Dear editor,
Paraquat (PQ) can cause acute lung injury and irreversible pulmonary fibrosis, without specific antidotes. Respiratory failure is the main cause of death among patients with PQ poisoning. Currently, the treatment involves the use of adsorbents, intensive hemoperfusion, antioxidants, immunosuppressive therapy, respiratory support by mechanical ventilation, and other symptomatic and supportive treatments.[1-3] The mortality of patients with severe PQ poisoning remains high. More effective and safer therapies remain to be explored. Few studies reported performing extracorporeal membrane oxygenation (ECMO) and subsequent lung transplantation with either successful or poor clinical outcome in extremely critical patients with PQ poisoning.[4,5] This study aims to summarize the clinical experience and reflect on the therapeutic prospect of ECMO in respiratory failure or cardiopulmonary failure caused by fatal PQ poisoning.
METHODS
We conducted a retrospective and observational study of five patients receiving ECMO on admission to the First Affiliated Hospital of Zhejiang University after PQ poisoning between 2011 and 2018. All patients were diagnosed with PQ poisoning according to their history of excessive ingestion of 20% PQ solution and strongly positive semi-quantitative urine dithionite tests. All patients had no severe related primary diseases of the heart, lung, liver, or kidney before poisoning. The treatment protocols were carried out in accordance with the principles of the Declaration of Helsinki.
RESULTS
The baseline information and clinical characteristics of these patients are shown in Table 1. Four patients, except patient 1, had fever and only patient 5 manifested as dyspnea on admission. Symptomatic and supportive treatments were applied immediately for all five patients, such as gastric lavage, antioxidants of glutathione and acetylcysteine, immunosuppressive therapy of methylprednisolone and cyclophosphamide, stomach and liver protection, and infection control. All patients performed continuous renal replacement therapy (CRRT) and/or hemoperfusion (HP). As shown in Table 1, patient 1 ingested 150 mL PQ solution, which was far beyond the lethal dose. Vasoactive drugs of dopamine and noradrenaline were administered to improve circulatory failure immediately after poisoning. The venous-arterial (V-A) ECMO was performed proactively at 21 hours after poisoning, even though she had not displayed apparent dyspnea and pulmonary abnormality, according to the computed tomography (CT) scan. Furthermore, the other four patients developed dyspnea and a sharp decline in oxygen saturation (SaO2) during hospitalization. Severe changes were observed in the bilateral pulmonary CT scan images. The life-threatening cardiac and/or respiratory failure developed and ECMO was subsequently performed. Patient 2 had sudden cardiac arrest and acute respiratory distress syndrome; therefore, V-A ECMO was performed to provide both circulatory and respiratory support. Patients 3, 4, and 5 manifested symptoms as respiratory failure without circulatory failure and venous-venous (V-V) ECMO was applied. The specific characteristics of ECMO are shown in Table 1.
Table 1 Clinical characteristics of the five patients in whom extracorporeal membrane oxygenation was performed after fatal paraquat poisoning
