INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a life-threatening syndrome with arterial hypoxemia, severe pulmonary edema, and impaired alveolar-capillary barriers caused by dysregulated inflammation.[1] The hospital mortality rate is 34.9% to 46.1%.[2] There is also a high prevalence of ARDS among patients admitted to intensive care units (ICUs). However, effective treatment is still lacking despite conventional management strategies, such as the limitation of tidal volume, maintenance of adequate high positive end-expiratory pressure (PEEP), advanced infection management, and supportive therapies.[3,4]
Our previous study[5] indicated that corticosteroids could decrease the risk of ARDS in community-acquired pneumonia patients. Corticosteroids have also been reported to reduce the 28-day mortality rate in septic patients.[6] Animal studies[7,8] showed that corticosteroid treatment could reduce acute-phase mortality and ameliorate fibrosis in acute lung injury rat models. Corticosteroids might exert immunosuppressive properties to alleviate lung damage by inhibiting pro-inflammatory cytokine transcription[9] and promoting the secretion of anti-inflammatory mediators.[10] However, the therapeutic effect of corticosteroids has been conflicting in clinical trials.
Many randomized controlled trials (RCTs) reported that low-dose corticosteroids demonstrated the promise to reduce mortality in ARDS patients.[11,12,13,14] However, a trial conducted by Tongyoo et al[15] showed that low-dose hydrocortisone therapy failed to confer a significant survival benefit compared with placebo. So we aim to assess whether low-dose corticosteroids have beneficial therapeutic effects on decreasing mortality in ARDS patients by conducting a systematic review and meta-analysis.
METHODS
Protocol and registration
We performed this meta-analysis based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[16] and registered (ID: CRD42019130222) it at the PROSPERO website (http://www.crd.york.ac.uk/PROSPERO). The study was performed in accordance with the Cochrane Collaboration and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines, and the GRADE system was used to assess the quality of the evidence.[17]
Search strategy
Literature searches were conducted independently by YQC and XFD. The databases, including Medline (www.ncbi.nlm.nih.gov/pubmed), EMBASE (https://www.embase.com), and Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (https://www.cochranelibrary.com/central), were searched for articles published from their inception to May 2, 2020. We combined MeSH/Emtree and title/abstract keywords, such as “steroids”, “glucocorticoids”, “corticosteroids”, “acute lung injury”, “respiratory distress syndrome, adult”, “acute respiratory failure” and “shock lung”, to identify all RCTs and observational studies on corticosteroid treatment in ARDS patients (supplemental Table 1). Moreover, we attempted to find other potentially relevant studies by reviewing the references of eligible articles.
Table 1 Summary of identified RCT studies
| Study | Country | Center | Number of patients | Female/ male | Age, years (mean±SD) | Initial daily dosea | Time of treatment initiation | Duration of treatment (days) | Primary outcome | Secondary outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | MV free days (mean±SD) | New infection rate | ||||||||||||||
| SG | NG | SG | NG | SG | NG | SG | NG | SG | NG | |||||||
| Meduri et al[11] (2007) | USA | M | 63 | 28 | 44/47 | 50.1±15.3 | 53.2±15.3 | Infusion of Me 1 mg/(kg·d) | Early severe ARDS | Up to 28 | 15/63 | 12/28 | 16.5±10.1 | 8.7±10.2 | 27/63 | 16/28 |
| Meduri et al[12] (1998) | USA | M | 16 | 8 | 15/9 | 47.0±39.0 | 51.0±6.6 | Intravenous pushing Me 2 mg/(kg·d) | Unresolving ARDS | Up to 32 | 2/16 | 5/8 | NA | NA | 12/16 | 6/8 |
| Rezk et al[13] (2013) | Egypt | M | 18 | 9 | 4/23 | NA | NA | Infusion of Me 1 mg/(kg·d) | Early ARDS | Up to 28 | 0/18 | 3/9 | NA | NA | NA | NA |
| Villar et al[14] (2020) | Spain | M | 139 | 138 | 86/191 | 56.0±14.0 | 58.0±15.0 | Intravenously receiving Dex 20 mg/d | Early ARDS | Up to 10 | 29/139 | 50/138 | 12.3±9.9 | 7.5±9.0 | 33/139 | 35/138 |
| Tongyoo et al[15] (2016) | Thailand | S | 98 | 99 | 96/101 | 64.5±17.3 | 64.3±16.0 | Intravenous bolus Hy 200 mg/d | Early ARDS | 7 | 22/98 | 27/99 | 12.0±9.7 | 9.7±10.0 | 34/98 | 41/99 |
| Annane et al[21] (2006) | France | M | 85 | 92 | NA | NA | NA | Hy 200 mg/d and Flu 50 μg | Early ARDS | 7 | 54/85 | 67/92 | 4.9±8.4 | 3.1±6.9 | 12/85 | 12/92 |
| Steinberg et al[22] (2006) | USA | M | 89 | 91 | 82/98 | 49.0±19.0 | 49.2±16.5 | Intravenously receivng Me 2 mg/(kg·d) | Unresolving ARDS | Up to 25 | 26/89 | 26/91 | 11.2±9.4 | 6.8±8.5 | 25/89 | 43/91 |
| Liu et al[23](2012) | China | S | 12 | 14 | 7/19 | 69.8±14.9 | 55.9±15.3 | Intravenous bolus Hy 300 mg/d | Early ARDS | 7 | 3/12 | 6/14 | 13.9±11.3 | 12.8±11.3 | 3/12 | 2/14 |
RCT: randomized controlled trial; M: multi-center; S: single-center; ARDS: acute respiratory distress syndrome; SG: steroid group; NG: non-steroid group; SD: standard deviation; NA: not available; Me: methylprednisolone; Hy: hydrocortisone; Flu: fludrocortisone; Dex: dexamethasone; MV: mechanical ventilation; calculated on the basis of 4 mg of methylprednisolone=20 mg of hydrocortisone=5 mg of prednisone; a: a loading dose was administered in five trials, including hydrocortisone 200 mg,[
Eligibility criteria
This meta-analysis included studies that met the following population, intervention, comparators, outcomes, and study (PICOS) criteria: (1) population: adult patients with a definite ARDS diagnosis; (2) intervention: low-dose corticosteroid therapy was used after ARDS onset (low-dose corticosteroids meant that patients were treated with less than 250 mg prednisolone or equivalent per day); (3) comparison intervention: placebo or conventional therapy; (4) outcomes: either in-hospital or 28-day mortality, ventilator-free days, adverse events such as new infections, gastrointestinal bleeding, and hyperglycemia; and (5) study design: RCTs or observational studies. Observational studies were included to confirm the conclusions drawn from the RCTs. If the studies lacked our outcomes of interest or included nonhuman subjects or patients under 18 years old, they were excluded. If the full text was a commentary or review, it was also excluded.
