INTRODUCTION
Percutaneous coronary intervention (PCI) is currently an effective and widely accepted treatment for patients with coronary artery disease (CAD). Despite advances in medical and interventional treatment modalities, many patients develop angina pectoris due to myocardial underperfusion.[1] In previously published reports, recurrent angina developed in 20%-30% of patients within one year following bare-metal stent (BMS) implantation.[2,3] Similar findings were observed in patients treated with drug-eluting stent (DES)[1] or bioresorbable vascular scaffold (BVS).[4,5]
Stent thrombosis and in-stent restenosis (ISR) are the two major causes of stent failure. However, DES registries and randomized trials have typically reported stent thrombosis rates less than 1% at one-year follow-up. Moreover, the rate of clinically relevant ISR was only 5% at one-year follow-up.[6] These structural mechanisms do not provide an adequate explanation for the striking 50% rate of persistent or recurrent angina following successful PCI.[7]
Then why do patients develop recurrent angina pectoris without restenosis after PCI? Previous studies have demonstrated that endothelial dysfunction[8] or microcirculation disorders[9] could reduce myocardial perfusion in patients without stent thrombosis or ISR. Such coronary microvascular impairment, as detected by increased microvascular resistance, has been verified as a pathogenetic factor of myocardial ischemia and as an independent predictor of poor clinical outcome in patients with cardiovascular disease.[10,11] There are several methods to assess coronary flow tardiness caused by microcirculation disorders. The index of microcirculatory resistance (IMR) is a new but somewhat invasive method commonly used in clinical practice to evaluate functional coronary microcirculation.[12,13] Notably, IMR requires coronary catheterization at a moderately high cost. In contrast, thrombolysis in myocardial infarction (TIMI) frame count is a relatively simple and economic procedure[14,15] that can be performed by a standardized review of the coronary angiogram. In addition to the assessment of epicardial coronary circulation, it has been reported to effectively and accurately evaluate functional microcirculation.[16]
The study is to investigate predictors of recurrent angina pectoris within one year in patients who have undergone successful coronary revascularization using PCI, but on repeat angiography have no need for secondary revascularization.
METHODS
Inclusion and exclusion
The cohort study comprised 3,837 patients with CAD, enrolled in Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, China, from January 2007 to June 2019. They had undergone successful PCI; some of them had redeveloped angina pectoris within one year after the procedure, but had no need for revascularization on repeat coronary angiography.
The inclusion criteria were: (1) using PCI, patients underwent complete coronary revascularization; (2) repeat coronary angiography was performed within one year± three months following PCI; (3) the TIMI flow grade at initial PCI reached level 3; (4) on repeat angiography, there was no stent restenosis or new stenosis, and no additional stent implantation was required. Exclusion criteria were: (1) severe heart failure, defined by an ejection fraction (EF) <40% or N-terminal pro-B-type natriuretic peptide (NT-proBNP) >2,000 pg/mL; (2) patients with co-morbidities that may have resulted in angina pectoris, such as left ventricular hypertrophy, valvopathy, or cardiomyopathy; (3) patients with atrial fibrillation or severe uncontrolled rhythm disturbance on an electrocardiogram.
The study was conducted according to the Declaration of Helsinki and was approved by the ethics committee of Sir Run Run Shaw Hospital.
Procedures and the primary and secondary endpoints
The initial PCI procedure was performed as per accepted, current general guidelines. All patients were treated according to standard guidelines with statin, aspirin, clopidogrel, or ticagrelor. The types of stents, implantation techniques, and the techniques of intravascular imaging were determined by operator preferences. All patients were followed up with telephone interview after PCI, but 18.7% of patients were lost due to the wrong telephone number or refusal. We consecutively enrolled the patients with follow-up angiogram, and vessel diameter stenosis <70% without the evidence of ischemia or fractional flow reserve (FFR) >0.80 was defined for this study as “no PCI intervention indicated”.
The TIMI frame count method was used to quantify blood flow through the coronary circulation and coronary microvasculature function.[16] Current studies have used TIMI frame counts of the left anterior descending (LAD) to determine the coronary flow velocity, because TIMI frame counts of the left circumflex and right coronary artery could confuse the results as patients might have either left crown dominant or right crown dominant coronary arteries. The TIMI frame count was performed using the methods as described in the literature.[17] Two investigators extracted the data independently, and the average of the two results was recorded. If the difference between the two measurements was greater than 10%, a third researcher would provide input, and the average of the two closest results was recorded.
The primary endpoint was defined as the development of recurrent angina pectoris within one year following PCI. The secondary endpoint was defined as the TIMI frame count in the follow-up angiogram.
Definitions
The TIMI frame count was defined as the number of cineframes required for contrast to the first reach standardized distal coronary landmarks in the artery.[17] Angina pectoris was a clinical syndrome characterized by discomfort in the chest, jaw, shoulder, back, or arms, typically elicited by exertion or emotional stress and relieved by rest or nitroglycerin.[18]
Statistical analysis
Statistical analysis was performed using the SPSS statistical package, version 24.0 (Chicago, Illinois, USA). Categorical variables were expressed as numbers (percentage) and compared using Chi-square test. Continuous variables were expressed as mean±standard deviation (SD) or median and interquartile range and compared using Student’s t-test or non-parametric Mann-Whitney U-test according to whether the continuous variables conformed to a normal distribution. Univariate and multivariate logistic regressions were performed to assess the risk factors for recurrent angina pectoris. In addition, multivariate linear regression was performed to identify the risk factors of TIMI frame counts. All reported P-values were two-sided, and the P-values <0.05 were considered statistically significant.
RESULTS
Baseline characteristics of patients with and without recurrent angina
Notably, 53.5% of patients in our cohort developed recurrent angina pectoris within one year following PCI; patients were 64.3±10.1 years old, 72.0% were male, 65.4% had hypertension, 22.8% had diabetes mellitus, and 24.7 % were current smokers.
Compared with patients who did not suffer recurrent angina pectoris, those who did were significantly older (62.97±10.10 years old vs. 65.45±9.90 years old, P<0.001), and had a higher incidence of hypertension (62.7% vs. 67.7%, P=0.002). In the group with recurrent angina pectoris, patients had a higher level of C-reactive protein (CRP), fewer of them achieved a low-density lipoprotein cholesterol (LDL-C) target goal of <1.8 mmol/L (P for both <0.05), and fewer were taking ezetimibe (4.3% vs. 2.7%, P=0.007). Specifically, the TIMI frame count was higher (coronary flow was slower) in the patients with recurrent angina pectoris (20.78±7.29 frames vs. 21.98±7.06 frames, P<0.001).
Predictors of post-PCI angina pectoris
Multivariate logistic regression showed that female sex (adjusted odds ratio [OR] 1.236, 95% confidence interval [CI] 1.044-1.464, P=0.014), older age (OR 1.313, 95% CI 1.221-1.412, P<0.001), current smoking (OR 1.210, 95% CI 1.026-1.428, P=0.024), and LDL-C level ≥1.8 mmol/L (OR 1.194, 95% CI 1.036-1.376, P=0.015) were associated with an increased risk of recurrent angina pectoris within one year following PCI. It was also demonstrated that a higher TIMI frame count (OR 1.026, 95% CI 1.017-1.036, P<0.001) was significantly correlated with post-PCI angina pectoris (Table 1).
Table 1 Logistics regression for post-PCI angina pectoris at one-year follow-up
| Variables | Univariate regression | Multivariate regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age | 1.281 | 1.201-1.367 | <0.001 | 1.313 | 1.221-1.412 | <0.001 |
| Female | 1.150 | 0.996-1.328 | 0.057 | 1.236 | 1.044-1.464 | 0.014 |
| BMI | 1.011 | 0.990-1.033 | 0.299 | 1.018 | 0.995-1.041 | 0.135 |
| Current smoking | 1.015 | 0.875-1.177 | 0.842 | 1.210 | 1.026-1.428 | 0.024 |
| Diabetes | 0.894 | 0.767-1.040 | 0.147 | 0.865 | 0.738-1.014 | 0.073 |
| Hypertension | 1.243 | 1.087-1.422 | 0.002 | 1.120 | 0.967-1.297 | 0.130 |
| CRP | 1.008 | 0.998-1.018 | 0.112 | 1.004 | 0.993-1.015 | 0.472 |
| PLT | 0.999 | 0.998-1.000 | 0.079 | 0.999 | 0.998-1.000 | 0.125 |
| LDL-C ≥1.8 mmol/L | 1.172 | 1.024-1.341 | 0.022 | 1.194 | 1.036-1.376 | 0.015 |
| SUA | 1.001 | 1.000-1.001 | 0.115 | 1.000 | 1.000-1.001 | 0.414 |
| Beta blocker | 1.150 | 1.012-1.306 | 0.032 | 1.210 | 1.057-1.386 | 0.006 |
| CCB | 1.130 | 0.909-1.405 | 0.271 | 1.088 | 0.862-1.375 | 0.447 |
| Dual antiplatelet | 0.989 | 0.862-1.134 | 0.873 | 1.025 | 0.887-1.185 | 0.737 |
| TIMI frame count | 1.024 | 1.015-1.033 | <0.001 | 1.026 | 1.017-1.036 | <0.001 |
| Multiple vessels PCI | 0.890 | 0.731-1.085 | 0.250 | 0.867 | 0.697-1.079 | 0.202 |
| Total stent length | 1.000 | 0.997-1.002 | 0.730 | 1.000 | 0.997-1.003 | 0.951 |
| Stent size >2.5 mm | 1.103 | 0.888-1.370 | 0.377 | 1.181 | 0.939-1.486 | 0.154 |
| EF | 0.997 | 0.990-1.003 | 0.307 | 0.998 | 0.991-1.006 | 0.680 |
BMI: body mass index; CRP: C-reactive protein; PLT: platelet; LDL-C: low-density lipoprotein cholesterol; SUA: serum uric acid; CCB: calcium channel blocker; EF: ejection fraction; TIMI: thrombolysis in myocardial infarction; PCI: percutaneous coronary intervention; OR: odds ratio; CI: confidence interval.
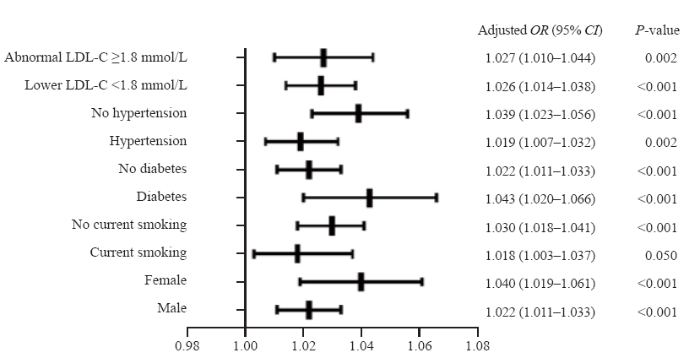
Quite impressively, a higher TIMI frame count of the LAD was consistently correlated with the incidence of post-PCI angina pectoris irrespective of the subgroup examined. This proved true (P for all <0.05, Figure 1) in patients whether they had hypertension (OR 1.019, 95% CI 1.007-1.032, P=0.002) or not (OR 1.039, 95% CI 1.023-1.056, P<0.001), diabetes (OR 1.043, 95% CI 1.020-1.066, P<0.001) or not (OR 1.022, 95% CI 1.011-1.033, P<0.001), female (OR 1.040, 95% CI 1.019-1.061, P<0.001) or male (OR 1.022, 95% CI 1.011-1.033, P<0.001), smoking (OR 1.018, 95% CI 1.003-1.037, P=0.050) or not (OR 1.030, 95% CI 1.018-1.041, P<0.001), and whether they achieved the LDL-C target goal (OR 1.026, 95% CI 1.014-1.038, P<0.001) or not (OR 1.027, 95% CI 1.010-1.044, P=0.002).
Figure 1.
Figure 1.
Multiple logistic regression of TIMI frame count of LAD and post-PCI angina in predefined subgroups. LDL-C: low-density lipoprotein cholesterol; OR: odds ratio; CI: confidence interval; LAD: left anterior descending; TIMI: thrombolysis in myocardial infarction; PCI: percutaneous coronary intervention.
Predictors of TIMI flow frame count
The univariate linear regression analysis revealed that the older age, female sex, body mass index (BMI), current smoking, diabetes mellitus, serum uric acid (SUA), and dose of beta-blocker were associated with a higher TIMI frame count, but hypertension and dual antiplatelet treatment were negatively correlated with a higher TIMI frame count (Table 2).
Table 2 Linear regression for TIMI frame count of LAD at one-year follow-up
| Variables | Simple regression | Multiple regression | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | P-value | β | 95% CI | P-value | |
| Age | 0.558 | 0.350-0.767 | <0.001 | 0.317 | 0.074-0.560 | 0.011 |
| Female | 1.877 | 1.397-2.357 | <0.001 | 1.651 | 1.079-2.223 | <0.001 |
| Body mass index | 0.199 | 0.124-0.275 | <0.001 | 0.155 | 0.077-0.233 | <0.001 |
| Current smoking | 0.863 | 0.333-1.394 | 0.001 | 0.042 | -0.522-0.607 | 0.884 |
| Diabetes | 0.722 | 0.272-1.295 | 0.010 | 0.717 | 0.178-1.256 | 0.009 |
| Hypertension | -0.930 | -1.412- -0.448 | <0.001 | -0.712 | -1.215- -0.209 | 0.006 |
| CRP | -0.007 | -0.040-0.027 | 0.692 | -0.013 | -0.045-0.020 | 0.440 |
| PLT | 0.000 | -0.004-0.004 | 0.928 | 0.001 | -0.004-0.005 | 0.814 |
| LDL-C ≥1.8 mmol/L | 0.199 | -0.286-0.684 | 0.420 | 0.017 | -0.467-0.501 | 0.946 |
| SUA | 0.005 | 0.002-0.007 | <0.001 | 0.001 | -0.002-0.003 | 0.593 |
| Beta blocker | 0.389 | -0.068-0.847 | 0.095 | 0.050 | -0.526-0.627 | 0.864 |
| CCB | -0.193 | -0.971-0.585 | 0.626 | 0.096 | -0.699-0.891 | 0.813 |
| Dual antiplatelet | -0.427 | -0.921-0.066 | 0.089 | -0.537 | -1.035- -0.040 | 0.034 |
| Multiple vessels PCI | 0.241 | -0.470-0.951 | 0.506 | 0.104 | -0.636-0.845 | 0.783 |
| Total stent length | -0.005 | -0.014-0.005 | 0.332 | -0.002 | -0.012-0.008 | 0.689 |
| Stent size >2.5 mm | 0.424 | -0.357-1.204 | 0.287 | 0.281 | -0.502-1.064 | 0.482 |
| EF | 0.003 | -0.021-0.027 | 0.825 | 0.015 | -0.009-0.040 | 0.216 |
TIMI: thrombolysis in myocardial infarction; LAD: left anterior descending; CRP: C-reactive protein; PLT: platelet; LDL-C: low-density lipoprotein cholesterol; SUA: serum uric acid; CCB: calcium channel blocker; PCI: percutaneous coronary intervention;EF: ejection fraction; CI: confidence interval.
After correction for the confounding factors screened from the univariate analysis, the multivariable linear regression analysis revealed that the female sex, older age, diabetes, and BMI were associated with a higher TIMI frame count. Patients with hypertension and patients that received standard dual antiplatelet therapy were negatively correlated with a higher TIMI frame count (Table 2).
DISCUSSION
This is a large study to investigate the incidence of recurrent angina pectoris and statistically identify risk factors for recurrent angina in patients without restenosis following successful and complete coronary revascularization at initial PCI. The major findings were: (1) the TIMI frame count was significantly correlated with post-PCI angina pectoris; (2) female sex, older age, current smoking, and LDL-C level ≥1.8 mmol/L were associated with an increased risk of recurrent angina pectoris following PCI; (3) female sex, older age, diabetes, and an elevated BMI were correlated with an increased TIMI frame count, while hypertension and standard dual antiplatelet therapy were negatively correlated with a higher TIMI frame count.
Despite advances in medical and interventional treatments, many patients develop recurrent angina pectoris without coronary artery stenosis following complete coronary revascularization at initial PCI.[19] In the present study, 53.5% of patients developed recurrent angina pectoris following PCI without revascularization at one-year follow-up.
In recent studies, an elevated BMI[20] and a higher number of stents[19] increased the risk of developing recurrent angina pectoris after PCI, whereas the administration of nicorandil reduced the risk.[21] The current study is consistent with this report as we found that an elevated BMI correlated with a higher TIMI frame count. Nicorandil was not included in the present study due to its extremely low usage in patients following PCI.
A study reported cardiac pain after PCI in the absence of ischemic events.[7] A more intense long-term endothelial dysfunction[8] was proposed as a possible mechanism. Similarly, microcirculation disorders[9] after coronary artery stenting have also been indicated. The TIMI frame count has been demonstrated to be useful in detecting coronary flow changes in patients with stent implantation[22] or impaired coronary microcirculation in patients who have impaired flow and increased burden of coronary atherosclerosis.[23] Previous observations have reported that females,[11,24,25] elderly patients, diabetes mellitus patients,[26,27] and patients with an elevated BMI[28] have been more likely to develop coronary microcirculation dysfunction. These reports are consistent with our study. Angina pectoris without recurrent coronary artery occlusion had a significant correlation with microcirculation disorders. After PCI, the reperfusion of ischemic tissue can cause widespread microvascular dysfunction that significantly exacerbates cardiovascular damage.[29] Platelets are critical mediators of inflammation during reperfusion injury, and a hyperactive platelet phenotype may contribute to exaggerated microcirculation dysfunction. Standard dual antiplatelet therapy can effectively inhibit platelet activation, reduce damage to microcirculation function, and improve myocardial blood perfusion.[30] This could provide the basis for our finding that dual antiplatelet therapy reduced the TIMI frame count. Hypertensive patients have been shown to have higher baseline coronary velocity as compared with healthy controls,[31] again consistent with this study.
The study intended to identify risk factors for recurrent angina pectoris following PCI. Some patient factors cannot be altered, such as sex and age. However, the risk of developing post-PCI recurrent angina might be altered by quitting smoking to decrease endothelial inflammation and improve coronary microcirculation. Additionally, changes in diet, lifestyle, and pharmacological treatments to control weight and reduce both LDL-C and blood glucose are realistic and valuable. These measures have been shown to be effective in improving coronary microcirculation, and reduce the incidence of recurrent angina. As noted previously, standard dual antiplatelet therapy can effectively inhibit platelet activation, reduce damage to microcirculation function, and improve myocardial perfusion.
This study has several limitations. First, as a single-center retrospective study, residual confounding or selection bias cannot be completely ruled out. Second, as a retrospective study, not all patients underwent follow-up angiography. Patients who developed post-PCI angina pectoris reliably returned not only for follow-up, but for remedial action. Patients without recurrent angina pectoris were less likely to return. Third, a part of patients without angina would like to take non-invasive examinations such as coronary CTA in the one-year follow-up. These would lead to a certain degree of selection bias.
CONCLUSIONS
Female sex, older age, diabetes mellitus, and elevated BMI are associated with a higher TIMI frame count, which could develop coronary microcirculation dysfunction and recurrent angina pectoris after PCI. In addition, risk factors of current smoking and LDL-C level ≥1.8 mmol/L are statistically associated with recurrent angina pectoris. Treatment with dual antiplatelet therapy is negatively correlated with a higher TIMI frame count and the risk of recurrent angina pectoris.
Funding: This study was supported by Zhejiang Natural Science Foundation (LY18H020007).
Ethical approval: The study was approved by the Ethics Committee of Sir Run Run Shaw Hospital.
Conflicts of interest: The authors declare no competing interests.
Contributors: TX and YL contributed equally to this work. TX and YL proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
Reference
Temporal trends in patient-reported angina at 1 year after percutaneous coronary revascularization in the stent era: a report from the National Heart, Lung, and Blood Institute-sponsored 1997-2006 dynamic registry
DOI:10.1161/CIRCOUTCOMES.109.869131
URL
PMID:20031899
[Cited within: 2]

