INTRODUCTION
Suspension trauma syndrome (STS) was first described in a report of autopsies of people who died suspended by a harness in Austria and Spain.[1] There were minimal findings related to traumatic injuries, and it was therefore concluded that the cause of death was due to shock.[1] STS is also known as harness hang syndrome, harness-induced trauma, harness-induced pathology, and orthostatic shock while suspended. It is defined as the development of presyncope symptoms and loss of consciousness if the human body is held motionless in a vertical position forsome time.[2] People with prolonged suspension in a harness can die in an expedited fashion and without significantly associated trauma. This syndrome can occur in people who practise activities using harness systems with a dorsal or chest point of attachment as used by painters and builders of high-rise buildings, towers, bridges, marine platforms, power plants, or with a frontal waist point of attachment in sports and recreational activities such as mountaineering, rock climbing, skydiving, paragliding, via ferrata, canyoning, and caving.[3] It can evolve rapidly leading to the state of unconsciousness and eventually death.[3] The term “harness suspension” was originally used to describe STS, but it is not really the harness that is at issue. Pain and respiratory compromise are the issue more than the trauma per se, and any condition that decreases central intravascular volume prior to suspension (i.e., dehydration, hypothermia, fatigue) increases risk.
The objectives of this study are to review the current literature to describe the pathophysiology of STS, identify predisposing factors, and discuss the controversies regarding its management.
METHODS
Data sources and search strategy
A review of the literature published in English and Spanish languages from 1972 to 2020 on suspension trauma was performed. The preference of the languages is based on the ability of the authors to speak those. An exhaustive review of articles published in Pubmed, Medline, Cochrane Library, MeSH, UpToDate, and Google Scholar was executed. The search words used were harness syndrome, suspension syndrome, suspension trauma, suspension shock, harness hang syndrome, suspension stress, reflow syndrome, orthostatic intolerance, orthostatic syndrome, rescue death, rock climbing, harness accident, combinations of these words, and the use of “and” or “or” when feasible. This work was reported in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Assessment of Multiple Systematic Reviews (AMSTAR) guidelines.
Study selection criteria
Articles referring to STS associated with other injury mechanisms such as traumatic impact injuries, drowning, asphyxiation or bleeding, as well as isolated clinical case reports, pediatric population, and publications other than English or Spanish were excluded.
Data extraction
The first author (PP) supervised the entire process from the selection of qualifying articles to the way the extraction took place. A set of data was independently extracted by one international research fellow (IRF-SEV), and then verified by PP. In turn, PP extracted the second set of data, and then it was verified by IRF-SEV. The data extracted from the articles included in this review were organized using a data extraction table with the following categories: author, year of publication, type of study, number of patients, age, gender, and modality of injury.
Risk of bias
An assessment of risk of bias was performed per each individual study, using the Newcastle-Ottawa Quality Assessment Scale for both case-control studies and cohort studies as valid evaluation tool.
RESULTS
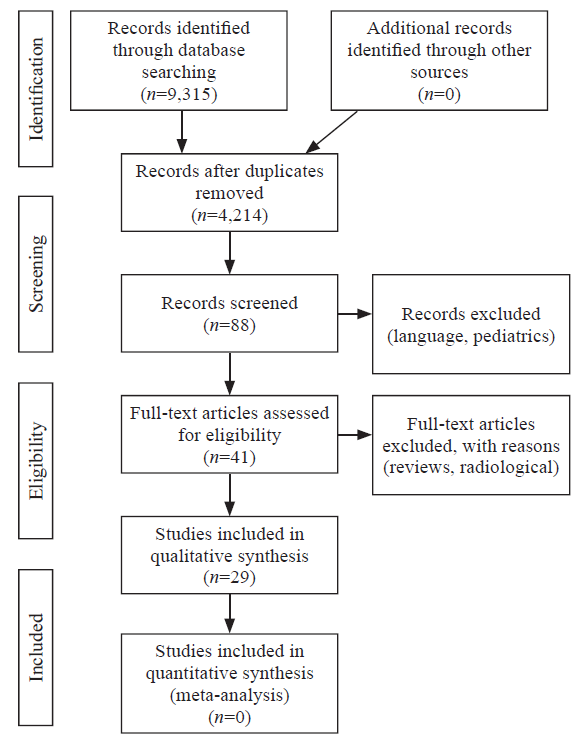
Forty-one articles related to STS were identified. Of these, 29 articles related to mechanism, pathophysiology and management of individuals who suffered prolonged suspension trauma without associated traumatic injuries were included in the study. The PRISMA flow diagram is shown in Figure 1. The Newcastle-Ottawa Quality Assessment Scale was used.