Study selection and data extraction
YQC and XFD independently investigated and reviewed the titles and/or abstracts of all retrieved studies to identify eligible articles and then recorded the reasons for exclusion. Then, HYL and DW independently collected the characteristics of all eligible studies, including the first author, country, study design, multi-/single-center status, number of patients, female/male patient ratio, mean age, initial daily corticosteroid type and dose, time of treatment initiation, duration of treatment, definition of ARDS, primary and secondary outcomes, and indicators of the disease severity. Any disagreements were resolved through discussions among XJZ, LFL, QCK, LXW, and TWS.
Definitions
The number of ventilator-free days was defined as the number of days on which an ARDS patient was alive and free from mechanical ventilation after receiving corticosteroid treatment. The new infection rate was defined as a normally sterile site with a positive culture.
Quality assessment
The quality of the RCTs was evaluated using the method recommended by the Cochrane Collaboration. The study was deemed to have a high risk of bias if more than one item was assessed as having a high risk of bias. If all domains of the outcome indicators were assessed as having a low risk of bias, the study was deemed to have a low risk of bias. A quality assessment of the studies reporting adverse events was also performed.[18] The Newcastle-Ottawa Scale (NOS) was used to assess the risk of bias in the included cohort studies.[19] A maximum of nine points could be obtained, with three points as the maximum for selection, three points as the maximum for design and analysis comparability, and three points as the maximum for the assessment of outcomes. Studies with a total score more than seven were considered to be of high quality, whereas scores of 4-6 indicated moderate quality, and scores less than 4 indicated low quality. The GRADE approach, which provided a clear and concise methodology to support the strength of a recommendation, was used to estimate and summarize the quality of the evidence. Trial sequential analysis (TSA)[20] was used to adjust for random error and estimate the sample size required for the meta-analysis. This method ensures that the results of comprehensive analyses are reliable.
Statistical analysis
All statistical analyses were conducted with Review Manager (RevMan, version 5.3, Cochrane Collaboration); Stata 14.0 (College Station, Texas, USA, Serial number: 401406267051); and the TSA program (version 0.9 beta, https://www.ctu.dk/tsa). For binary variables, we chose the odds ratio (OR) and 95% confidence intervals (CI) to express the treatment effect results. A fixed effect model or a random effect model with the inverse-variance method was used to calculate the pooled OR. The continuous variables were computed as the standardized mean difference (SMD) and 95% CI according to the reported mean and standard deviation. The heterogeneity of the included studies was evaluated by the I2 statistic: values of 0%-25% indicated no heterogeneity, 25%-50% mild heterogeneity, 50%-75% moderate heterogeneity, and 75%-100% high heterogeneity. A sensitivity analysis is a method of evaluating the effect of a single study on the pooled effect size. It is usually applied to estimate the robustness and reliability of the results.
RESULTS
Study selection
The initial literature search yielded 2,365 potentially relevant studies, and we identified two additional articles by reviewing the references of the selected studies. After removing 496 duplicate records, we preliminarily screened the titles and abstracts of the remaining 1,869 publications and found that 25 full-text articles met the inclusion criteria. Finally, we identified eight RCTs[11-15,21-23] and six cohort studies[24,25,26,27,28,29] in the systematic review and meta-analysis.
The records were excluded on title or abstract for reasons: not relevant population or study settings (n=1,704); experimental or animal studies (n=48); non-adult studies (n=12); review, letter comment, case report, and meta-analysis (n=80). The full-text articles were excluded for reasons: high-dose use of corticosteroids (n=5), use of corticosteroids before ARDS (n=2), no interested outcomes (n=4) (supplementary Figure 1).
Figure 1.
Figure 1.
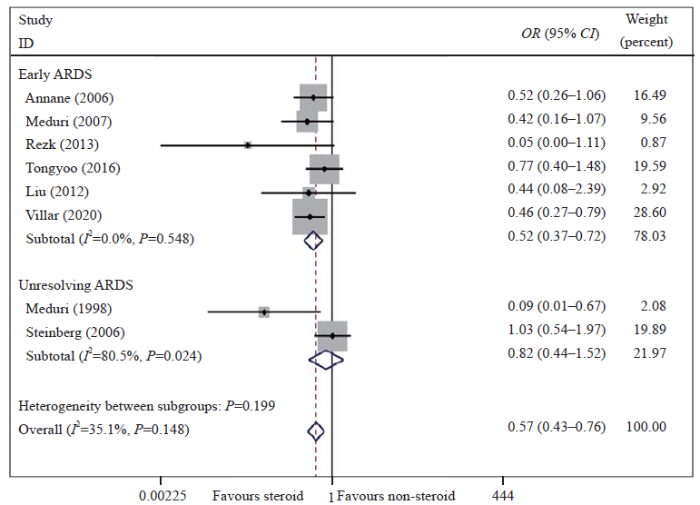
Meta-analysis of overall pooled and subgroup OR with 95% CI across RCTs for the primary outcomes in patients with ARDS. Forest plot showing the significance of the relationship between the use of low-dose corticosteroids and mortality in patients with early and unresolving ARDS according to the fixed effects model; RCTs: randomized controlled trials; OR: odds ratio; CI: confidence interval; ARDS: acute respiratory distress syndrome.
