaban (Grade 2B), apixaban (Grade 2B), or edoxaban (Grade 2B) over vitamin K antagonist (VKA) therapy, and suggest VKA therapy over low-molecular-weight heparin (LMWH; Grade 2C). For VTE and cancer, we suggest LMWH over VKA (Grade 2B), dabigatran (Grade 2C), rivaroxaban (Grade 2C), apixaban (Grade 2C), or edoxaban (Grade 2C). We have not changed recommendations for who should stop anticoagulation at 3 months or receive extended therapy. For VTE treated with anticoagulants, we recommend against an inferior vena cava filter (Grade 1B). For DVT, we suggest not using compression stockings routinely to prevent PTS (Grade 2B). For subsegmental pulmonary embolism and no proximal DVT, we suggest clinical surveillance over anticoagulation with a low risk of recurrent VTE (Grade 2C), and anticoagulation over clinical surveillance with a high risk (Grade 2C). We suggest thrombolytic therapy for pulmonary embolism with hypotension (Grade 2B), and systemic therapy over catheter-directed thrombolysis (Grade 2C). For recurrent VTE on a non-LMWH anticoagulant, we suggest LMWH (Grade 2C); for recurrent VTE on LMWH, we suggest increasing the LMWH dose (Grade 2C).Of 54 recommendations included in the 30 statements, 20 were strong and none was based on high-quality evidence, highlighting the need for further research.Copyright © 2016 American College of Chest Physicians. All rights reserved.
A contemporary approach to thrombolytic therapy for pulmonary embolism
Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis
DOI:10.1001/jama.2014.5990
PMID:24938564
[Cited within: 2]

Thrombolytic therapy may be beneficial in the treatment of some patients with pulmonary embolism. To date, no analysis has had adequate statistical power to determine whether thrombolytic therapy is associated with improved survival, compared with conventional anticoagulation.To determine mortality benefits and bleeding risks associated with thrombolytic therapy compared with anticoagulation in acute pulmonary embolism, including the subset of hemodynamically stable patients with right ventricular dysfunction (intermediate-risk pulmonary embolism).PubMed, the Cochrane Library, EMBASE, EBSCO, Web of Science, and CINAHL databases from inception through April 10, 2014.Eligible studies were randomized clinical trials comparing thrombolytic therapy vs anticoagulant therapy in pulmonary embolism patients. Sixteen trials comprising 2115 individuals were identified. Eight trials comprising 1775 patients specified inclusion of patients with intermediate-risk pulmonary embolism.Two reviewers independently extracted trial-level data including number of patients, patient characteristics, duration of follow-up, and outcomes.The primary outcomes were all-cause mortality and major bleeding. Secondary outcomes were risk of recurrent embolism and intracranial hemorrhage (ICH). Peto odds ratio (OR) estimates and associated 95% CIs were calculated using a fixed-effects model.Use of thrombolytics was associated with lower all-cause mortality (OR, 0.53; 95% CI, 0.32-0.88; 2.17% [23/1061] vs 3.89% [41/1054] with anticoagulants; number needed to treat [NNT] = 59) and greater risks of major bleeding (OR, 2.73; 95% CI, 1.91-3.91; 9.24% [98/1061] vs 3.42% [36/1054]; number needed to harm [NNH] = 18) and ICH (OR, 4.63; 95% CI, 1.78-12.04; 1.46% [15/1024] vs 0.19% [2/1019]; NNH = 78). Major bleeding was not significantly increased in patients 65 years and younger (OR, 1.25; 95% CI, 0.50-3.14). Thrombolysis was associated with a lower risk of recurrent pulmonary embolism (OR, 0.40; 95% CI, 0.22-0.74; 1.17% [12/1024] vs 3.04% [31/1019]; NNT = 54). In intermediate-risk pulmonary embolism trials, thrombolysis was associated with lower mortality (OR, 0.48; 95% CI, 0.25-0.92) and more major bleeding events (OR, 3.19; 95% CI, 2.07-4.92).Among patients with pulmonary embolism, including those who were hemodynamically stable with right ventricular dysfunction, thrombolytic therapy was associated with lower rates of all-cause mortality and increased risks of major bleeding and ICH. However, findings may not apply to patients with pulmonary embolism who are hemodynamically stable without right ventricular dysfunction.
Predictors of major hemorrhage following fibrinolysis for acute pulmonary embolism
PMID:16377297
[Cited within: 1]

One hundred four patients at Brigham and Women's Hospital who received alteplase for acute pulmonary embolism were evaluated. Major bleeding occurred in 20 patients (19.2%). The principal site of bleeding was unknown in 9 (45.0%), gastrointestinal in 6 (30.0%), retroperitoneal in 3 (15.0%), intracranial in 1 (5.0%), and splenic in 1 (5.0%). Independent predictors of major hemorrhage were the administration of catecholamines for systemic arterial hypotension (odds ratio [OR] 115, 95% confidence interval [CI] 9.4 to 1,410.9, p < 0.001), cancer (OR 16.0, 95% CI 3.2 to 80, p = 0.004), diabetes mellitus (OR 9.6, 95% CI 1.7 to 54, p = 0.010), and elevated international normalized ratio before fibrinolysis (OR 5.8, 95% CI 1.5 to 22, p = 0.012).
A survey of ventilation strategies during cardiopulmonary resuscitation
DOI:10.5847/wjem.j.1920-8642.2019.04.005 URL [Cited within: 1]
Anticoagulation practices during venovenous extracorporeal membrane oxygenation for respiratory failure. A systematic review
DOI:10.1513/AnnalsATS.201605-364SR URL [Cited within: 1]
Bleeding, transfusion, and mortality on extracorporeal life support: ECLS working group on thrombosis and hemostasis
DOI:10.1016/j.athoracsur.2015.07.046
PMID:26443879
[Cited within: 1]