Figure 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
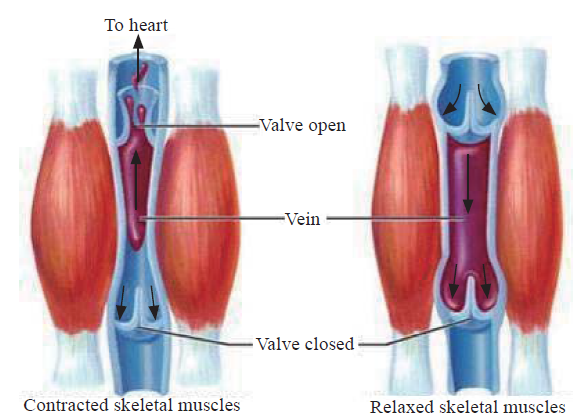
Controversies were found regarding the physiopathological aspects involved in STS. Predisposing factors related to the type of harness and differences in the recommendations in the initial management to reduce the risk of post-rescue death were identified. As for the physiopathology of this syndrome, one theory[4] supports the sequestration of blood in the lower extremities by a deficient muscular pump (Figure 2) causing low cardiac output, while a second theory[4] supports metabolic waste accumulation and hyperkalemia when anaerobic muscular metabolism is produced. Another theory[5] is that harnesses with a dorsal hook may be a trigger of positional asphyxia and vascular compression of arteries and femoral veins exerted by the harness in the inguinal region causing a decrease in venous return as opposed to ventral hook harnesses in which this effect is less severe.
Figure 2.
Figure 2.
Lower extremity skeletal-muscle pump.
The first series[4] of cases of death due to STS was published in 1970. A research group studied the cause of death in ten climbers with no physical injury who were suspended on their own harnesses from 90 minutes up to eight hours. Eight patients were rescued and extricated and survived from 30 minutes to 11 days after their rescue. Eventually, all eight patients died.
In 1972, another series[3] of cases was published in which ten out of 23 climbers died after being suspended on their harness, although they did not suffer any traumatic injuries (e.g., fractures or solid organ damage). Damisch and Schauer[6] in 1985 performed 46 suspension tests on various types of harnesses for up to 10 minutes. No one lost consciousness, but two individuals with harnesses with dorsal hooks had to stop the test by presenting undetectable blood pressure between five and nine minutes of suspension. Harry[7] conducted a study on the type of harness used in parachuting. During this study, one of the participants lost consciousness after six minutes of suspension on a body harness.
A group of French speleology doctors also performed autopsies on patients who died suspended by a harness. This group initially indicated that 10 of the 12 individuals died from hypothermia, but in contradiction to their theory, many of these subjects had lost consciousness very quickly. For this reason, they decided to conduct a study reproducing the same circumstances in a laboratory. The participants were asked to act as if they were unconscious hanging from a harness. The first two participants effectively lost consciousness at seven and thirty minutes, respectively, and the study was stopped. Later on, during a second attempt of the same study but only modifying certain parameters, a participant lost consciousness after six minutes. They concluded that hypothermia was not the cause of death in these patients.[8]
Pathophysiology of suspension trauma
One of the hypotheses that explain STS is the accumulation of blood in the lower extremities due to gravity and proximal compression, which diminishes venous return. This compression causes a reduction in cardiac preload and a concomitant decrease in cardiac output and tissue perfusion. Eventually, this effect is manifested clinically as a loss of consciousness and cardiac arrest. This physiological phenomenon has been observed during military parades, in operating rooms and historically during crucifixions.[9-11] Regarding the pathophysiological aspects involved, certain conditions that endanger the life of these patients have been established.
Compression of blood circulation in limbs
Due to the prolonged suspension in an upright position, these individuals have an accumulation of blood volume in their lower extremities, reaching up to 20% of the total blood volume and causing orthostatic hypotension. Further, the compression of the femoral veins by the harness causes a profound decrease in preload.[12]
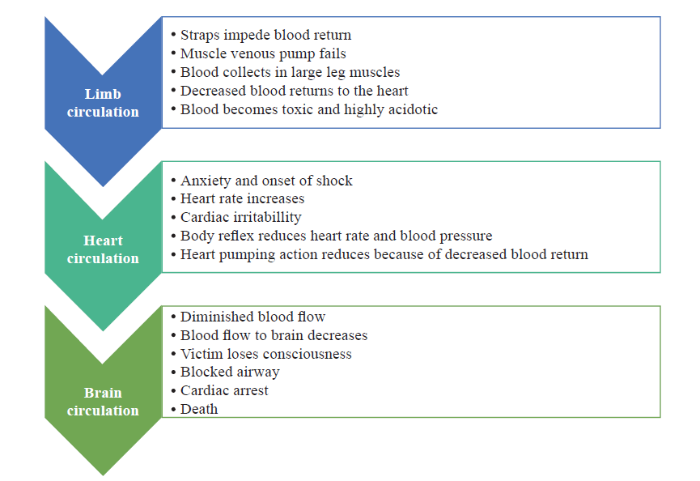
Under normal conditions, acidosis from anaerobic metabolism decreases vascular resistance to allow an increase in blood flow, thus increasing the supply of oxygen and while eliminating waste products. However, when the lower extremities are immobilized in suspension by the harness, blood sequestration in the peripheral region, which contains highly acidotic venous blood, is markedly increased. Figure 3 shows the sequence of this syndrome.