Study characteristics
In total, 999 and 799 ARDS patients were enrolled in the included eight RCTs and six cohort studies, respectively. In the eight RCTs, 520 patients received a low-dose of corticosteroid treatment. The time of corticosteroid therapy initiation was in the early phase of ARDS (within seven days of ARDS onset) in nine studies and in unresolving ARDS (more than seven days after ARDS onset) in the other five studies. We regarded more than seven days as prolonged corticosteroid use and no more than seven days as the short-term administration. The included studies reported the raw data or adjusted OR for mortality (in-hospital or 28-day), ventilator-free days, and adverse events. The main baseline characteristics of RCT studies are shown in Table 1, supplementary Table 2, and supplementary Table 3.
Quality assessment
Three trials[14,15,22] had a low risk of bias, four trials[12,13,21,23] were considered to have an unclear risk of bias, and one trial[11] was judged as having a high risk of bias because of the open-label methylprednisolone use. The summary quality scores of adverse events were eight in one study,[14] six in four studies,[13,15,21,22,23] and five in two studies[11,12] because they did not predefine adverse events or adequate statistical analysis of potential confounders (supplementary Figure 2). The NOS was used to assess the quality of the included cohort studies, and six eligible cohort studies[24,25,26,27,28,29] were regarded as being of high quality (supplementary Table 4).
Figure 2.
Figure 2.
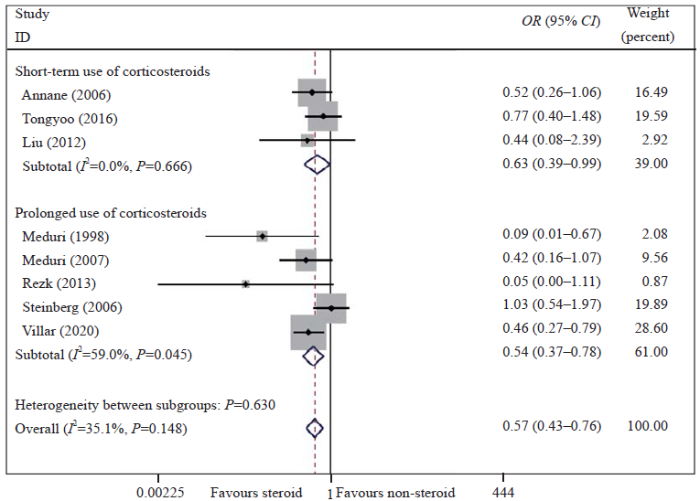
Meta-analysis of overall pooled and subgroup OR with 95% CI across RCTs for the primary outcomes in patients with ARDS. Forest plot showing the significance of the relationships between the prolonged use and short-term use of low-dose corticosteroids and mortality in ARDS patients according to the fixed effects model; RCTs: randomized controlled trials; OR: odds ratio; CI: confidence interval; ARDS: acute respiratory distress syndrome.
Primary outcomes
As determined with the fixed effects model, the pooled OR for in-hospital or 28-day mortality in the eight RCTs is shown in Figure 1 and Figure 2. The results showed that the mortality rate was lower in the corticosteroid treatment group than in the control group (OR 0.57, 95% CI 0.43-0.76, I2=35.1%, P=0.148). The GRADE assessment showed that the quality of the evidence was moderate (supplementary Table 5). We also conducted a TSA and found that the cumulative evidence crossed the traditional significant difference line and the TSA threshold, although our sample did not meet the expected sample size. The TSA results showed that no more experiments were needed, and confirmed our conclusions (supplementary Figure 3). Therefore, our conclusions were robust and reliable. The cohort studies also indicated that low-dose corticosteroids reduced mortality in ARDS patients (OR 0.51, 95% CI 0.27-0.95, I2=66.7%, P=0.010) (supplementary Figure 4).
Secondary outcomes
Six RCTs[11,14,15,21-23] reported the ventilator-free days. The results suggested that corticosteroid use may reduce the duration of mechanical ventilation (SMD 0.40, 95% CI 0.27-0.53, I2=26.1%, P=0.239) (supplementary Figure 5). Seven RCTs[11,12,14,15,21,22,23] reported that low-dose corticosteroid administration may reduce the rate of new infections (OR 0.73, 95% CI 0.55-0.98, I2=0%, P=0.470). However, the administration of low-dose corticosteroids was not associated with other adverse events including hyperglycemia and gastrointestinal bleeding (supplementary Figure 6).
Subgroup and sensitivity analyses
To reduce the mild heterogeneity, we performed a subgroup analysis according to the initiation time of corticosteroid treatment and the duration of treatment. The results showed that the mortality was significantly reduced in patients who received low-dose corticosteroids in the early phase of ARDS (OR 0.52, 95% CI 0.37-0.72, I2=0%, P=0.548) but not in those with unresolving ARDS (OR 0.82, 95% CI 0.44-1.52, I2=80.5%, P=0.024) (Figure 1). We also found that a prolonged treatment course was beneficial to patient survival (OR 0.54, 95% CI 0.37-0.78, I2=59.0%, P=0.045) (Figure 2). The sensitivity analysis was conducted by omitting a single study to evaluate the impact of any given study on the pooled ORs and 95% CIs. The sensitivity analysis outcomes indicated that the results were robust and reliable (supplementary Figures 7 and 8).
DISCUSSION
This meta-analysis included eight RCTs and six observational cohort studies and explored the treatment effect of low-dose corticosteroids. We found that low-dose corticosteroid administration could reduce mortality in ARDS patients, especially when the corticosteroids were administered in the early phase of ARDS and the course of treatment was prolonged. More ventilator-free days and a lower rate of new infections were also associated with corticosteroid use. Our analysis indicated that the prolonged low-dose corticosteroid treatment might be an appropriate therapy for patients with early ARDS.