BACKGROUND: Percutaneous coronary intervention (PCI) has witnessed rapid technological advancements, resulting in improved safety and effectiveness over time. Little, however, is known about the temporal impact on patient-reported symptoms and quality of life after PCI. METHODS AND RESULTS: Temporal trends in post-PCI symptoms were analyzed using 8879 consecutive patients enrolled in the National Heart, Lung, and Blood Institute-sponsored Dynamic Registry (wave 1: 1997 [bare metal stents], wave 2: 1999 [uniform use of stents], wave 3: 2001 [brachytherapy], wave 4, 5: 2004, 2006 [drug eluting stents]). Patients undergoing PCI in the recent waves were older and more often reported comorbidities. However, fewer patients across the waves reported post-PCI angina at one year (wave 1 to 5: 24%, 23%, 18%, 20%, 20%; P(trend)<0.001). The lower risk of angina in recent waves was explained by patient characteristics including use of antianginal medications at discharge (relative risk [95% CI] for waves 2, 3, 4 versus 1: 1.0 [0.9 to 1.2], 0.9 [0.7 to 1.1], 1.0 [0.8 to 1.3], 0.9 [0.7 to 1.1]). Similar trend was seen in the average quality of life scores over time (adjusted mean score for waves 1 to 5: 6.2, 6.5, 6.6 and 6.6; P(trend)=0.01). Other factors associated with angina at 1 year included younger age, female gender, prior revascularization, need for repeat PCI, and hospitalization for myocardial infarction over 1 year. CONCLUSIONS: Favorable temporal trends are seen in patient-reported symptoms after PCI in routine clinical practice. Specific subgroups, however, remain at risk for symptoms at 1 year and thus warrant closer attention.
Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease
DOI:10.1056/NEJM200104123441502
URL
PMID:11297702
[Cited within: 1]