Figure 3.
Figure 3.
Progression of suspension trauma.
Impaired cardiac and respiratory functions
In addition to the decrease in pre-load, after-load and cardiac output, there is a release of adrenaline into the bloodstream as a result of the fear and anxiety experienced by the individual who is suspended. Initially, there is an increase in the frequency and intensity of cardiac contractions as a compensatory mechanism in an attempt to maintain cerebral blood flow, which then leads to the presence of pre-syncopal symptoms, such as nausea, dizziness, sweating, confusion, loss of vision, buzzing, and vertigo. This condition is known as distributive shock and includes a decrease in heart rate, blood pressure, and cardiac output.[13,14] Madsen et al[15] reported in their study of 69 patients pre-syncopal symptoms presenting at 27 minutes after the start of the test with heart rates ranging from 30 to 57 beats per minute.
The decrease in blood pressure mentioned above causes stimulation of baro-receptors and activates the sympathetic autonomic system. This produces an increase in heart rate and blood pressure as a temporary response. However, the decrease in intracardiac volume may induce the presence of paradoxical bradycardia and hypotension as seen in the Bezold-Jarisch reflex.[16]
Peripheral vasoconstriction plays a central role in maintaining blood pressure. Convertino[17] found that the control of systemic vascular resistance was greater in high tolerant orthostasis participants compared with low, in line with greater elevations in the circulating vasopressor hormones (vasopressin, angiotensin, and norepinephrine).
In a recent investigation, Lanfranconi et al[18] reported that participants who did not develop STS were able to activate remedial responses through the O2 transport and utilization chain of various intensities to defend brain oxidative metabolism needs. On the other hand, suspension triggered an imbalanced response of respiratory and cardiovascular reflexes leading to critical cerebral hypoxia. Their findings indicate that the ability to cope with hanging motionless in harness occurs in people developing less marked as well as more rapidly fading of fluctuations in both respiratory and cardiovascular reflex responses. Conversely, wider fluctuations in control of variations of the ventilation and blood pressure result in a progressive decrease in tolerance to suspension, while the imbalance leading to cerebral hypoxia is an early phenomenon (10-12 minutes from the start of the suspension test) irreversibly ending in syncopal event.
Brain circulation failure
STS is a risk that specifically affects wide ranges of situations. An irreversible orthostatic stasis could lead to death if a prompt rescue is not performed. Lanfranconi et al[19] performed a suspension test on 40 adults lasting almost half an hour. They came to the conclusion that the participants who developed STS failed to activate cardiovascular reflexes that usually safeguarded O2 availability to match the metabolic needs of the brain tissue. As was previously described, syncope occurs as a compensatory action. As a result, when an individual falls to the ground, his or her horizontal position allows the lower extremities, heart, and brain to remain at the same level thus restoring blood flow. On the contrary, suspended individuals are unable to counteract this condition by remaining relatively upright in their harnesses. The decrease in heart rate and blood pressure caused by increased vagal autonomic response further causes a deficiency of blood flow. Loss of consciousness may also block the airway, depending on the position of the head.
Post-rescue death
There is no established hypothesis in the literature about the mechanism of post-rescue death. Published reviews indicate that the acidotic blood volume produced through anaerobic metabolism is accumulated in the veins of lower extremities and returns to the heart abruptly, which may lead to heart failure. Although acidosis may temporarily depress cardiac contractility, it has little or no effect on heart rhythm. Therefore, changes in pH are unlikely to cause sudden death.[20]
Cardiac dysrhythmia is caused by sinus arrhythmia and premature ventricular contractions, as seen in the electrocardiograms (EKG) in the experimental study by Stuhlinger et al.[21] Allister[22] reported the case of a man who suffered crush injuries of his legs for eight hours and suffered cardiac arrest one hour after his release. Potassium released from damaged muscle cells is one of the most important blood components present in suspension syndrome. On presentation, the pH was 7.15 and during resuscitation the potassium level was 8.0 mEq/L with peaked T waves. The patient was treated with bicarbonate and insulin with glucose and survived.