In 1998, a pilot RCT[12] assessed the effect of low-dose methylprednisolone on unresolving ARDS patients and described a beneficial association between low-dose corticosteroids and reduced mortality in ARDS patients; moreover, a RCT[11] conducted in five hospitals showed that the mortality was significantly reduced after administering infusions of low-dose corticosteroids to patients with early ARDS, which was consistent with our outcomes. Chen et al[30] also found that the use of corticosteroids in patients with severe community-acquired pneumonia could reduce the mortality compared with the placebo. Some studies[15,22] showed no significant survival benefits in ARDS patients who used corticosteroids compared with those who did not. This meta-analysis provides evidence that the administration of low-dose corticosteroids might reduce mortality after the onset of ARDS.
The pathological processes of ARDS are divided into two phases: acute exudation (less than seven days after ARDS onset) and proliferation. Many fluids and proteins accumulate in the lung, resulting in pulmonary interstitial edema in the acute exudative stage. At this time, corticosteroids could inhibit inflammation and facilitate the repair of pulmonary epithelial cells.[31] This may be the reason why corticosteroids improve the prognosis of early ADRS patients. In addition, alveolar type II (ATII) cells and interstitial cells proliferate, and collagen is deposited in the lung, which leads to fibrosis in the lung as ARDS progresses.[32] Fibrosis is significantly associated with lung failure and an increased mortality rate. It was reported that methylprednisolone did not improve the histological characteristics of end-stage fibrotic lungs.[33] Therefore, the corticosteroid use may be associated with reduced mortality in early but not unresolving ARDS. In addition, the duration of corticosteroid treatment is another determinant of efficacy. The local and systemic inflammation in ARDS can last more than two weeks,[34] and long-term immune disorders require longer courses of corticosteroid treatment. Two studies[35,36] have reported that the prolonged use of corticosteroids improved pulmonary inflammation better than any other interventions in ARDS.
A meta-analysis is a means of assessing the overall effect by pooling the data from multiple studies and performing a statistical analysis. The pooled meta-analysis results have significant heterogeneity when the differences among the outcomes of the individual studies are greater than expected. Heterogeneity is considered acceptable among RCTs when the overall I2 statistic is less than 50%. Although one RCT was assessed as having a high risk of bias with regard to the mortality rate, this was not enough to influence the interpretation of the results. The overall risk of bias was low in the included RCTs and cohort studies; thus, the degree of methodological heterogeneity was small.
The present meta-analysis had some advantages. First, the definitions of ARDS were consistent. Baseline characteristics, including the initial daily dose, initiation time, and treatment duration, were clearly recorded in the included studies, which reduced the influence of those factors on the results. Second, observational studies were used to confirm the conclusions extracted from the RCTs. Finally, sensitivity analysis showed that results were robust and reliable.
However, this systematic review also had several limitations. First, only eight RCTs met eligibility criteria, even though we attempted to retrieve all relevant literature. Second, a small sample size of 999 ARDS patients was included. However, the Cochrane risk of bias assessment and GRADE assessment showed that our conclusions were supported. In addition, the TSA suggested that the cumulative score statistic crossed the traditional significant difference line and TSA threshold. Although the sample did not meet the expected sample size, confirmed conclusions were obtained. Third, the heterogeneity of ARDS (infectious and noninfectious) and whether combined with shock made it difficult to explain the effect of low-dose corticosteroids. However, we failed to extract relevant data to perform subgroup analyses. Therefore, additional high-quality studies on these aspects are needed.
CONCLUSIONS
This meta-analysis shows that the low-dose corticosteroid use is associated with the reduced mortality and more ventilator-free days, especially in patients with early ARDS and prolonged treatment.
ACKNOWLEDGMENTS
We would like to thank the Chinese Evidence-Based Medicine Center, West China Hospital, Sichuan University, for providing the Stata 14.0 statistical software.
Funding: This study was supported by the United Fund of National Natural Science Foundation of China (U2004110); the Leading Talent Fund in Science and Technology Innovation in Henan Province (194200510017); the Science and Technology People-Benefit Project of Zhengzhou (2019KJHM0001); the Special Fund for Young and Middle-Aged Medical Research from China International Medical Foundation (Z-2018-35); the Integrated Thinking Research Fund from China International Medical Foundation (Z-2016-23-2001); the Fund for Mechanism Study on Gabexate Mesilate in Treating Sepsis and Septic Shock (2019-hx-45).
Ethical approval: Not needed.
Conflicts of interest: The authors have no conflicts of interest to disclose.
Contributors: YQC and XFD contributed equally to this article. All the authors contributed substantially to the study. TWS, YQC, and XFD conceived of the study. HYL and DW contributed to the data interpretation. YQC and XFD contributed to the study protocol and wrote the article. XJZ, LFL, QCK, LXW, and TWS revised the article. All authors approved the final version.
All the supplementary files in this paper are available at www.wjem.com.cn.
Reference
Acute respiratory distress syndrome: the Berlin Definition
DOI:10.1001/jama.2012.5669
PMID:22797452
[Cited within: 1]

The acute respiratory distress syndrome (ARDS) was defined in 1994 by the American-European Consensus Conference (AECC); since then, issues regarding the reliability and validity of this definition have emerged. Using a consensus process, a panel of experts convened in 2011 (an initiative of the European Society of Intensive Care Medicine endorsed by the American Thoracic Society and the Society of Critical Care Medicine) developed the Berlin Definition, focusing on feasibility, reliability, validity, and objective evaluation of its performance. A draft definition proposed 3 mutually exclusive categories of ARDS based on degree of hypoxemia: mild (200 mm Hg < PaO2/FIO2 ≤ 300 mm Hg), moderate (100 mm Hg < PaO2/FIO2 ≤ 200 mm Hg), and severe (PaO2/FIO2 ≤ 100 mm Hg) and 4 ancillary variables for severe ARDS: radiographic severity, respiratory system compliance (≤40 mL/cm H2O), positive end-expiratory pressure (≥10 cm H2O), and corrected expired volume per minute (≥10 L/min). The draft Berlin Definition was empirically evaluated using patient-level meta-analysis of 4188 patients with ARDS from 4 multicenter clinical data sets and 269 patients with ARDS from 3 single-center data sets containing physiologic information. The 4 ancillary variables did not contribute to the predictive validity of severe ARDS for mortality and were removed from the definition. Using the Berlin Definition, stages of mild, moderate, and severe ARDS were associated with increased mortality (27%; 95% CI, 24%-30%; 32%; 95% CI, 29%-34%; and 45%; 95% CI, 42%-48%, respectively; P < .001) and increased median duration of mechanical ventilation in survivors (5 days; interquartile [IQR], 2-11; 7 days; IQR, 4-14; and 9 days; IQR, 5-17, respectively; P < .001). Compared with the AECC definition, the final Berlin Definition had better predictive validity for mortality, with an area under the receiver operating curve of 0.577 (95% CI, 0.561-0.593) vs 0.536 (95% CI, 0.520-0.553; P < .001). This updated and revised Berlin Definition for ARDS addresses a number of the limitations of the AECC definition. The approach of combining consensus discussions with empirical evaluation may serve as a model to create more accurate, evidence-based, critical illness syndrome definitions and to better inform clinical care, research, and health services planning.
Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries
DOI:10.1001/jama.2016.0291 URL [Cited within: 1]
The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia
DOI:10.1007/s00134-016-4325-4 URL [Cited within: 1]
A pulmonary source of infection in patients with sepsis-associated acute kidney injury leads to a worse outcome and poor recovery of kidney function
DOI:10.5847/wjem.j.1920-8642.2020.01.003 URL [Cited within: 1]
Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis
DOI:10.1378/chest.15-1733 URL [Cited within: 1]
Association of corticosteroid treatment with outcomes in adult patients with sepsis: a systematic review and meta-analysis
DOI:10.1001/jamainternmed.2018.5849
PMID:30575845
[Cited within: 1]

Although corticosteroids are widely used for adults with sepsis, both the overall benefit and potential risks remain unclear.To conduct a systematic review and meta-analysis of the efficacy and safety of corticosteroids in patients with sepsis.MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched from inception until March 20, 2018, and updated on August 10, 2018. The terms corticosteroids, sepsis, septic shock, hydrocortisone, controlled trials, and randomized controlled trial were searched alone or in combination. Randomized clinical trials (RCTs) were included that compared administration of corticosteroids with placebo or standard supportive care in adults with sepsis.Meta-analyses were conducted using a random-effects model to calculate risk ratios (RRs) and mean differences (MDs) with corresponding 95% CIs. Two independent reviewers completed citation screening, data abstraction, and risk assessment.Twenty-eight-day mortality.This meta-analysis included 37 RCTs (N = 9564 patients). Eleven trials were rated as low risk of bias. Corticosteroid use was associated with reduced 28-day mortality (RR, 0.90; 95% CI, 0.82-0.98; I2 = 27%) and intensive care unit (ICU) mortality (RR, 0.85; 95% CI, 0.77-0.94; I2 = 0%) and in-hospital mortality (RR, 0.88; 95% CI, 0.79-0.99; I2 = 38%). Corticosteroids were significantly associated with increased shock reversal at day 7 (MD, 1.95; 95% CI, 0.80-3.11) and vasopressor-free days (MD, 1.95; 95% CI, 0.80-3.11) and with ICU length of stay (MD, -1.16; 95% CI, -2.12 to -0.20), the sequential organ failure assessment score at day 7 (MD, -1.38; 95% CI, -1.87 to -0.89), and time to resolution of shock (MD, -1.35; 95% CI, -1.78 to -0.91). However, corticosteroid use was associated with increased risk of hyperglycemia (RR, 1.19; 95% CI, 1.08-1.30) and hypernatremia (RR, 1.57; 95% CI, 1.24-1.99).The findings suggest that administration of corticosteroids is associated with reduced 28-day mortality compared with placebo use or standard supportive care. More research is needed to associate personalized medicine with the corticosteroid treatment to select suitable patients who are more likely to show a benefit.
Effects of different corticosteroid doses and durations on smoke inhalation-induced acute lung injury and pulmonary fibrosis in the rat
DOI:10.1016/j.intimp.2019.03.051
[Cited within: 1]

Excessive inflammation induced by cytokine storm and coagulation disorders is considered the primary characteristic of smoke inhalation-induced acute lung injury (SI-ALI). Glucocorticoids such as methylprednisolone (MP) are commonly used to treat patients with inflammatory diseases; however, the management of ALI or acute respiratory distress syndrome (ARDS) remains controversial. We explored the effects of different MP doses and durations in a rat SI-ALI model. SI-ALI model rats had a high mortality rate and severe lung injury with proinflammatory, procoagulant, and pro-fibrotic changes. We found that a medium MP dose (4 mg/kg) markedly improved survival rates compared with low (0.4 mg/kg) and high (40 mg/kg) doses in the acute phase. A medium dose significantly attenuated lung injury, and reduced proinflammatory cytokine production and neutrophil infiltration into alveoli. Both medium and high MP doses improved coagulation and fibrinolysis conditions compared with low-dose MP. We also explored the effect of different durations of MP treatment on attenuating fibrotic changes in late-phase SI-ALI Pro-fibrotic chemokine levels were gradually increased, followed by an increase in collagen and fibrin deposition after smoke inhalation. Three and 7-day MP treatments significantly attenuated this process, which was reflected by a reduction in pro-fibrotic chemokine levels. There was no significant difference between 3- and 7-day treatments. We report that a medium MP (4 mg/kg) dose significantly reduced inflammation and coagulation disorders, as well as acute-phase mortality. Three-day MP treatment may sufficiently attenuate fibrotic changes in late-phase SI-ALI.