BACKGROUND: The recent recognition that coronary-artery stenting has improved the short- and long-term outcomes of patients treated with angioplasty has made it necessary to reevaluate the relative benefits of bypass surgery and percutaneous interventions in patients with multivessel disease. METHODS: A total of 1205 patients were randomly assigned to undergo stent implantation or bypass surgery when a cardiac surgeon and an interventional cardiologist agreed that the same extent of revascularization could be achieved by either technique. The primary clinical end point was freedom from major adverse cardiac and cerebrovascular events at one year. The costs of hospital resources used were also determined. RESULTS: At one year, there was no significant difference between the two groups in terms of the rates of death, stroke, or myocardial infarction. Among patients who survived without a stroke or a myocardial infarction, 16.8 percent of those in the stenting group underwent a second revascularization, as compared with 3.5 percent of those in the surgery group. The rate of event-free survival at one year was 73.8 percent among the patients who received stents and 87.8 percent among those who underwent bypass surgery (P<0.001 by the log-rank test). The costs for the initial procedure were $4,212 less for patients assigned to stenting than for those assigned to bypass surgery, but this difference was reduced during follow-up because of the increased need for repeated revascularization; after one year, the net difference in favor of stenting was estimated to be $2,973 per patient. CONCLUSION: As measured one year after the procedure, coronary stenting for multivessel disease is less expensive than bypass surgery and offers the same degree of protection against death, stroke, and myocardial infarction. However, stenting is associated with a greater need for repeated revascularization.
Medical therapy in patients undergoing percutaneous coronary intervention: results from the ROSETTA registry
URL
PMID:12915928
[Cited within: 1]