Blaisdell[23] in their study found platelet and fibrin residues in the lungs of these individuals after blood reperfusion and interpreted it as a delayed inflammatory response rather than a cause of sudden death.
Pulmonary embolism after periods of blood stasis may present a clinical resemblance to sudden death, but Patscheider[24] found no clots or mechanical obstructions in the autopsies described in his study.
Predisposing factors
Suspension time
The onset of this syndrome is completely unpredictable, but a 2006 Occupational Safety and Health Administration (OSHA) guideline defined increased mortality to be suspended for more than thirty minutes.[25]
Height
It has been reported that when individuals are suspended more than five feet from the ground, the risk of STS increases.[4]
Age and gender
Weight
Increased weight has led to the decreased mean arterial pressure in the lab setting.[1]
Types of harness
There are several types of harnesses used by individuals who work at height. These include chest, dorsal, seat type, positioning, and full-body harnesses. There are controversies about which type of harness may portend an increased risk for this pathology to occur. Some groups indicate that harnesses may cause cardiorespiratory involvement as they cause positional asphyxia. The dorsal harness produces a decrease in blood flow creating functional hypovolemia by limiting the physiological movement of the rib cage and diaphragm. This decreases the respiratory volume and increases intrathoracic pressure resulting in a decrease in preload and cardiac output.[26]
Beverly et al[13] reported an increased frequency of this syndrome in patients with dorsal harnesses. They conducted a prospective randomized study on the differences according to the types of harness (frontal or dorsal) for 30 minutes, and concluded that full-body harness was the best tolerated (range 5-30 minutes) when compared to dorsal or belt harnesses. Another finding was that staying in suspension for 30 minutes using the frontal-type harness did not result in significant hemodynamic changes. The duration of the test was shorter in those who wore dorsal harnesses, because many of them experienced dizziness, indicating the onset of more severe hemodynamic dysfunction. In individuals with dorsal harnesses, an increase in heart rate was noted as a compensatory autonomic response to the decrease in pulse pressure and heart volume. This decrease in pulse pressure, when associated with the presence of dizziness, may indicate the onset of poor cerebral perfusion. When comparing the results with the frontal harness, no changes in heart rate were identified in this group. On the other hand, the assessment of mean blood pressure showed an increase in number without seeing a clear decrease in pulse pressure. Biologic markers of muscle damage were also evaluated. The results showed that in the dorsal harness group, an increase of aspartate aminotransferase was seen in plasma. This result could be explained by the compressive effect on the groin exerted by harness strips causing muscular ischemia in this site. Turner et al[27] supported the theory of this group. They determined that 50% of individuals wearing dorsal harnesses experienced symptoms after 31 minutes of suspension.
Management of suspension trauma
Some authors have questioned the existence of suspension trauma, but it is clear that persons suspended in a harness can die more quickly than expected and with no significant trauma. Clearly, this is a shock syndrome in its early phase complicated by rhabdomyolysis in its late phase survivors.[1] The shock is secondary to failure of the venous pump to return sufficient volume to central circulation. Treatment starts with immediate rescue of a suspended person, always following Advanced Trauma Life Support® (ATLS®) guidelines. Oxygen and immediate intravenous fluid administration to prevent crush syndrome is appropriate but should not delay rescue. Early first-aid care appears to be vital, even before the arrival of professional emergency medical care.[28] Research teams recommend starting the rescue of these patients promptly. If the patient is conscious, he or she should be instructed to move his or her legs vigorously upwards, thus creating muscle work to mitigate the pre-syncopal symptoms.
If unconscious, the patient should be released from the harness as soon as possible. In this situation, it is recommended to keep the patient in a semi-fowler position by keeping his or her torso in an elevated position at approximately an angle of 30-40º, and then slowly moving him or her to the supine position in a period between 30 and 45 minutes. There is controversy regarding the initial management since the lack of oxygenated blood received by the brain during this suspension period is potentially lethal. Certain groups claim that it is even riskier to place these patients in the supine position immediately after the rescue due to the reflux that can cause death by producing an acute overload of volume to the right ventricle by massive return of blood. In a study[21] of ten volunteer individuals who remained suspended by a harness until they showed signs of circulatory collapse and were immediately placed horizontally, it was identified that some of the individuals presented with sinus arrhythmia and ventricular extrasystoles. The authors concluded that the rapid venous return of accumulated blood could induce cardiac failure and death. Currently, there is no scientific evidence to support this theory that justifies changes in the guideline’s management and principles of life support.[9]
On the contrary, Blaisdell[23] contradicted the theory of reflux death syndrome. When studying the pathophysiology of reperfusion syndrome in skeletal muscle ischemia, they noted that suffering from lack of blood flow in muscle cells caused edema and further increased ischemia. The early restoration of blood circulation will limit the additional loss of volume in the interstitium by reducing edema. The authors suggest that the patient should be released from the harness and placed supine as soon as possible to restore oxygenated blood flow to the damaged muscle. Maintaining the vertical position in these individuals will simply worsen muscle damage and hypovolemic shock. These patients should be transferred to a hospital following the guidelines of ATLS®.