Dexamethasone attenuated endotoxin-induced acute lung injury through inhibiting expression of inducible nitric oxide synthase
DOI:10.3233/CH-2009-1162 URL [Cited within: 1]
Corticosteroids for ARDS
PMID:20473257
[Cited within: 1]

The aim of this study was to describe the role of glucocorticoids in immune modulation during critical illness and to review clinical trials and recent meta-analyses of glucocorticoids in early and late acute respiratory distress syndrome (ARDS). Selected reviews of publications, clinical trials, and meta-analyses were considered for the study. Activation of the adrenal axis is an important component of the compensatory anti-inflammatory response to critical illness. A recent meta-analysis of high doses of corticosteroids in patients with or at risk for ARDS demonstrated a trend for greater risk of the development of ARDS and a fatal outcome. Additional meta-analyses of four randomized trials and five cohort studies in patients with established ARDS or pneumonia indicated an overall mortality benefit with corticosteroids but this finding was not confirmed in another meta-analysis limited to randomized trials and excluding the trial focused on pneumonia. Lung function is improved and the duration of mechanical ventilation is reduced with prolonged administration of lower doses. In conclusion, short-duration, high-dose glucocorticoid therapy is not effective in preventing ARDS and may be harmful. Lower doses for persistent ARDS improve lung function and shorten the duration of mechanical ventilation but the impact on long-term mortality is unclear. Additional trials are needed to determine if corticosteroids improve important clinical outcomes before they can be recommended for the routine use of patients with unresolved ARDS.
Induction of cytokine receptors by glucocorticoids: functional and pathological significance
PMID:9745359
[Cited within: 1]

Current concepts on the role of glucocorticoid hormones in the regulation of inflammatory and immune responses depict this role as being inhibitory. Over the past decade, however, a large variety of studies have shown that glucocorticoids also exert stimulatory effects on immune function, suggesting that the present concept of the role of glucocorticoids in the immune system in not sufficient and needs to be extended. Here, Jan Wiegers and Hans Reul ask how these apparently paradoxical effects fit together and what their functional and pathological significance might be.
Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial
DOI:10.1378/chest.06-2100 URL [Cited within: 10]
Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial
DOI:10.1001/jama.280.2.159 URL [Cited within: 6]
Effects of methyl prednisolone in early ARDS
DOI:10.1016/j.ejcdt.2013.02.013 URL [Cited within: 6]
Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial
DOI:S2213-2600(19)30417-5
PMID:32043986
[Cited within: 7]

There is no proven specific pharmacological treatment for patients with the acute respiratory distress syndrome (ARDS). The efficacy of corticosteroids in ARDS remains controversial. We aimed to assess the effects of dexamethasone in ARDS, which might change pulmonary and systemic inflammation and result in a decrease in duration of mechanical ventilation and mortality.We did a multicentre, randomised controlled trial in a network of 17 intensive care units (ICUs) in teaching hospitals across Spain in patients with established moderate-to-severe ARDS (defined by a ratio of partial pressure of arterial oxygen to the fraction of inspired oxygen of 200 mm Hg or less assessed with a positive end-expiratory pressure of 10 cm HO or more and FiO of 0·5 or more at 24 h after ARDS onset). Patients with brain death, terminal-stage disease, or receiving corticosteroids or immunosuppressive drugs were excluded. Eligible patients were randomly assigned based on balanced treatment assignments with a computerised randomisation allocation sequence using blocks of 10 opaque, sealed envelopes to receive immediate treatment with dexamethasone or continued routine intensive care (control group). Patients in the dexamethasone group received an intravenous dose of 20 mg once daily from day 1 to day 5, which was reduced to 10 mg once daily from day 6 to day 10. Patients in both groups were ventilated with lung-protective mechanical ventilation. Allocation concealment was maintained at all sites during the trial. Primary outcome was the number of ventilator-free days at 28 days, defined as the number of days alive and free from mechanical ventilation from day of randomisation to day 28. Secondary outcome was all-cause mortality 60 days after randomisation. All analyses were done according to the intention-to-treat principle. This study is registered with ClinicalTrials.gov, NCT01731795.Between March 28, 2013, and Dec 31, 2018, we enrolled 277 patients and randomly assigned 139 patients to the dexamethasone group and 138 to the control group. The trial was stopped by the data safety monitoring board due to low enrolment rate after enrolling more than 88% (277/314) of the planned sample size. The mean number of ventilator-free days was higher in the dexamethasone group than in the control group (between-group difference 4·8 days [95% CI 2·57 to 7·03]; p<0·0001). At 60 days, 29 (21%) patients in the dexamethasone group and 50 (36%) patients in the control group had died (between-group difference -15·3% [-25·9 to -4·9]; p=0·0047). The proportion of adverse events did not differ significantly between the dexamethasone group and control group. The most common adverse events were hyperglycaemia in the ICU (105 [76%] patients in the dexamethasone group vs 97 [70%] patients in the control group), new infections in the ICU (eg, pneumonia or sepsis; 33 [24%] vs 35 [25%]), and barotrauma (14 [10%] vs 10 [7%]).Early administration of dexamethasone could reduce duration of mechanical ventilation and overall mortality in patients with established moderate-to-severe ARDS.Fundación Mutua Madrileña, Instituto de Salud Carlos III, The European Regional Development's Funds, Asociación Científica Pulmón y Ventilación Mecánica.Copyright © 2020 Elsevier Ltd. All rights reserved.
Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial
PMID:27741949
[Cited within: 9]

Authors of recent meta-analyses have reported that prolonged glucocorticoid treatment is associated with significant improvements in patients with severe pneumonia or acute respiratory distress syndrome (ARDS) of multifactorial etiology. A prospective randomized trial limited to patients with sepsis-associated ARDS is lacking. The objective of our study was to evaluate the efficacy of hydrocortisone treatment in sepsis-associated ARDS.In this double-blind, single-center (Siriraj Hospital, Bangkok), randomized, placebo-controlled trial, we recruited adult patients with severe sepsis within 12 h of their meeting ARDS criteria. Patients were randomly assigned (1:1 ratio) to receive either hydrocortisone 50 mg every 6 h or placebo. The primary endpoint was 28-day all-cause mortality; secondary endpoints included survival without organ support on day 28.Over the course of 4 years, 197 patients were randomized to either hydrocortisone (n = 98) or placebo (n = 99) and were included in this intention-to-treat analysis. The treatment group had significant improvement in the ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen and lung injury score (p = 0.01), and similar timing to removal of vital organ support (HR 0.74, 95 % CI 0.51-1.07; p = 0.107). After adjustment for significant covariates, day 28 survival was similar for the whole group (HR 0.80, 95 % CI 0.46-1.41; p = 0.44) and for the larger subgroup (n = 126) with Acute Physiology and Chronic Health Evaluation II score <25 (HR 0.57, 95 % CI 0.24-1.36; p = 0.20). With the exception of hyperglycemia (80.6 % vs. 67.7 %; p = 0.04), the rate of adverse events was similar. Hyperglycemia had no impact on outcome.In sepsis-associated ARDS, hydrocortisone treatment was associated with a significant improvement in pulmonary physiology, but without a significant survival benefit.ClinicalTrials.gov identifier NCT01284452 . Registered on 18 January 2011.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement
DOI:10.1016/j.ijsu.2010.02.007 URL [Cited within: 1]
What is “quality of evidence” and why is it important to clinicians?