| Variables | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age (years) | 15 | 14 | 45 | 25 | 33 |
| Gender | Female | Female | Male | Female | Female |
| Ingestion amount (mL) | 150 | 100 | 110 | 90 | 100 |
| Poisoning to admission time (hours) | 4 | 24 | 24 | 24 | 10 |
| Hospital stays (hours) | 58.0 | 79.0 | 72.5 | 365.5 | 298.0 |
| CRRT/HP | HP | HP | HP+CRRT | CRRT | HP+CRRT |
| Clinical manifestations before ECMO | |||||
| Dyspnea | No | Yes | Yes | Yes | Yes |
| SaO2 (%) | 97.8 | 60.5 | 88.1 | 92.0 | 70.0 |
| Creatinine (μmol/L) | 243 | 464 | 354 | 120 | 296 |
| PaO2 (mmHg) | 112 | 33 | 53 | 60 | 37 |
| pH | 7.40 | 7.44 | 7.45 | 7.45 | 7.45 |
| WBC (×109/L) | 16.6 | 9.2 | 33.9 | 6.7 | 16.6 |
| APACHE II score | 11 | 28 | 15 | 8 | 12 |
| CT scanning | No abnormality | Bilateral pulmonary multiple patchy blur | Bilateral pulmonary interstitial fibrosis | Bilateral pulmonary multiple patchy shadows with partial consolidation; bilateral pleural effusion | Bilateral pulmonary diffuse patchy shadows |
| Indications of ECMO | Circulatory failure | Cardiopulmonary failure | Respiratory failure | Respiratory failure | Respiratory failure |
| ECMO characteristics | |||||
| Poisoning to ECMO time (hours) | 21 | 96 | 72 | 144 | 72 |
| ECMO pattern | V-A | V-A | V-V | V-V | V-V |
| Bypass | LFA-LFV | RFA-LFV | RFV-RJV | RFV-RJV | RFV-RJV |
| ECMO complications | Hemorrhage at puncture point | Hemorrhagic shock | Oropharyngeal hemorrhage | No | Multiple organ hemorrhage |
| ECMO to death time (hours) | 41.0 | 7.0 | 24.5 | 245.5 | 236.0 |
| Death reasons | MOF | Hemorrhagic shock | MOF | Respiratory failure, giving up treatment | MOF, giving up treatment |
CRRT: continuous renal replacement therapy; HP: hemoperfusion; ECMO: extracorporeal membrane oxygenation; SaO2:oxygen saturation; PaO2: partial pressure of oxygen; WBC: white blood count; APACHE II: Acute Physiology and Chronic Health Evaluation II; CT: computed tomography; V-A: venous-arterial; V-V: venous-venous; LFA: left femoral artery; LFV: left femoral vein; RFA: right femoral artery; RFV: right femoral vein; RJV: right jugular vein; MOF: multiple organ failure.
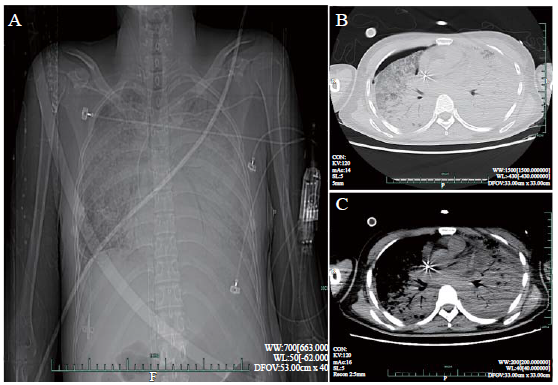
Hemorrhage is the main complication of ECMO. All patients, other than patient 4, presented hemorrhage at the site of puncture, oropharynx, lungs, or multiple organs. Notably, patient 2 displayed massive pulmonary hemorrhage during operation of ECMO and died of hemorrhagic shock after seven hours. V-V ECMO assisted patients 4 and 5 to maintain oxygenation for about ten days. Figure 1 shows the typical CT imaging manifestations after five days of ECMO in patient 4. However, because of high medical expenses and lack of donor lungs, both patients had to discontinue further treatments, and they died of respiratory failure and multiple organ failure (MOF), respectively. Unfortunately, all five patients did not survive.
Figure 1.
Figure 1.
Typical computed tomography imaging manifestations of patient 4 on day 5 of extracorporeal membrane oxygenation. A: X-ray indicated bilateral pulmonary infiltration; B and C: CT images revealed bilateral pulmonary diffuse exudation with partial consolidation and bilateral pleural effusion; pneumothorax was observed in the right pulmonary.