BACKGROUND: Previous studies have examined medication use among patients with coronary artery disease who have suffered an acute myocardial infarction (MI). However, little is known about medication use among patients with coronary artery disease who undergo percutaneous coronary intervention (PCI). OBJECTIVE: To examine the patterns of use of medical therapy among patients who undergo PCI; and to examine the determinants of medical therapy in these patients. METHODS: The Routine versus Selective Exercise Treadmill Testing after Angioplasty (ROSETTA) registry is a prospective multicentre study examining the use of functional testing after PCI. The medication use was examined among 787 patients who were enrolled in the ROSETTA registry at 13 clinical centres in five countries. RESULTS: Most patients were men (mean age 61+/-11 years, 76% male) who underwent single vessel PCI (85%) with stent implantation (58%). At admission, discharge and six months, rates of acetylsalicylic acid use were 77%, 96% and 93%, respectively (discharge versus six months, P<0.0001). Rates of use of other oral antiplatelet agents were 11%, 59% and 2% (P=0.02). For individual anti-ischemic medications, rates of use were as follows: beta-blockers 49%, 58% and 59% (P<0.0001); calcium antagonists 34%, 43% and 42% (P<0.0001); and nitrates 42%, 56% and 43% (P<0.0001). Rates of use of combination anti-ischemic medications were as follows: triple therapy 7%, 9% and 9% (P<0.0001); double therapy 34%, 47% and 38% (P<0.0001); monotherapy 36%, 36% and 41% (P<0.0001); and no anti-ischemic therapy 23%, 8% and 12% (P<0.0001). Rates of use of angiotensin-converting enzyme inhibitors were 25%, 33% and 32% (P<0.0001), and rates of use of lipid lowering agents were 41%, 52% and 61% (P<0.0001). CONCLUSIONS: Trials and guidelines statements have favourably affected the rates of use of acetylsalicylic acid and other antiplatelet agents after PCI. However, in spite of patients undergoing a successful revascularization procedure, physicians do not reduce the use of anti-ischemic medical therapy.
Everolimus-eluting bioresorbable scaffolds for coronary artery disease
DOI:10.1056/NEJMoa1509038
URL
PMID:26457558
[Cited within: 1]

BACKGROUND: In patients with coronary artery disease who receive metallic drug-eluting coronary stents, adverse events such as late target-lesion failure may be related in part to the persistent presence of the metallic stent frame in the coronary-vessel wall. Bioresorbable vascular scaffolds have been developed to attempt to improve long-term outcomes. METHODS: In this large, multicenter, randomized trial, 2008 patients with stable or unstable angina were randomly assigned in a 2:1 ratio to receive an everolimus-eluting bioresorbable vascular (Absorb) scaffold (1322 patients) or an everolimus-eluting cobalt-chromium (Xience) stent (686 patients). The primary end point, which was tested for both noninferiority (margin, 4.5 percentage points for the risk difference) and superiority, was target-lesion failure (cardiac death, target-vessel myocardial infarction, or ischemia-driven target-lesion revascularization) at 1 year. RESULTS: Target-lesion failure at 1 year occurred in 7.8% of patients in the Absorb group and in 6.1% of patients in the Xience group (difference, 1.7 percentage points; 95% confidence interval, -0.5 to 3.9; P=0.007 for noninferiority and P=0.16 for superiority). There was no significant difference between the Absorb group and the Xience group in rates of cardiac death (0.6% and 0.1%, respectively; P=0.29), target-vessel myocardial infarction (6.0% and 4.6%, respectively; P=0.18), or ischemia-driven target-lesion revascularization (3.0% and 2.5%, respectively; P=0.50). Device thrombosis within 1 year occurred in 1.5% of patients in the Absorb group and in 0.7% of patients in the Xience group (P=0.13). CONCLUSIONS: In this large-scale, randomized trial, treatment of noncomplex obstructive coronary artery disease with an everolimus-eluting bioresorbable vascular scaffold, as compared with an everolimus-eluting cobalt-chromium stent, was within the prespecified margin for noninferiority with respect to target-lesion failure at 1 year. (Funded by Abbott Vascular; ABSORB III ClinicalTrials.gov number, NCT01751906.).
Evaluation of a fully bioresorbable vascular scaffold in patients with coronary artery disease: design of and rationale for the ABSORB III randomized trial
URL PMID:26386787 [Cited within: 1]
Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014
URL PMID:26417060 [Cited within: 1]
Angina after percutaneous coronary intervention: the need for precision medicine
DOI:10.1016/j.ijcard.2017.07.105
URL
PMID:28807510
[Cited within: 2]