DOI:10.1136/bmj.39490.551019.BE URL [Cited within: 1]
Methodological shortcomings predicted lower harm estimates in one of two sets of studies of clinical interventions
DOI:10.1016/j.jclinepi.2006.02.021 URL [Cited within: 1]
Trial sequential analysis in systematic reviews with meta-analysis
DOI:10.1186/s12874-017-0315-7
PMID:28264661
[Cited within: 1]

Background: Most meta-analyses in systematic reviews, including Cochrane ones, do not have sufficient statistical power to detect or refute even large intervention effects. This is why a meta-analysis ought to be regarded as an interim analysis on its way towards a required information size. The results of the meta-analyses should relate the total number of randomised participants to the estimated required meta-analytic information size accounting for statistical diversity. When the number of participants and the corresponding number of trials in a meta-analysis are insufficient, the use of the traditional 95% confidence interval or the 5% statistical significance threshold will lead to too many false positive conclusions (type I errors) and too many false negative conclusions (type II errors).Methods: We developed a methodology for interpreting meta-analysis results, using generally accepted, valid evidence on how to adjust thresholds for significance in randomised clinical trials when the required sample size has not been reached.Results: The Lan-DeMets trial sequential monitoring boundaries in Trial Sequential Analysis offer adjusted confidence intervals and restricted thresholds for statistical significance when the diversity-adjusted required information size and the corresponding number of required trials for the meta-analysis have not been reached. Trial Sequential Analysis provides a frequentistic approach to control both type I and type II errors. We define the required information size and the corresponding number of required trials in a meta-analysis and the diversity (D-2) measure of heterogeneity. We explain the reasons for using Trial Sequential Analysis of meta-analysis when the actual information size fails to reach the required information size. We present examples drawn from traditional meta-analyses using unadjusted naive 95% confidence intervals and 5% thresholds for statistical significance. Spurious conclusions in systematic reviews with traditional meta-analyses can be reduced using Trial Sequential Analysis. Several empirical studies have demonstrated that the Trial Sequential Analysis provides better control of type I errors and of type II errors than the traditional naive meta-analysis.Conclusions: Trial Sequential Analysis represents analysis of meta-analytic data, with transparent assumptions, and better control of type I and type II errors than the traditional meta-analysis using naive unadjusted confidence intervals.
Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome
DOI:10.1097/01.CCM.0000194723.78632.62 URL [Cited within: 6]
Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome
DOI:10.1056/NEJMoa051693 URL [Cited within: 6]
The effect of stress dose glucocorticoid on patients with acute respiratory distress syndrome combined with critical illness-related corticosteroid insufficiency
Established acute respiratory distress syndrome: benefit of corticosteroid rescue therapy
PMID:9730790
[Cited within: 2]

The mortality of the acute respiratory distress syndrome (ARDS) is still very high. There is some evidence based on case series suggesting that corticosteroids may be beneficial in the chronic fibroproliferative state of the disease. In a retrospective study we analysed the data of 31 non-trauma ARDS patients who had been on mechanical ventilation for at least 7 days. Thirteen cases received corticosteroids at a dosage equivalent to 100-250 mg methylprednisolone at the discretion of the attending physician in charge. Apart from corticosteroid administration, supportive care was identical in the treated and non-treated patient subgroups. Both groups were comparable regarding the relevant demographic, clinical and physiologic data, Apache-II score, radiological score, lung injury score and multiple organ failure score. Mortality in the treatment group was 38% (5/13) as opposed to 67% (12/18) in the untreated group (p = 0.117). There was a significant improvement in the PaO2/FIO2 ratio from a median of -26 to +5 mm Hg measured in a 48-hour period before and after corticosteroid treatment (p = 0.039). The response to corticosteroid therapy could not be predicted on the basis of clinical or physiologic data. Five patients responded within 48 h, and 3 showed a delayed response after 5-6 days. There were no significant complications attributable to corticosteroid treatment.Although the data of this first comparative study were retrospective, they suggest a beneficial effect of corticosteroid treatment in patients with established fibroproliferative ARDS. A prospective clinical trial, however, is highly warranted in order to fully elucidate the true effect of this therapeutic approach under controlled conditions.