Intravenous hydration should be initiated to increase diuresis and decrease the risk of renal failure, preferably with isotonic saline followed by hypotonic saline with bicarbonate if necessary. Hypoglycemia should be avoided by correcting blood glucose levels with 25 g intravenous bolus with 50% dextrose. It is not recommended to administer intravenous potassium until clearly indicated.[4]Appropriate pain relief should be administered to reduce stress due to pain and anxiety in these patients.
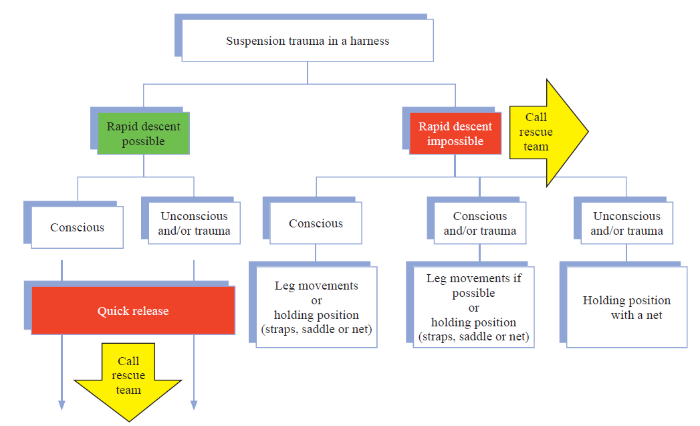
In addition, it is very important to perform an EKG in search of alterations such as the presence of peaked T waves suggesting hyperkalemia, prolongation of the QT interval, and widened QRS complexes. Blood pressure should be monitored since the presence of high blood pressure may indicate hyperkalemia and the onset of suspension syndrome. These patients should be immediately transferred to a hospital with appropriate knowledge of the management of trauma patients (Figure 4) and preferably with dialysis capabilities.
Figure 4.
Figure 4.
Algorithm for the initial management of suspension trauma patients.[26]
Complications
Rhabdomyolysis is a well-described complication of STS. It is produced by the compressive effect of the harness on the lower extremities, with the consequent decrease in blood flow, poor muscle irrigation, and hemostasis. Damaged muscle cells release myoglobin causing acute renal failure. Flora and Holzl[32] published a series of ten patients who died of STS, where seven of them died immediately after rescue, and one at 11 days after rescue from renal failure.
Limitations
The study has a number of limitations. This is a literature review with inherent problems. Due to the retrospective nature of the study based on several other authors’ publications, the authors were careful to select articles focused only on physiopathology, management, and prevention. Additionally, some of the referenced articles included in this review are a bit older, as only a few research studies have been published on this topic. These factors in combination encouraged the authors to review the subject and write this manuscript.
CONCLUSIONS
STS represents a risk for individuals performing suspension activities with a harness. Currently, the modernization of the harness design and the development of safety guidelines have made this activity safer than before. Several studies support the front-type harness over the dorsal hook harness. In order to apply the most appropriate recommendations, we recommend larger studies based on scientific evidence where the safety would also be a concern, as well as experimental animal models, in order to develop management and life support guidelines from a trauma and emergency medicine perspective.
As a take-home message, the basics of STS management can be summarized as follows: (1) remove the person from the rope: be sure that the scene is safe, if the patient can cooperate, ask to move and raise his/her legs; (2) lay the patient flat and start ATLS protocols with no delay: airway, breathing, circulation (ABC), plus hypothermia prevention; (3) oxygen, monitoring, intravenous fluid if available (alternate saline and half-normal saline with added bicarbonate); (4) remove the harness and transport the patient to a facility capable of dialysis if he or she has been suspended passively for more than two hours.
Funding: None.
Ethical approval: This study is a literature review, and there is no need for an informed consent.
Conflicts of interests: The authors declare that they have no competing interests.