Persistence or recurrence of angina after successful percutaneous coronary intervention (PCI) represent an important clinical issue involving from one fifth to one third of patients undergoing myocardial revascularization at one-year follow-up. A systematic approach to this syndrome is strongly needed. Precision medicine is particularly important in addressing angina after successful PCI because of the multiple underlying causes. Restenosis or coronary atherosclerosis progression explain symptom recurrence after successful PCI in some patients, while functional causes, including vasomotor abnormalities of epicardial coronary arteries and/or coronary microvascular dysfunction, explain symptoms in the remaining patients. In this review, we summarize the mechanisms of persistent or recurrent angina after PCI, proposing a diagnostic algorithm and a systematic therapeutic approach.
Endothelial dysfunction as predictor of angina recurrence after successful percutaneous coronary intervention using second generation drug eluting stents
DOI:10.1177/2047487318777435
URL
PMID:29785885
[Cited within: 2]

Background The role of endothelial dysfunction in predicting angina recurrence after percutaneous coronary intervention is unknown. Design We assessed the role of peripheral endothelial dysfunction measured by reactive-hyperaemia peripheral-artery tonometry (RH-PAT) in predicting recurrence of angina after percutaneous coronary intervention. Methods We enrolled consecutive patients undergoing percutaneous coronary intervention with second-generation drug-eluting stents. RH-PAT was measured at discharge. The endpoint was repeated coronary angiography for angina recurrence and/or evidence of myocardial ischaemia at follow-up. Patients with in-stent restenosis and/or significant de novo stenosis were defined as having angina with obstructed coronary arteries (AOCA); all other patients as having angina with non-obstructed coronary arteries (ANOCA). Results Among 100 patients (mean age 66.7 +/- 10.4 years, 80 (80.0%) male, median follow-up 16 (3-20) months), AOCA occurred in 14 patients (14%), ANOCA in nine patients (9%). Repeated coronary angiography occurred more frequently among patients in the lower RH-PAT index tertile compared with middle and upper tertiles (14 (41.2%) vs. 6 (18.2%) vs. 3 (9.1%), p = 0.006, respectively). ANOCA was more frequent in the lower RH-PAT index tertile compared with middle and upper tertiles. In the multivariate regression analysis, the RH-PAT index only predicted angina recurrence. The receiver operating characteristic curve of the RH-PAT index to predict the angina recurrence demonstrated an area under the curve of 0.79 (95% confidence interval: 0.69-0.89; p < 0.001), with a cut-off value of 1.705, having sensitivity 74% and specificity 70%. Conclusions Non-invasive assessment of peripheral endothelial dysfunction using RH-PAT might help in the prediction of recurrent angina after percutaneous coronary intervention, thus identifying patients who may need more intense pharmacological treatment and risk factor control.
Thermodilutional confirmation of coronary microvascular dysfunction in patients with recurrent angina after successful percutaneous coronary intervention
DOI:10.1016/j.cjca.2015.03.004
URL
PMID:26088108
[Cited within: 2]

BACKGROUND: Recurrent angina (RA) after percutaneous coronary intervention (PCI) remains a challenging problem that confronts cardiologists in routine clinical practice. In patients without epicardial coronary causes, RA is commonly speculated as resulting from coronary microvascular dysfunction. The aim of this study was to investigate the coronary microvascular function in patients with RA late after successful PCI and without epicardial stenosis at the time of repeat angiography. METHODS: We studied 39 consecutive patients with RA in whom PCI was successfully performed 6 to 12 months previously because of angina and single-vessel disease and without restenosis and disease progression at the time of repeat angiography. Twelve subjects without RA were recruited as the control group. Thermodilution-derived coronary flow reserve (CFR) and index of microvascular resistance (IMR) were measured using a pressure-temperature sensor-tipped coronary wire. The exercise treadmill test was performed according to the Bruce protocol. RESULTS: Patients with RA showed significantly higher IMR and lower CFR than control subjects, in the target arteries and in the reference vessels (P < 0.05). The hyperemic IMR was more remarkably increased in the target arteries than in the reference vessels (29.3 +/- 11.7 vs 24.4 +/- 9.7; P = 0.008). The hyperemic IMR was increased and the CFR was impaired more significantly in patients with a positive exercise treadmill test (P < 0.05). CONCLUSIONS: Using an intracoronary thermodilution method, to our knowledge, we have for the first time confirmed that, in patients who underwent successful coronary stenting and without epicardial stenosis at repeat angiography late after PCI, coronary microvascular dysfunction was responsible for the RA.
Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management
DOI:10.1161/CIRCULATIONAHA.109.900191 URL PMID:20516386 [Cited within: 1]
Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (women’s ischemia syndrome evaluation) study
DOI:10.1016/j.jacc.2010.01.054
URL
PMID:20579539
[Cited within: 2]

OBJECTIVES: We investigated whether coronary microvascular dysfunction predicts major adverse outcomes during follow-up among women with signs and symptoms of ischemia. BACKGROUND: Altered coronary reactivity occurs frequently in women evaluated for suspected ischemia, and the endothelium-dependent component is linked with adverse outcomes. Possible links between endothelium-independent microvascular coronary reactivity and adverse outcomes remain uncertain. METHODS: As part of the National Heart, Lung and Blood Institute-sponsored WISE (Women's Ischemia Syndrome Evaluation), we investigated relationships between major adverse outcomes and baseline coronary flow reserve (CFR) after intracoronary adenosine in 189 women referred to evaluate suspected ischemia. RESULTS: At a mean of 5.4 years, we observed significant associations between CFR and major adverse outcomes (death, nonfatal myocardial infarction, nonfatal stroke, or hospital stay for heart failure). An exploratory receiver-operator characteristic analysis identified CFR <2.32 as the best discriminating threshold for adverse outcomes (event rate 26.7%; and >or=2.32 event rate 12.2%; p = 0.01). Lower CFR was associated with increased risk for major adverse outcomes (hazard ratio: 1.16, 95% confidence interval: 1.04 to 1.30; p = 0.009). This held true among the 152 women without obstructive coronary artery disease (CAD) (hazard ratio: 1.20, 95% confidence interval: 1.05 to 1.38; p = 0.008). The CFR significantly improved prediction of adverse outcomes over angiographic CAD severity and other risk conditions. CONCLUSIONS: Among women with suspected ischemia and atherosclerosis risk factors, coronary microvascular reactivity to adenosine significantly improves prediction of major adverse outcomes over angiographic CAD severity and CAD risk factors. These findings suggest that coronary microvessels represent novel targets for diagnostic and therapeutic strategies to predict and limit adverse outcomes in women. (Women's Ischemia Syndrome Evaluation [WISE]; NCT00000554).
Coronary microvascular disease and clinical prognosis in deferred lesions: the index of microcirculatory resistance
DOI:10.3233/CH-189403
URL
PMID:30584125
[Cited within: 1]