DISCUSSION
Redox cycling is the main mechanism involved in PQ toxicity. PQ reacts with the abundant pulmonary oxygen to generate oxidative stress and deplete the body of antioxidants.[1] Therefore, oxygen therapy is usually not applied at the early stage to prevent aggravating injury by redox cycling. Previous studies have shown that ECMO has attracted more attention in severe poisoning, such as through irritant gases, PQ, and aluminum phosphide.[6,7,8] ECMO can preserve tissue perfusion by augmenting oxygenated venous blood via V-V ECMO, enhancing perfusion to critical organs, and improving oxygenation via V-A ECMO. Lung transplantation may be the most effective end-stage therapy, owing to irreversible pulmonary fibrosis induced by PQ, in which ECMO can be the transitional supportive therapy.[4,9] However, because of the severity of the disease and the lack of lung sources, mortality remains high. In addition, the most common complication of ECMO is hemorrhage at the cannulation site in V-V ECMO or at the surgical entry site in V-A ECMO, which may increase morbidity.[8]
Our department, as a poisoning treatment center, has successfully performed ECMO for approximately 30 critical patients each year in the past eight years. Moreover, our hospital is equipped to perform lung transplantation. According to previous clinical practice and studies, rare patient has survived after ingesting 40-60 mL of 20% PQ solution.[3] Patient 1 developed circulatory failure after ingesting 150 mL 20% PQ solution. The V-A ECMO was proactively performed to maintain circulatory stability and to reduce pulmonary oxygen transport, and thereby to decrease the generation of oxygen free radicals before the appearance of significant abnormalities in her pulmonary system. However, she still died of MOF. We speculate that other mechanisms of PQ intoxication might play an important role in addition to those of oxidative stress. The V-A ECMO showed a high risk of hemorrhage.[8] Both patient 1 and patient 2 in our study had hemorrhage. Patient 2 bled heavily during the operation of V-A ECMO and died of hemorrhagic shock. In the present study, V-V ECMO maintained ventilation and oxygenation in two patients for approximately ten days. These two patients may demonstrate the applicability of ECMO for patients with fatal PQ poisoning, although they abandoned treatments, owing to high costs and lack of donor lungs, and finally died. Moreover, a previous study[4] has reported one successful case that ECMO could be a bridge to lung transplantation for a patient with severe PQ poisoning.Therefore, we speculate that ECMO may be just a bridge treatment for lung transplantation, other than treating severe PQ poisoning, according to these five patients' clinical outcome.
CONCLUSIONS
Further clinical verification is needed regarding the effectiveness of ECMO in patients with fatal PQ poisoning to confirm whether EMCO is an effective life-saving therapy or just grasp for straws. The optimal characteristics in PQ patients, as well as the timing and patterns of ECMO, should be further explored. Prohibitive costs and insufficiency of donor lungs are also prominent inevitable problems. In addition, clinicians should consider key points during the operation of ECMO to decrease the occurrence of complications and provide enough time for cardiopulmonary support.
Funding: This work is supported by the Foundation of Key Discipline Construction of Zhejiang Province for Traditional Chinese Medicine (2017-XK-A36) and the Medical and Health Science Foundation of Zhejiang Province (2019327552).
Ethical approval: This study was approved by the Ethical Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (2018369).
Conflicts of interest: No any benefits have been received from a commercial party related directly or indirectly to the study.
Contributors: MXF proposed and wrote the first draft. Both authors contributed to the design and interpretation of the study and to further drafts.
Reference
Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment
PMID:18161502
[Cited within: 1]

Paraquat dichloride (methyl viologen; PQ) is an effective and widely used herbicide that has a proven safety record when appropriately applied to eliminate weeds. However, over the last decades, there have been numerous fatalities, mainly caused by accidental or voluntary ingestion. PQ poisoning is an extremely frustrating condition to manage clinically, due to the elevated morbidity and mortality observed so far and due to the lack of effective treatments to be used in humans. PQ mainly accumulates in the lung (pulmonary concentrations can be 6 to 10 times higher than those in the plasma), where it is retained even when blood levels start to decrease. The pulmonary effects can be explained by the participation of the polyamine transport system abundantly expressed in the membrane of alveolar cells type I, II, and Clara cells. Further downstream at the toxicodynamic level, the main molecular mechanism of PQ toxicity is based on redox cycling and intracellular oxidative stress generation. With this review we aimed to collect and describe the most pertinent and significant findings published in established scientific publications since the discovery of PQ, focusing on the most recent developments related to PQ lung toxicity and their relevance to the treatment of human poisonings. Considerable space is also dedicated to techniques for prognosis prediction, since these could allow development of rigorous clinical protocols that may produce comparable data for the evaluation of proposed therapies.