Contributors: PP proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
Reference
Risks and management of prolonged suspension in an Alpine harness
DOI:10.1016/j.wem.2010.10.008 URL [Cited within: 5]
Suspension trauma
DOI:10.1136/emj.2007.046391 URL [Cited within: 1]
Suspension síndrome: a potencially fatal vagally mediated circulatory collapse—an experimental randomized crossover trial
DOI:10.1007/s00421-019-04126-5 URL [Cited within: 4]
Dangerous suspension. Understanding suspension syndrome and prehospital treatment for those at risk
DOI:10.1016/S0197-2510(09)70265-7 URL [Cited within: 5]
Clinical update: suspension trauma
DOI:10.1016/j.wem.2010.12.006 URL [Cited within: 1]
Does the horizontal position increase risk of rescue death following suspension trauma?
DOI:10.1136/emj.2008.064931
PMID:19934143
[Cited within: 2]

It is widely believed that placing a patient who has been subjected to suspension trauma in a horizontal position after rescue may cause rescue death. The discussion whether position is important has been dominated by non-medical personnel. Subsequently, this has led to a general advice on emergency treatment of these patients, which may cause incorrect or even fatal treatment.To determine whether there is any medical evidence supporting that horizontal positioning after suspension trauma may cause rescue death, the authors located publications, reports, expert opinions and other sources of information addressing the acute treatment of suspension trauma. These sources were then evaluated.Several thousand hits regarding suspension trauma were located on the internet and five articles on the PubMed. Although most of them warned of the dangers of rescue death brought about by assuming the horizontal position after prolonged suspension, the authors found no clinical studies, and none of the sources offered any conclusive evidence as to whether the horizontal position increases the risk of rescue death. Neither the authors, nor the suspension trauma experts who were contacted, had ever experienced or heard of case reports supporting the causal relation between the horizontal position and rescue death.After evaluating the current literature, the authors found no support for the view that the horizontal position may be potentially fatal for patients exposed to suspension trauma. In the absence of any evidence to the contrary, the authors suggest that the initial management of patients who have had suspension trauma should follow normal guidelines for the acute care of traumatised patients, without special modifications.
On the physical death of Jesus Christ
DOI:10.1001/jama.1986.03370110077025 URL
Medical theories on the cause of death in crucifixion
DOI:10.1177/014107680609900416 URL [Cited within: 1]
Suspension trauma: a clinical review
Harness suspension stress: Physiological and safety assessment
DOI:10.1097/JOM.0000000000001459
PMID:30256303
[Cited within: 2]

: Hanging motionless in a full body harness may result in unwanted events, such as acute hypotension and syncope, which has been termed harness suspension stress (HSS). The etiology of HSS has not been explored, and it is unknown if the type of harness influences the HSS response.Evaluate hemodynamics, subjective discomfort, and biological markers of muscle damage during 30-minutes suspension; and evaluate differences between harness attachment (frontal or dorsal).Heart rate, blood pressure, biological markers of muscle damage, and subjective discomfort were measured.Trial time was shorter in the dorsal versus frontal point of attachment. Hemodynamic shift resulted in the dorsal trial which indicated possible perfusion abnormalities.Hemodynamic adjustments contributed to early termination observed in the dorsal trial. A frontal point of attachment may be more suitable for extended harness exposure.
Cardiorespiratory response to free suspension simulating the situation between fall and rescue in a rock-climbing accident
DOI:10.1580/1080-6032(1996)007[0109:CRTFSS]2.3.CO;2 URL [Cited within: 1]
Tolerance to head-up tilt and suspension with elevated legs
The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart
PMID:6826948
[Cited within: 1]

The concept of depressor reflexes originating in the heart was introduced by von Bezold in 1867 and was later revived by Jarisch. The Bezold-Jarisch reflex originates in cardiac sensory receptors with nonmyelinated vagal afferent pathways. The left ventricle, particularly the inferoposterior wall, is a principal location for these sensory receptors. Stimulation of these inhibitory cardiac receptors by stretch, chemical substances or drugs increases parasympathetic activity and inhibits sympathetic activity. These effects promote reflex bradycardia, vasodilation and hypotension (Bezold-Jarisch reflex) and also modulate renin release and vasopressin secretion. Conversely, decreases in the activity of these inhibitory sensory receptors reflexly increase sympathetic activity, vascular resistance, plasma renin activity and vasopressin. Long regarded as pharmacologic curiosities, it is now clear that reflexes originating in these inhibitory cardiac sensory receptors are important to the pathophysiology of many cardiovascular disorders. This paper reviews the role of inhibitory cardiac sensory receptors in several clinical states including 1) bradycardia, hypotension and gastrointestinal disorders with inferoposterior myocardial ischemia and infarction, 2) bradycardia and hypotension during coronary arteriography, 3) exertional syncope in aortic stenosis, 4) vasovagal syncope, 5) neurohumoral excitation in chronic heart failure, and 6) the therapeutic effects of digitalis.