While fractional flow reserve (FFR) is a good diagnostic index to assess the myocardial ischemia, coronary flow reserve (CFR) and the index of microcirculatory resistance (IMR) can be used to address microvascular status without any significant epicardial disease. The independent predictors for FFR and IMR are totally different and acts differently on the macro- and micro-vascular dysfunction. In high FFR patients, low CFR and high IMR which indicates the presence of overt microvascular disease demonstrated poor prognosis. Thus, comprehensive physiological assessments using FFR, CFR and IMR could improve the ability to discriminate patients at high risk of future events.
The effect of elective implantation of the ABSORB bioresorbable vascular scaffold on coronary microcirculation: serial assessment using the index of microcirculatory resistance
DOI:10.1111/micc.12521
URL
PMID:30561875
[Cited within: 1]

INTRODUCTION: Stenting with metal stents can affect microcirculatory function. The impact of BVS on the microvascular network has not been studied. METHODS: A total of 30 patients with bifurcation disease of Medina (X,X,0) scheduled for elective PCI with the ABSORB BVS were studied. Pressure wire studies were performed before and after scaffold implantation and at a mean follow-up of 9 months. At each time point, FFR, IMR, and CFR were calculated using the thermodilution method. RESULTS: Following scaffold implantation, FFR change from pre-PCI, post-PCI and follow-up was 0.76, 0.92 and 0.91, respectively (P < 0.001 from pre to post-PCI and P = 0.91 from post-PCI to follow-up). There was a statistically significant improvement between pre- and post-procedural IMR (median 27.7 to 17.9, P = 0.02) and CFR (median 2.2 to 2.9, P = 0.02). Median IMR at follow-up (23.6) remained numerically lower than pre-procedure but this was not statistically significant (P = 0.05). Similarly, while median CFR at follow-up remained at post-procedural level (2.9), this effect did not reach statistical significance (P = 0.06). CONCLUSION: There is an immediate reduction in microvascular resistance after elective BVS implantation but this effect is not sustained long term.
Use of the TIMI frame count in the assessment of coronary artery blood flow and microvascular function over the past 15 years
DOI:10.1007/s11239-008-0220-3
URL
PMID:18425623
[Cited within: 1]

Since its introduction, the TIMI frame count method has contributed to the understanding of the pathophysiology of coronary artery disease. In this article, the evolution of the TFC method and its applicability in the assessment of various therapeutic modalities are described.
Effects of intracoronary injection of nicorandil and tirofiban on myocardial perfusion and short-term prognosis in elderly patients with acute ST-segment elevation myocardial infarction after emergency PCI
DOI:10.5847/wjem.j.1920-8642.2020.03.005
URL
PMID:32351648
[Cited within: 1]

BACKGROUND: This study investigated the effects of the intracoronary injection of nicorandil and tirofiban on myocardial perfusion and short-term prognosis in elderly patients with acute ST-segment elevation myocardial infarction (STEMI) after emergency percutaneous coronary intervention (PCI). METHODS: Seventy-eight STEMI patients with age >65 years who underwent emergency PCI were consecutively enrolled. These patients received conventional PCI and were randomly divided into a control group and a treatment group (n=39 per group). The control group received an intracoronary injection of tirofiban followed by a maintenance infusion for 36 hours after surgery. The treatment group received intracoronary injection of tirofiban and nicorandil, and then intravenous infusion of tirofiban and nicorandil 36 hours after surgery. The following parameters were measured: TIMI grade, corrected TIMI frame count (cTFC), TIMI myocardial perfusion grade (TMPG), ST-segment resolution (STR) rate 2 hours post-operatively, resolution of ST-segment elevation (STR) at 2 hours postoperatively, peak level of serum CK-MB, left ventricular end diastolic diameter (LVEDD) and left ventricular ejection fraction (LVEF) at 7-10 days postoperatively, and major adverse cardiac events (MACEs) in-hospital and within 30 days post-operatively. RESULTS: Compared with the control group, more patients in the treatment group had TIMI 3 and TMPG 3, and STR after PCI was significantly higher. The treatment group also had significantly lower cTFC, lower infarction relative artery (IRA), lower peak CK-MB, and no reflow ratio after PCI. The treatment group had significantly higher LVEDD and LVEF but lower incidence of MACEs than the control group. CONCLUSION: The intracoronary injection of nicorandil combined with tirofiban can effectively improve myocardial reperfusion in elderly STEMI patients after emergency PCI and improve short-term prognoses.
Coronary microvascular dysfunction in patients with microvascular angina: analysis by TIMI frame count
URL PMID:16220069 [Cited within: 2]
TIMI frame count: a quantitative method of assessing coronary artery flow
DOI:10.1161/01.cir.93.5.879
URL
PMID:8598078
[Cited within: 2]

BACKGROUND: Although the Thrombolysis in Myocardial Infarction (TIMI) flow grade is valuable and widely used qualitative measure in angiographic trials, it is limited by its subjective and categorical nature. METHODS AND RESULTS: In normal patients and patients with acute myocardial infarction (MI) (TIMI 4), the number of cineframes needed for dye to reach standardized distal landmarks was counted to objectively assess an index of coronary blood flow as a continuous variable. The TIMI frame-counting method was reproducible (mean absolute difference between two injections, 4.7 +/- 3.9 frames, n=85). In 78 consecutive normal arteries, the left anterior descending coronary artery (LAD) TIMI frame count (36.2 +/- 2.6 frames) was 1.7 times longer than the mean of the right coronary artery (20.4 +/- 3.0) and circumflex counts (22.2 +/- 4.1, P < .001 for either versus LAD). Therefore, the longer LAD frame counts were corrected by dividing by 1.7 to derive the corrected TIMI frame count (CTFC). The mean CTFC in culprit arteries 90 minutes after thrombolytic administration followed a continuous unimodal distribution (there were not subpopulations of slow and fast flow) with a mean value of 39.2 +/- 20.0 frames, which improved to 31.7 +/- 12.9 frames by 18 to 36 hours (P < .001). No correlation existed between improvements in CTFCs and changes in minimum lumen diameter (r=-.05, P=.59). The mean 90-minute CTFC among nonculprit arteries (25.5 +/- 9.8) was significantly higher (flow was slower) compared with arteries with normal flow in the absence of acute MI (21.0 +/- 3.1, P < .001) but improved to that of normal arteries by 1 day after thrombolysis (21.7 +/- 7.1, P=NS). CONCLUSIONS: The CTFC is a simple, reproducible, objective and quantitative index of coronary flow that allows standardization of TIMI flow grades and facilitates comparisons of angiographic end points between trials. Disordered resistance vessel function may account in part for reductions in flow in the early hours after thrombolysis.
Management of stable angina: a commentary on the European Society of Cardiology guidelines
DOI:10.1177/2047487316648475
URL
PMID:27222385
[Cited within: 1]