Treatment outcome of combined continuous venovenous hemofiltration and hemoperfusion in acute paraquat poisoning: a prospective controlled trial
Predictive value of the maximum serum creatinine value and growth rate in acute paraquat poisoning patients
DOI:10.1038/s41598-018-29800-0 URL [Cited within: 1]
Successful extracorporeal membrane oxygenation therapy as a bridge to sequential bilateral lung transplantation for a patient after severe paraquat poisoning
DOI:10.3109/15563650.2015.1082183 URL [Cited within: 3]
Tissue concentration of paraquat on day 32 after intoxication and failed bridge to transplantation by extracorporeal membrane oxygenation therapy
DOI:10.1186/2050-6511-14-45 URL [Cited within: 1]
Extracorporeal membrane oxygenation for poisonings reported to U.S. poison centers from 2000 to 2018: an analysis of the national poison data system
DOI:10.1097/CCM.0000000000004401 URL [Cited within: 1]
Impact of extra-corporeal membrane oxygenation on outcome of aluminium phosphide poisoning complicated with myocardial dysfunction
DOI:10.1080/15563650.2019.1584297 URL [Cited within: 1]
Extracorporeal membrane oxygenation in the treatment of poisoned patients
DOI:10.3109/15563650.2013.800876
PMID:23697460
[Cited within: 3]

Although extracorporeal membrane oxygenation (ECMO) was used in many patients following its introduction in 1972, most hospitals had abandoned this experimental treatment for adult patients. Recently, improvements in the ECMO circuitry rendered it more biocompatible. The surprisingly low mortality in patients with severe acute respiratory distress syndrome who were treated with ECMO in the influenza A/H1N1 pandemic of 2009 resurrected interest in ECMO in many intensive care units around the world.This article reviews the different techniques of ECMO, the indications, contraindications and complications of its use, its role in poisoned patients and the ethics of its use.We searched Pubmed, Toxnet, Cochrane database and Embase from 1966 to September 2012 using the search terms (''extracorporeal membrane oxygenation'', 'extracorporeal life support', 'ECMO', 'ECLS', 'assist-device', and 'intox*' or 'poison*'). These searches identified 242 papers of which 116 described ECMO in conditions other than intoxications or were reviews. In total 46 publications selected for this manuscript were case reports or case series involving poisoned patients. ECMO TECHNIQUES: Two types of ECMO are used: veno-venous ECMO (VV-ECMO) or veno-arterial ECMO (VA-ECMO). VV-ECMO is used for patients with severe ARDS to secure adequate oxygenation of the organs while protecting the lungs from harmful ventilation pressures or prolonged inspiratory fraction of oxygen. VA-ECMO can be used whenever the patient remains in shock despite adequate fluid resuscitation and is refractory to administration of inotropes and vasopressors.The organ support that can be applied with ECMO makes it especially useful in patients with severe poisoning as the clinical impact of the intoxication is often temporary; ECMO can be used as a 'bridge to recovery'.Absolute contraindications are uncontrolled coagulopathy and severe intracranial bleeding, which precludes the use of anticoagulation therapy. Relative contraindications to ECMO include advanced age, severe irreversible brain injury, untreatable metastatic cancer, severe organ dysfunction (some suggest a Sequential Organ Failure Assessment (SOFA) score > 15), and high pressure positive pressure ventilation for more than 7 days.The most common complication of ECMO is either bleeding at the cannulation site (in VV-ECMO) or bleeding at the surgical entry site (in VA-ECMO). Overall bleeding complications currently occur in 10-36% patients, and intracranial haemorrhage is seen in up to 6% of patients. ECMO should be reserved, therefore, for the most severely ill poisoned patients with a high risk of death. ECMO in poisoned patients. There are no randomised trials of ECMO in poisoned patients with refractory shock or who have ARDS caused by an intoxication. VV-ECMO can be considered in patients with type l and ll respiratory failure. In patients with life-threatening haemodynamic instability, VA-ECMO can be considered when shock persists despite volume administration, inotropes and vasoconstrictors, and (sometimes) intra-aortic balloon counterpulsation. Typical examples include poisoning due to calcium channel antagonists, beta-blockers, tricyclic antidepressants, chloroquine and colchicine. ETHICS OF ECMO USE: It is only ethical to use such a costly intervention (£19,252 and US$ 31,000 per quality-adjusted life year) if the treatment has a real purpose such as a 'bridge to recovery', a 'bridge to transplant', or a 'bridge to permanent assist device' (in the case of persistent cardiac failure).In the last decade, ECMO equipment has improved considerably, rendering it more biocompatible, and it has been used more frequently as an assist device for patients needing oxygenation as well as circulatory support. ECMO is considered a good salvage therapy for patients who are severely poisoned with ARDS or refractory circulatory shock.
Extracorporeal life support as a bridge to lung transplantation
DOI:10.1016/j.ccm.2011.02.005 URL [Cited within: 1]