Neurohumoral mechanisms associated with orthostasis: reaffirmation of the significant contribution of the heart rate response
DOI:10.3389/fphys.2014.00236
PMID:25071585
[Cited within: 1]

The inability to compensate for acute central hypovolemia underlies the clinical development of orthostatic hypotension and instability (e.g., syncope). Although neuro-humoral control of both cardiac output and peripheral vascular resistance contributes to hemodynamic stability during orthostasis, a notion has been proposed that the failure of adequate peripheral vascular constriction rather than cardiac responses represents the primary mechanism underlying the development of orthostatic intolerance. This review article provides an opportunity to present compelling evidence captured over the past 30 years in our laboratory to support the concept that neural-mediated tachycardia during orthostasis in healthy individuals represents a critical response to tolerating acute reduction in central blood volume in addition to, and independent of, peripheral vascular constriction. In this review paper, data are presented from experiments using graded lower body negative pressure (LBNP) as a method to induce orthostatic intolerance in two experimental human models: (1) comparison of heart rate and autonomic responses in individuals with relatively high and low tolerance to LBNP; and (2) vagal and sympathetic blockade of cardiac neural control. These experiments revealed that: (1) greater elevations in heart rate are associated with higher orthostatic (LBNP) tolerance; (2) higher orthostatic heart rate is associated with greater sympathetic nerve activity and withdrawal of vagally-mediated cardiac baroreflex response; and (3) non-specific sympathetic blockade causes a pronounced reduction in heart rate and LBNP tolerance. Cardiac parasympathetic withdrawal contributes to protection against development of hypotension during the initial seconds of transition to an orthostatic challenge, while the primary mechanism by which tachycardia defends orthostatic stability in healthy subjects for extended durations is mediated predominantly through sympathetic adrenergic control.
Impact of hanging motionless in harness on respiratory and blood pressure reflex modulation in mountain climbers
DOI:10.1089/ham.2018.0089
PMID:31009248
[Cited within: 1]

Harness hang syncope (HHS) is a risk that specifically affects safety of harness users in mountain climbing. To evaluate individual patterns of breathing resulting from deranged cardiovascular reflexes triggering a syncopal event when a mismatch between cerebral O demand and supply is present. Forty healthy participants [aged 39.1 (8.2) years] were enrolled in a motionless suspension test while hanging in harness. Respiratory gas exchange values were analyzed to assess the pattern of breathing (EpInW, respiratory elastic power) and cardiovascular parameters were monitored (BP, blood pressure). Four participants experienced HHS after 30.0 (7.6) minutes, with an early manifestation of loss of control of both a sustainable EpInW and BP, starting after 10-12 minutes. Among the other participants, two different reactions were observed during suspension: (1) group G1 tolerated 32.7 (11.4) minutes of suspension by a favorable adaptation of the EpInW and BP parameters and (2) group G2 showed significantly shorter time of suspension 24.0 (10.4) minutes with unfavorable increase in EpInW and BP. Greater resistance to HHS occurs in people developing less marked fluctuations of both respiratory and cardiovascular reflex responses. Conversely, wider fluctuations both in control of EpInW and BP were observed in the event of decreased suspension tolerance or in syncopal events.
The elusive path of brain tissue oxygenation and cerebral perfusion in harness hang syncope in mountain climbers
DOI:10.1089/ham.2017.0028
PMID:28981369
[Cited within: 1]

Lanfranconi, Francesca, Luca Pollastri, Giovanni Corna, Manuela Bartesaghi, Massimiliano Novarina, Alessandra Ferri, and Giuseppe Andrea Miserocchi. The elusive path of brain tissue oxygenation and cerebral perfusion in harness hang syncope in mountain climbers. High Alt Med Biol. 18:363-371, 2017.Harness hang syncope (HHS) is a risk that specifically affects wide ranges of situations requiring safety harnesses in mountains. An irreversible orthostatic stasis could lead to death if a prompt rescue is not performed. We aimed at evaluating the risk of developing HHS and at identifying the characteristics related to the pathogenesis of HHS.Forty adults (aged 39.1 [8.2] years) were enrolled in a suspension test lasting about 28.7 (11.4) minutes. We measured cardiovascular parameters, and near infrared spectroscopy (NIRS) was used to assess cerebral hypoxia by changes in the concentration of oxyhemoglobin (Δ[HbO]) and de-oxyhemoglobin (Δ[HHb]). In the four participants who developed HHS: (1) systolic and diastolic blood pressure showed ample oscillations with a final abrupt drop (∼30 mmHg); (2) Δ[HbO] increased after 8-12 minutes of suspension and reached a plateau before HHS; and (3) Δ[HHb] decreased with a final abrupt increase before syncope.Participants who developed HHS failed to activate cardiovascular reflexes that usually safeguard O availability to match the metabolic needs of the brain tissue. Since cerebral hypoxia was detected as an early phenomenon by Δ[HbO] and Δ[HHb] changes, NIRS measurement appears to be the most important parameter to monitor the onset of HHS.