In 2013 the European Society of Cardiology (ESC) released new guidelines on the management of stable coronary artery disease. These guidelines update and replace the previous ESC guidelines on the management of stable angina pectoris, issued in 2006. There are several new aspects in the 2013 ESC guidelines compared with the 2006 version. This opinion paper provides an in-depth interpretation of the ESC guidelines with regard to these issues, to help physicians in making evidence-based therapeutic choices in their routine clinical practice. The first new element is the definition of stable coronary artery disease itself, which has now broadened from a 'simple' symptom, angina pectoris, to a more complex disease that can even be asymptomatic. In the first-line setting, the major changes in the new guidelines are the upgrading of calcium channel blockers, the distinction between dihydropyridines and non-dihydropyridine calcium channel blockers, and the presence of important statements regarding the combination of calcium channel blockers with beta-blockers. In the second-line setting, the 2013 ESC guidelines recommend the addition of long-acting nitrates, ivabradine, nicorandil or ranolazine to first-line agents. Trimetazidine may also be considered. However, no clear distinction is made among different second-line drugs, despite different quality of evidence in favour of these agents. For example, the use of ranolazine is supported by strong and recent evidence, while data supporting the use of the traditional agents appear relatively scanty.
Chest pain after percutaneous coronary intervention in patients with stable angina
URL PMID:27574412 [Cited within: 2]
The relationship of body mass index to percutaneous coronary intervention outcomes: does the obesity paradox exist in contemporary percutaneous coronary intervention cohorts? Insights from the British Cardiovascular Intervention Society Registry
URL PMID:28683933 [Cited within: 1]
Nicorandil prevents microvascular dysfunction resulting from PCI in patients with stable angina pectoris: a randomised study
URL PMID:24457276 [Cited within: 1]
The superiority of TIMI frame count in detecting coronary flow changes after coronary stenting compared to TIMI flow classification
URL PMID:12368511 [Cited within: 1]
Correlation between the TIMI risk score and high-risk angiographic findings in non-ST-elevation acute coronary syndromes: observations from the platelet receptor inhibition in ischemic syndrome management in patients limited by unstable signs and symptoms (PRISM-PLUS) trial
DOI:10.1016/j.ahj.2004.08.042
URL
PMID:15894966
[Cited within: 1]

BACKGROUND: The TIMI risk score for unstable angina and non-ST elevation myocardial infarction is an effective tool for predicting the risk of death and ischemic events among patients with non-ST elevation acute coronary syndromes, as well as for identifying those who are likely to benefit most from low-molecular-weight heparin and glycoprotein IIb/IIIa inhibition. METHODS: To explore the pathobiologic basis for this interaction, we evaluated the relationship between the risk score, assessed at presentation, and angiographic findings among patients with non-ST elevation acute coronary syndromes. Angiographic data regarding thrombus, epicardial flow, and lesion severity were available for 1491 patients from the angiographic substudy of PRISM-PLUS. RESULTS: Patients with risk scores of 5 to 7 (N = 435) were more likely to have a severe culprit stenosis (81% vs 58%, P < .001) and multivessel disease (80% vs 43%, P < .001) compared to those with scores of 0 to 2 (N = 220). The probability of left main disease (P = .01), visible thrombus, and impaired flow in the culprit lesion also increased progressively with rising risk scores (P < .001). Of the risk indicators that comprise the score, history of coronary disease, advanced age, and ST changes showed the strongest association with severe epicardial disease. Positive biomarkers of necrosis, ST changes, and prior aspirin use emerged as stronger correlates of visible thrombus and/or impaired culprit artery flow. CONCLUSIONS: The TIMI risk score identifies patients who are more likely to have intracoronary thrombus, impaired flow, and increased burden of coronary atherosclerosis. These findings likely explain in part the particular benefit of potent antithrombin and antiplatelet agents among patients with higher risk scores.
Cardiac magnetic resonance imaging for myocardial perfusion and diastolic function-reference control values for women
DOI:10.3978/j.issn.2223-3652.2015.09.03
URL
PMID:26885495
[Cited within: 1]

Angina, heart failure with preserved ejection fraction (HFpEF) and coronary microvascular dysfunction (CMD) in the absence of obstructive coronary artery disease (CAD) are more common in women and are associated with adverse cardiovascular prognosis. Cardiac magnetic resonance imaging (CMRI) is established for assessment of left ventricular (LV) morphology and systolic function and is increasingly used to assess myocardial perfusion and diastolic function. Indeed, stress CMRI allows measurement of myocardial perfusion reserve index (MPRI) using semi-quantitative techniques, and quantification of LV volumetric filling patterns provides valuable insight into LV diastolic function. The utility of these two techniques remains limited, because reference control values for MPRI and LV diastolic function in asymptomatic middle-aged, women have not previously been established. To address this limitation, we recruited twenty women, without clinical cardiovascular disease or cardiovascular risk factors, with normal maximal Bruce protocol exercise treadmill testing. Subjects underwent CMRI (1.5 tesla) using a standardized protocol of adenosine stress and rest perfusion and LV cinematic imaging. Commercially available with automated CMRI segmentation was used for calculation of MPRI, LV filling profiles, and ejection fraction. Mean age was 54+/-9 years and mean body mass index was 25+/-4 kg/m(3). The exercise treadmill testing results demonstrated a normotensive group with normal functional capacity and hemodynamic response. We report reference control values for semi-quantitative MPRI as well as measures of LV systolic and diastolic function including ejection fraction, stroke volume, peak filling rate (PFR), PFR adjusted for end-diastolic volume (EDV) and stroke volume, time to PFR, and EDV index. The data herein provide reference values for MPRI and diastolic function in a cohort of healthy, middle-aged of women. These reference values may be used for comparison with a variety of patient populations, including women with CMD and HFpEF.
Cardiac syndrome X and microvascular coronary dysfunction
DOI:10.1016/j.tcm.2012.07.014
URL
PMID:23026403
[Cited within: 1]