Bleeding may occur frequently during adult extracorporeal life support; however, there are no detailed investigations of bleeding events, red blood cell transfusion, and their impact on mortality. The purpose of our study was to characterize the incidence of bleeding and red blood cell transfusion during adult extracorporeal life support and examine the impact on mortality.We performed a retrospective analysis of adult extracorporeal life support patients over approximately a 3-year period. The incidence of bleeding events and transfusions were recorded. Unadjusted and adjusted multivariate logistic regression analyses were performed to estimate the odds of inhospital mortality among patients with bleeding and for each red blood cell unit transfused. Ninety-day survival was compared between patients who bled and those who did not.Serious bleeding events occurred in 74 of 132 patients (56.1%), and the rate of bleeding was 10 events per 100 days. The crude odds ratio for inhospital mortality in patients who bled was 2.22 (95% confidence interval [CI]: 1.00 to 4.94, p = 0.05); and for each unit of red blood cells transfused, it was 1.03 (95% CI: 1.01 to 1.04, p = 0.005). The adjusted odds ratios for bleeding and red blood cell transfusions were 0.90 (95% CI: 0.37 to 2.19, p = 0.82) and 1.03 (95% CI: 1.00 to 1.06, p = 0.04). There was a trend toward decreased 90-day survival among patients who bled compared with patients who did not (46.7% versus 64.9%, p = 0.08).Bleeding and red blood cell transfusion occur frequently during adult extracorporeal life support, but only the amount of red blood cell transfusion is associated with inhospital mortality after controlling for confounding variables.Copyright © 2016 The Society of Thoracic Surgeons. Published by Elsevier Inc. All rights reserved.
Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation
DOI:10.1186/s13054-017-1655-8 URL [Cited within: 1]
Extracorporeal membrane oxygenation in acute massive pulmonary embolism: a case series and review of the literature
DOI:10.1177/0267659118786830
PMID:30009670
[Cited within: 1]

Extracorporeal membrane oxygenation (ECMO) has been used to stabilize patients with massive pulmonary embolism though few reports describe this approach. We describe the presentation, management and outcomes of patients who received ECMO for massive pulmonary embolism (PE) in our pulmonary embolism response team (PERT) registry.We enrolled a consecutive cohort of patients with confirmed PE for whom PERT was activated and selected patients treated with ECMO. We prospectively captured clinical, therapeutic and outcome data at the time of PERT activation and during the follow-up period for up to 365 days.Thirteen patients who had PERT activation with confirmed PE diagnosis have undergone ECMO since the initiation of our PERT program in 2012. The mean age was 49 ± 19 years. Six (46%) patients were female. All the patients had cardiac arrest, either as an initial presentation or in-hospital cardiac arrest after presentation. All the patients exhibited right ventricular (RV) dilation on echocardiogram with RV hypokinesis. Eight (62%) patients received systemic thrombolysis with intravenous tissue plasminogen activator (tPA) and three (23%) patients underwent catheter-directed thrombolysis therapy using the EKOS system (EKOS Corporation, Bothell, WA, USA). Four (31%) patients underwent surgical embolectomy. Mean ECMO duration was 5.5 days, ranging from 2-18 days. Thirty-day mortality was 31% and one-year mortality was 54%.Patients with massive pulmonary embolism who suffer a cardiac arrest have high morbidity and mortality. ECMO can be used in conjunction with systemic thrombolysis, catheter-directed therapy or as a bridge to surgical embolectomy.
Massive pulmonary embolism requiring extracorporeal life support treated with catheter-based interventions
PMID:23258138
[Cited within: 1]

When pulmonary embolism (PE) develops, circulatory collapse and hypoxia are caused at the same time. The rapid and proper use of extracorporeal life support (ECLS) can improve the mortality rate of patients with collapsed massive PE. No study has examined the influence of treatment that involved adding catheter based-intervention to ECLS with massive collapsed PE. Thirty-five patients with massive PE were examined, and 10 of these patients were placed on ECLS. Eight of the 10 patients placed on ECLS for massive PE were female, and the median age was 61 years. Seven patients had in-hospital onset PE and 3 patients out-of-hospital onset PE. Their underlying conditions were a cerebral infarction (3 patients), coronary artery disease (5 patients), collagen disease (one patient), postoperative state (3 patients), and lung disease (2 patients). Pulmonary angiographic findings showed that a filling defect or complete occlusion was observed in all 10 patients in the proximal lobular arteries, 6 of which had large thrombi stretching to the main pulmonary arteries. All patients underwent thrombolysis. Percutaneous catheter embolus fragmentation and/or thrombectomy were undertaken in 7 patients. All patients required red blood cell transfusion for cannulation site bleeding. The mean duration of ECLS bypass was 48 ± 44 hours. The 30 day mortality rate was 30%. The current study clarified the characteristics of patients with massive PE requiring ECLS. These patients have extensive pulmonary thromboemboli, thus, the aggressive use of catheter-based intervention appears to have beneficial effects for massive PE requiring ECLS.