The effects of acid-base disturbances on cardiovascular and pulmonary function
PMID:4599247 [Cited within: 1]
Cardiac arrest after crush injury
PMID:6411209 [Cited within: 1]
The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review
PMID:12453699
[Cited within: 2]

There are two components to the reperfusion syndrome, which follows extremity ischemia. The local response, which follows reperfusion, consists of limb swelling with its potential for aggravating tissue injury and the systemic response, which results in multiple organ failure and death. It is apparent that skeletal muscle is the predominant tissue in the limb but also the tissue that is most vulnerable to ischemia. Physiological and anatomical studies show that irreversible muscle cell damage starts after 3 h of ischemia and is nearly complete at 6 h. These muscle changes are paralleled by progressive microvascular damage. Microvascular changes appear to follow rather than precede skeletal muscle damage as the tolerance of capillaries to ischemia vary with the tissue being reperfused. The more severe the cellular damage the greater the microvascular changes and with death of tissue microvascular flow ceases within a few hours-the no reflow phenomenon. At this point tissue swelling ceases. The inflammatory responses following reperfusion varies greatly. When muscle tissue death is uniform, as would follow tourniquet ischemia or limb replantation, little inflammatory response results. In most instances of reperfusion, which follows thrombotic or embolic occlusion, there will be a variable degree of ischemic damage in the zone where collateral blood flow is possible. The extent of this region will determine the magnitude of the inflammatory response, whether local or systemic. Only in this region will therapy be of any benefit, whether fasciotomy to prevent pressure occlusion of the microcirculation, or anticoagulation to prevent further microvascular thrombosis. Since many of the inflammatory mediators are generated by the act of clotting, anticoagulation will have additional benefit by decreasing the inflammatory response. In instances in which the process involves the bulk of the lower extremity, amputation rather than attempts at revascularization may be the most prudent course to prevent the toxic product in the ischemic limb from entering the systemic circulation.
Postural asphyxia or positional asphyxia due to abdominal suspension: a diagnosis of exclusion?
Suspension tolerance in a full-body safety harness, and a prototype harness accessory
DOI:10.1080/15459620801894386
PMID:18247226
[Cited within: 1]

Workers wearing full-body safety harnesses are at risk for suspension trauma if they are not rescued in 5 to 30 min after a successfully arrested fall. Suspension trauma, which may be fatal, occurs when a person's legs are immobile in a vertical posture, leading to the pooling of blood in the legs, pelvis, and abdomen, and the reduction of return blood flow to the heart and brain. To measure suspension tolerance time, 22 men and 18 women with construction experience were suspended from the chest D-ring (CHEST) and back D-ring (BACK) of full-body, fall-arrest harnesses. Fifteen men and 13 women from the original group of subjects were then suspended using a newly developed National Institute for Occupational Safety and Health harness accessory (ACCESS), which supports the upper legs. Midthigh circumference changes were 1.4 and 1.9 cm, changes in minute ventilation were 1.2 and 1.5 L/min, changes in heart rate (HR) were 15.1 and 21.6 bpm, and changes in mean arterial pressure were 5.1 and -2.6 mmHg (p < or = 0.05) for all subjects during CHEST and BACK, respectively. Kaplan-Meier median suspension time for all subjects for the CHEST condition was 29 min (range 4-60 min) and 31 min (range 5-56 min) for the BACK condition. The 95th percentile for suspension time was 7 min for CHEST and 11 min for BACK. Cox regression revealed that body weight had a statistically significant effect on the time until experiencing a medical end point (p < or = 0.05) during the BACK condition. Mean (+/- SD) suspension time was 58 +/- 6 min (range 39-60 min) for all subjects for the ACCESS condition. There were no terminations due to medical symptoms during the ACCESS suspension, changes in physiological variables were small, and 85% of ACCESS subjects completed 60-min suspensions. These data provide information on motionless suspension tolerance time to standards-setting organizations and demonstrate the potential of a prototype harness accessory to delay or prevent suspension trauma.
Proposal of an effective algorithm to manage suspension trauma in the field
Suspension trauma: those writing fall protection plans must understand the hazards of harness Hang syndrome in fall protection
Will your safety harness kill you?
PMID:12674991
Fatal and non-fatal accidents involving falls into the rope. In: Proceeding of the Second International Conference of Mountain Rescue Doctors