Women with cardiac chest pain indicated by signs and symptoms of myocardial ischemia in the absence of obstructive CAD are often labelled as cardiac syndrome X (CSX). A subset of patients with CSX may have symptoms of ischemia due to microvascular dysfunction. Angina due to microvascular coronary dysfunction (MCD) is an etiologic mechanism in women with vascular dysfunction. New data provide improve understanding of coronary vascular dysfunction and resultant myocardial ischemia that characterize MCD among patients with cardiac syndrome X. MCD has an adverse prognosis and health care cost expenditure comparable to obstructive CAD. The high prevalence of this condition, particularly in women, adverse prognosis and substantial health care costs, coupled with a lack of evidence regarding treatment strategies, places MCD as a research priority area.
Coronary microvascular dysfunction in rheumatoid arthritis compared to diabetes mellitus and association with all-cause mortality
Correction to: adenosine stress CMR T1-mapping detects early microvascular dysfunction in patients with type 2 diabetes mellitus without obstructive coronary artery disease
DOI:10.1186/s12968-017-0406-y
URL
PMID:29212500
[Cited within: 1]

In the original publication of this article [1] Fig. 1 was incorrect due to the use of a colour bar with wrong range in error.
Overweight status is associated with extensive signs of microvascular dysfunction and cardiovascular risk
DOI:10.1038/srep32282
URL
PMID:27578554
[Cited within: 1]

The aim of this present study was to investigate if overweight individuals exhibit signs of vascular dysfunction associated with a high risk for cardiovascular disease (CVD). One hundred lean and 100 overweight participants were recruited for the present study. Retinal microvascular function was assessed using the Dynamic Retinal Vessel Analyser (DVA), and systemic macrovascular function by means of flow-mediated dilation (FMD). Investigations also included body composition, carotid intimal-media thickness (c-IMT), ambulatory blood pressure monitoring (BP), fasting plasma glucose, triglycerides (TG), cholesterol levels (HDL-C and LDL-C), and plasma von Willebrand factor (vWF). Overweight individuals presented with higher right and left c-IMT (p = 0.005 and p = 0.002, respectively), average 24-h BP values (all p < 0.001), plasma glucose (p = 0.008), TG (p = 0.003), TG: HDL-C ratio (p = 0.010), and vWF levels (p = 0.004). Moreover, overweight individuals showed lower retinal arterial microvascular dilation (p = 0.039) and baseline-corrected flicker (bFR) responses (p = 0.022), as well as, prolonged dilation reaction time (RT, p = 0.047). These observations emphasise the importance of vascular screening and consideration of preventive interventions to decrease vascular risk in all individuals with adiposity above normal range.
Thromboinflammatory functions of platelets in ischemia-reperfusion injury and its dysregulation in diabetes
DOI:10.1055/s-0037-1613694
URL
PMID:29294493
[Cited within: 1]

Ischemia-reperfusion (IR) injury is a common complication of a variety of cardiovascular diseases, including ischemic stroke and myocardial infarction (MI). While timely re-establishment of blood flow in a thrombosed artery is the primary goal of acute therapy in these diseases, paradoxically, reperfusion of ischemic tissue can cause widespread microvascular dysfunction that significantly exacerbates organ damage. Reperfusion injury is associated with activation of the humoral and cellular components of the hemostatic and innate immune systems and also with excessive reactive oxygen species production, endothelial dysfunction, thrombosis, and inflammation. Platelets are critical mediators of thromboinflammation during reperfusion injury and a hyperactive platelet phenotype may contribute to an exaggerated IR injury response. This is particularly relevant to diabetes which is characteristically associated with hyperactive platelets, significantly worse IR injury, increased organ damage, and increased risk of death. However, the mechanisms underlying vulnerability to IR injury in diabetic individuals is not well defined, nor the role of
Cost-effectiveness of different durations of dual-antiplatelet use after percutaneous coronary intervention
DOI:10.1016/j.cjca.2017.10.004
URL
PMID:29275879
[Cited within: 1]

BACKGROUND: There is uncertainty regarding the optimal duration of dual-antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI). Our goal was to evaluate the cost-effectiveness of different durations of DAPT. METHODS: We created a probabilistic patient-level Markov microsimulation model to assess the discounted lifetime costs and quality-adjusted life years (QALYs) of short duration (3-6 months: short-duration group) vs standard therapy (12 months: standard-duration group) vs prolonged therapy (30-36 months: long-duration group) in patients undergoing PCI. RESULTS: The majority of patients in the model underwent PCI for stable angina (47.1%) with second-generation drug-eluting stents (62%) and were receiving clopidogrel (83.6%). Short-duration DAPT was the most effective strategy (7.163 +/- 1.098 QALYs) compared with standard-duration DAPT (7.161 +/- 1.097 QALYs) and long-duration DAPT (7.156 +/- 1.097 QALYs). However, the magnitude of these differences was very small. Similarly, the average discounted lifetime cost was CAN$24,859 +/- $6533 for short duration, $25,045 +/- $6533 for standard duration, and $25,046 +/- $6548 for long duration. Thus, in the base-case analysis, short duration was dominant, being more effective and less expensive. However, there was a moderate degree of uncertainty, because short duration was the preferred option in only approximately 55% of simulations at a willingness to pay threshold of $50,000. CONCLUSIONS: Based on a stable angina cohort receiving clopidogrel with second-generation stents, a short duration of DAPT was marginally better. However, the differences are minimal, and decisions about duration of therapy should be driven by clinical data, patient risk of adverse events, including bleeding, and cardiovascular events.
Coronary flow reserve in patients with resistant hypertension
DOI:10.1007/s00392-016-1043-4
URL
PMID:27747373
[Cited within: 1]

Resistant hypertension is associated with increased risk for cardiovascular events. Coronary flow reserve (CFR) is impaired in patients with hypertension and an independent predictor of cardiac mortality. However, there are no published data on CFR in the subset of treatment-resistant hypertension. The aim of this study was to assess CFR in patients with resistant hypertension. Twenty-five consecutive patients with primary resistant hypertension, scheduled for renal denervation, 25 matched patients with controlled hypertension, and 25 healthy controls underwent transthoracic colour Doppler echocardiography at rest and during adenosine infusion. Patients with hypertension were pair-matched with regard to age, sex, ischemic heart disease, diabetes mellitus, smoking status, and body-mass index. Healthy controls were selected according to age and sex. Mean flow velocity was measured in the left coronary anterior descending artery. Baseline mean flow velocities were similar in patients with controlled and resistant hypertension. CFR was significantly lower in patients with resistant hypertension as compared to individuals with non-resistant hypertension (2.7 +/- 0.6 vs. 3.1 +/- 0.8; p = 0.03). Systolic office blood pressure was significantly higher in patients with resistant hypertension (169 +/- 20 vs. 144 +/- 21 mm Hg; p < 0.01). Heart rate, ventricular mass, and ejection fraction were similar in the two groups. Healthy controls showed significantly lower baseline velocity, higher CFR, and lower blood pressure as compared to hypertensives. Resistant hypertension was associated with impaired CFR as compared to individuals with non-resistant hypertension indicating impaired cardiac microvascular function which may contribute to the increased risk of adverse outcome in patients with resistant hypertension.