Low-dose steroid therapy at an early phase of postoperative acute respiratory distress syndrome
DOI:10.1016/j.athoracsur.2004.07.079 URL [Cited within: 2]
Effects of methylprednisolone infusion on markers of inflammation, coagulation, and angiogenesis in early acute respiratory distress syndrome
DOI:10.1097/CCM.0b013e318232da5e URL [Cited within: 2]
Late steroid therapy in primary acute lung injury
DOI:10.1007/s001340051199 URL [Cited within: 2]
Corticosteroids in treatment of aspiration-related acute respiratory distress syndrome: results of a retrospective cohort study
DOI:10.1186/s12890-016-0194-4 URL [Cited within: 2]
Effect of corticosteroid therapy in the early phase of acute respiratory distress syndrome: a propensity-matched cohort study
DOI:10.3904/kjim.2019.153 URL [Cited within: 2]
Efficacy and safety of glucocorticoids in the treatment of community-acquired pneumonia: a meta-analysis of randomized controlled trials
DOI:10.5847/wjem.j.1920-8642.2015.03.002 URL [Cited within: 1]
Acute respiratory distress syndrome
DOI:10.1038/s41572-019-0069-0 URL [Cited within: 1]
Understanding ARDS-associated fibroproliferation
DOI:10.1007/s00134-014-3613-0 URL [Cited within: 1]
Corticosteroid rescue treatment of progressive fibroproliferation in late ARDS
PMID:8181346
[Cited within: 1]

Pulmonary fibroproliferation (PFP) is directly or indirectly the leading cause of death in patients with late ARDS. We previously reported our experience using intravenous corticosteroids (IVC) in 8 patients with late ARDS and now have expanded our observation to a total of 25 patients with severe fibroproliferation (mean lung injury score [LIS] 3) and progressive respiratory failure (RF). Thirteen patients had open-lung biopsy before treatment. Patients were started on IVC treatment (IVCT) an average of 15 +/- 7.5 days into mechanical ventilation (MV). Significant physiologic improvement (SPI) to IVCT was defined as a reduction in LIS of greater than 1 point or an increase in PaO2:FIO2 ratio of greater than 100. We observed three patterns of response: rapid responders (RR) had an SPI by day 7 (n = 15); delayed responders (DR) had an SPI by day 14 (n = 6); nonresponders (NR) were without SPI by day 14 (n = 4). Overall the following significant mean changes were seen within 7 days of IVCT: LIS from 3 to 2 (p = 0.001), PaO2:FIO2 from 162 to 234 (p = 0.0004), PEEP from 11 to 6.8 cm H2O (p = 0.001), chest radiograph score from 3.8 to 3.0 (p = 0.009), and VE from 16 to 13.6 L/min (p = 0.01). Development of pneumonia was related to the pattern of response. Surveillance bronchoscopy was effective in identifying pneumonia in eight afebrile patients. Nineteen of 25 (76 percent) patients survived the ICU admission. Comparisons were made between survivors (S) and nonsurvivors (NS) and among the three groups of responders. At the time ARDS developed, no physiologic or demographic variable could discriminate between S and NS. At the time of IVCT, only liver failure was more frequent in nonsurvivors (p = 0.035). Histologic findings at open-lung biopsy and pattern of physiologic response clearly predicted outcome. The presence of preserved alveolar architecture (p = 0.045), myxoid type fibrosis (p = 0.045), coexistent intraluminal bronchiolar fibrosis (p = 0.0045), and lack of arteriolar subintimal fibroproliferation (p = 0.045) separated S from NS. ICU survival rate was 86 percent in responders and 25 percent in nonresponders (p = 0.03). Only one death resulted from refractory respiratory failure.
Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study
PMID:17698689
[Cited within: 1]

Severe sepsis is common and frequently fatal, and community-acquired pneumonia (CAP) is the leading cause. Although severe sepsis is often attributed to uncontrolled and unbalanced inflammation, evidence from humans with infection syndromes across the breadth of disease is lacking. In this study we describe the systemic cytokine response to pneumonia and determine if specific patterns, including the balance of proinflammatory and anti-inflammatory markers, are associated with severe sepsis and death.This is a cohort study of 1886 subjects hospitalized with CAP through the emergency departments in 28 US academic and community hospitals. We defined severe sepsis as CAP complicated by new-onset organ dysfunction, following international consensus conference criteria. We measured plasma tumor necrosis factor, IL-6 (interleukin 6), and IL-10 levels daily for the first week and weekly thereafter. Our main outcome measures were severe sepsis and 90-day mortality.A total of 583 patients developed severe sepsis (31%), of whom 149 died (26%). Systemic cytokine level elevation occurred in 82% of all subjects with CAP. Mean cytokine concentrations were highest at presentation, declined rapidly over the first few days, but remained elevated throughout the first week, beyond resolution of clinical signs of infection. Cytokine levels were highest in fatal severe sepsis and lowest in CAP with no severe sepsis. Unbalanced (high/low) cytokine patterns were unusual (4.6%) and not associated with decreased survival. Highest risk of death was with combined high levels of the proinflammatory IL-6 and anti-inflammatory IL-10 cytokine activity (hazard ratio, 20.5; 95% confidence interval, 10.8-39.0) (P<.001).The circulating cytokine response to pneumonia is heterogeneous and continues for more than a week after presentation, with considerable overlap between those who do and do not develop severe sepsis. Unbalanced activation is uncommon, and mortality is highest when both proinflammatory and anti-inflammatory cytokine levels are high.
Cytokine production and monocyte HLA-DR expression as predictors of outcome for patients with community-acquired severe infections
PMID:14715564
[Cited within: 1]

This study was performed to evaluate the impact of pro- and anti-inflammatory molecules and human leukocyte antigen DR (HLA-DR) expression as markers of immune status for the final outcome of septic patients. The study included 30 patients with severe sepsis due to community-acquired infections. Concentrations of tumor necrosis factor alpha (TNF-alpha), interleukin-6 (IL-6), IL-8, IL-10, and transforming growth factor beta1 (TGF-beta1) in serum, as well as monocyte HLA-DR expression, were determined on admission and on days 3, 10, 13, and 17 during hospitalization. Of the 30 patients enrolled, 13 survived, while 17 died during their hospital stay. All patients had significantly lower HLA-DR expression and higher pro- and anti-inflammatory cytokine levels than healthy individuals. HLA-DR expression was significantly decreased in nonsurvivors at almost all time points. In nonsurvivors, higher levels in serum of TNF-alpha on days 13 and 17; IL-6 levels on day 3; and IL-10 on days 3, 10, and 13 were found. Baseline levels of TGF-beta1 were significantly higher in survivors. Independent risk factors of mortality were IL-10 levels on days 3 and 10, while monocyte HLA-DR expression on admission was a good predictor for survival. Several pro- and anti-inflammatory cytokines are oversynthesized during severe infections, especially in patients with a poor outcome. Monocyte HLA-DR expression is an early and constant predictive marker for survival in severe sepsis, while serum IL-10 levels on days 3 and 10 have negative prognostic value for the final outcome.
Systemic inflammation-associated glucocorticoid resistance and outcome of ARDS
DOI:10.1196/annals.1321.004 URL [Cited within: 1]